Abstract
As the obesity epidemic has worsened, its impact on lung health and disease has become progressively evident. The interactions between obesity and the accompanying metabolic syndrome and diseases such as asthma, pneumonia, and acute respiratory distress syndrome (ARDS) have proven complex and often counterintuitive in human studies. Hence, there is a growing need for relevant experimental approaches to understand the interactions between obesity and the lung. To this end, researchers have increasingly exploited mouse models combining both obesity and lung diseases, including ARDS, pneumonia, and asthma. Such models have both complemented and advanced the understanding we have gained from clinical studies and have allowed elegant dissections of obesity’s effects on the pathogenesis of lung disease. Yet these models come with several critically important caveats that we must reflect on when interpreting their results.
The prevalence of obesity, especially extreme obesity (body mass index [BMI] ≥ 40 kg/m2), has been increasing rapidly over the past 2 decades in the United States and other developed countries (1). More than one-third of the American population is obese, and >5% is extremely obese (2). The public health consequences of this rise in obesity are considerable because obesity is associated with significant morbidities and increased all-cause mortality in both men and women (1). Although the effects of obesity and the metabolic syndrome on cardiovascular and endocrinological disease are well documented, the impact of these entities on the incidence, manifestations, and response to treatment of diseases of the lung is only beginning to be appreciated. Clinical studies have indicated that, among other diseases, obesity may have profound effects on asthma, acute respiratory distress syndrome (ARDS), and lung infection (as recently witnessed in the H1N1 pandemic). Given the rapid and what appears to be inexorable rise in obesity in the United States and beyond, how obesity alters the lung in health and disease is critically important to researchers and clinicians alike. However, studies to date suggest that these effects may be complex and at times counterintuitive. Hence, there is a growing need for relevant experimental approaches to understand the interactions between obesity and the lung.
The use of mouse modeling to investigate lung disease dates back to the studies of pneumococcal virulence by Wollstein and Meltzer (3) of more than 100 years ago (4). Subsequent murine models have been developed to dissect a broad array of lung diseases, including ARDS, pneumonia, pulmonary fibrosis, lung cancer, chronic obstructive pulmonary disease, and asthma, to mention but a few (5–10). Mouse modeling has evolved simultaneously to examine obesity and the consequent metabolic syndrome, as manifested by glucose intolerance, dyslipidemia, and a host of other metabolic and inflammatory abnormalities (11), and such models have been pivotal in our understanding of the vascular effects of obesity (12). The most commonly used obese mouse models are either mutant strains that are spontaneously hyperphagic, including ob/ob mice (leptin deficient) (13), db/db mice (deficient in the long form of the leptin receptor) (14), and CPEfat/fat mice (carboxypeptidase-E deficient) (15), or, increasingly, diet-induced models of obesity, typically using high compared with low fat chows (typically 45–60% fat versus 10% fat) over a period of weeks to months to induce obesity (16) (Table 1).
Table 1.
Overview of the Most Popular Murine Models of Obesity and Their Relative Metabolic Parameters
| Mouse Model | Obesity (Wks until Obese) | Dyslipidemia | Hyperglycemia | Leptin “Resistance” (Mutant) |
|---|---|---|---|---|
| Diet-induced obesity | +++ (20–25) | ++ | +/− | ++ |
| CPEfat/fat | ++ (12–15) | +++ | ++ | + |
| Db/db (ObRb−/−) | +++ (6–8) | ++ | +++ | (++)* |
| Ob/ob (Leptin−/−) | ++ (8–10) | + | +/− | (+++)† |
“Obesity” is categorized by mouse weight at time of separation from age/sex-matched lean control mouse weight by 20 g. Adapted by permission from Reference 36.
No long form of leptin receptor expressed.
No leptin expressed.
Recently, the intersection of these two areas of mouse modeling has been exploited to examine the effects of obesity and metabolic syndrome on several lung diseases, most prominently ARDS, pneumonia, and asthma. The results of these studies offer significant insight into initial clinical observations and suggest novel ways to dissect the complex interaction between obesity and the lung but they must be assessed with caution because of the limitations of such modeling.
Acute Lung Injury and ARDS
Although we remain at a relatively early stage in our understanding of obesity’s effects on acute lung injury and ARDS, mounting data suggest that a complex and as of yet incompletely understood interaction exists. Clinical studies examining the effects of diabetes on the risk of ARDS have shown a protective effect of this disease, yet no associated change in mortality from ARDS once established (17, 18). Less is known about obesity itself and the other facets of the metabolic syndrome. Studies controlling for diabetic status suggest that rising BMI is associated with an increased risk of the development of ARDS and other organ failures (19–21), as might be predicted from the baseline systemic proinflammatory state that accompanies obesity. Interestingly, however, substantial evidence also indicates that subsequent mortality from ARDS, and from critical illness in general, is, in fact, lower in obese patients (22–24). Although some controversy has accompanied these latter findings, additional studies carefully adjusting for potentially confounding diagnostic artifacts and process of care effects have confirmed this association (25, 26), and the limited clinical data available suggest that the witnessed survival advantage may be driven in part by an obesity-associated attenuation of the inflammatory milieu in patients with established ARDS (27).
Efforts using mouse models to examine obesity’s effects on ARDS pathogenesis have been similarly contradictory. Several studies have shown that obese mice develop less acute lung injury and inflammation after LPS (28), hyperoxia (29), or ozone exposures (30) than do lean control animals. Yet the findings are not uniform, with competing reports of increased lung inflammation and injury in obese mice after such exposures, even from the same investigators (31–34). Although disparate in their results, attempts to reconcile these studies may be instructive, and two variables in these models are worth highlighting in this regard: (1) the type and degree of initiating injury and (2) the timing of injury and examination. In the most severe exposure models, such as LPS (28) and hyperoxia (29), obesity appears to have an ameliorative effect on injury and inflammation, whereas in less injurious models such as acute ozone (30) and particulate (31) exposure, obesity appears to augment injury. Furthermore, there appears to be a reversal of obesity’s effects on lung injury as the duration (and perhaps severity) of precipitating exposure increases: for example, acute (3 h) versus subacute (72 h) ozone exposures yield diametrically opposite effects (increased and reduced, respectively) in the same model of obesity (30, 32). Lastly, the timing of examination after injury appears to influence the witnessed effects of obesity on injury. This is particularly clear in the setting of LPS injury, in which early times points (2–6 h) after injury appear to manifest a proinflammatory effect of obesity, but by 24 hours, the effect is opposite (28).
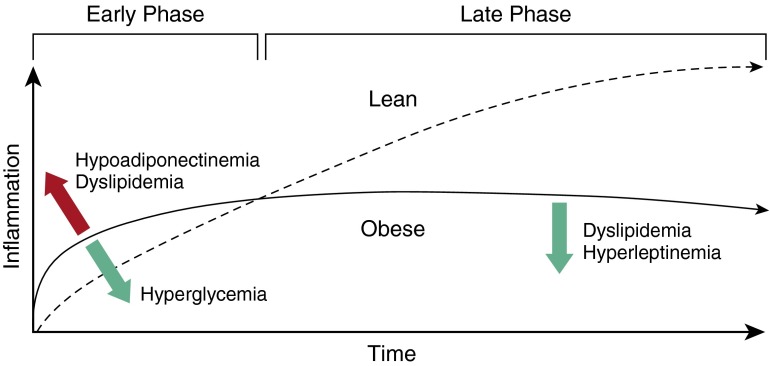
These findings may begin to reconcile the seemingly paradoxical findings in human obesity, in which susceptibility to ARDS appeared to be higher, yet the subsequent inflammatory state attenuated and survival improved. We must consider the possibility that obesity may alter ARDS pathogenesis by “priming” the lung for inflammatory insult and amplifying the early inflammatory response (thus lowering the threshold to initiate ARDS), while at the same time accelerating a subsequent transition to the recovery phase. How obesity may change the “inflammatory twitch” of the lung (35) is only beginning to be understood, but on the basis of recent mouse modeling, this appears to include both baseline pulmonary vascular “priming” (34) and neutrophil functional impairment (28, 36, 37), as well as other effects of elements of the metabolic syndrome, including hyperglycemia (29, 36), dyslipidemia (28, 36, 37), hypoadiponectinemia (34, 38), and hyperleptinemia (39). For example, recent studies by Shah and colleagues (34), examining several mouse strains that vary in their susceptibility to diabetes in a diet-induced obesity (DIO) model, have highlighted the complex interplay between glucose intolerance and lung injury. In these studies, obese diabetes-resistant mice showed augmented injury after LPS exposure compared with lean control mice, but this effect of obesity was reversed in the more diabetogenic strain. Although they confirm epidemiologic studies on the interaction between diabetes and ARDS, these findings suggest that the elements of the metabolic syndrome may counterbalance each other to shape the contour of the inflammatory twitch (Figure 1). Thus, the effects of obesity on lung inflammation after injury are likely to reflect an integration of the metabolic milieu in any given individual.
Figure 1.
Hypothetical effects of obesity on acute respiratory distress syndrome pathogenesis. Obesity and the metabolic syndrome may alter the kinetics of the “inflammatory twitch” in the lung after injury such that the course represents an “equilibrium” between the effects of multiple discrete elements of the metabolic syndrome. Apparent effects of hypoadiponectinemia, dyslipidemia, hyperglycemia, and hyperleptinemia are included as examples. Red upward arrow indicates increased inflammatory response. Green downward arrows indicate decreased inflammatory response.
Pneumonia
Obesity’s effects on pneumonia risk and severity have become well recognized only recently, after the H1N1 influenza pandemic. Although epidemiological studies of obesity and community-acquired bacterial pneumonia have been mixed, recent meta-analyses have shown a correlation between rising BMI and pneumonia risk (40), and studies of influenza A, particularly the H1N1 strain, have shown a clear correlation between obesity and influenza risk and severity (41, 42), as recently recognized by the CDC (43).
Mouse modeling of obese pneumonia has largely recapitulated the associations and uncertainties of clinical studies: clear obesity-associated susceptibility to and increased severity of influenza A infection have been shown (44–47), whereas obesity’s effects in bacterial pneumonia models have appeared variable, as recently reviewed by Mancuso (48). As might be anticipated from the above-noted findings in obese mouse models of sterile lung injury and inflammation, the pulmonary immune response to infectious pathogens also appears deranged. Pulmonary bacterial clearance and containment appear to be impaired in several obese mouse models, and studies have suggested a role for both neutrophil and macrophage dysfunction in this setting (36, 48). Despite likely impaired bacterial containment, the majority of studies have found little or no obesity-associated increase in lung viral titer in influenza infection, but instead demonstrate an overexuberant inflammatory response and accompanying lung injury (44–46). Whether this latter effect may reflect, in part, obesity-driven “priming” of the lung for inflammatory insult remains unclear. However, the adaptive immune response after both influenza infection and vaccination appears to be impaired in obese mice (47, 49), suggesting that a complex immune defect underlies obesity’s effects on influenza pathogenesis. In addition, using several strains of mutant mice, the roles of leptin signaling and its impairment have been implicated in obesity-associated alterations in the response to both bacterial and viral pneumonia (39, 50–52), as have diabetes and dyslipidemia (36, 37, 53), although these associations have yet to be demonstrated clearly in human pneumonia risk and severity. Variable expression of the metabolic syndrome in the mouse models studied is likely to contribute to the current lack of consensus on obesity’s effects, as demonstrated elsewhere in this issue (36) in a comparative study of Klebsiella pneumoniae pneumonia in DIO, db/db, CPEfat/fat, and ob/ob mouse models, in which the timing and degree of bacterial containment failure differed significantly between models. Clearly, much remains to be understood regarding obesity’s effect on the pathogenesis of pulmonary infection, but already it is apparent that such effects can be recapitulated in mouse models, suggesting that this approach is likely to be informative moving forward.
Asthma
As detailed elsewhere in this issue (54, 55), obesity has been associated with a greater prevalence and severity of asthma in human populations, and recent studies have strongly suggested that these associations reflect a causal connection, because weight gain has been shown to increase the risk of incident asthma and its severity (56, 57), whereas weight loss may ameliorate this effect (58, 59). Recent clinical studies have yielded tantalizing insights as to the possibility that “obese asthma” may reflect at least two distinct allergic and nonallergic phenotypes (60) and have suggested a differential role for discrete elements of the metabolic syndrome in altering both the innate and the adaptive arms of the immune response in asthma (61). However, for now, we remain far from a complete understanding of obesity’s effects on asthma pathogenesis and behavior.
Mouse models of obesity have perhaps contributed more to our growing understanding of obesity’s effects on asthma than to any other lung disease, with more than 100 publications currently indexed in PubMed on the subject. As reviewed previously by Shore (62), mouse models of obese asthma have suggested that obesity in the absence of allergic disease is associated with significant airways hyper-responsiveness (AHR), primarily on the basis of increased airways (not tissue) resistance, and is largely unaffected by the chest wall and abdominal obesity-driven mechanical factors that may contribute in human obese asthma. Furthermore, this effect appears to be dependent on the duration and/or degree of obesity, and, after ozone exposure, to be associated with an exaggerated airway inflammatory response (as discussed in the context of lung injury, above), as well as augmented airways resistance both at baseline and with methacholine challenge. Inflammatory mediators including tumor necrosis factor-α and IL-6 have been shown to contribute to this process, and although variable, elements of the metabolic syndrome, including hypercholesterolemia and altered leptin and adiponectin signaling, have been implicated in mouse models, with their primary effects appearing to be on innate and not adaptive immune responses (62, 63). The impact of obesity on allergic sensitization and challenge mouse models of asthma remains somewhat unclear, with divergent findings in the literature (62, 64).
More recent mouse models have advanced our understanding of the immune alterations underlying obese asthma and have suggested other physiological consequences of obesity and the metabolic syndrome that may affect asthma pathogenesis. In recent work from Kim and colleagues (65) using multiple gene-deleted mouse strains, diet-induced, NLRP3 inflammasome-mediated IL-1β release from tissue macrophages was shown to drive IL-17 production by innate lymphoid cells, leading to subsequent AHR. This demonstration of a “macrophage-innate lymphoid cell axis,” independent of adaptive immune response and possibly activated by the dyslipidemic environment of obesity, may explain, in part, the apparent presence of one or more “nonallergic” phenotypes of obese asthma. Similarly, the potential role of cerebral hyperinsulinemia has been shown, through elegant mouse models of intracerebroventricular insulin injection, to contribute to AHR via activation of cholinergic nerves in the dorsal motor nucleus of the vagus and subsequent parasympathetic outflow to the airways (66). Both these studies demonstrate the power of well-designed mouse models to dissect what often appears to be an impenetrable morass of metabolic and inflammatory signals in obesity.
Caveats and Pitfalls
One of the most important aspects of mouse modeling is knowing the limitations of the model. This is particularly true in mouse modeling of obese lung disease, in which not only the well-known issues of species differences (mouse versus man) and inaccurate disease recapitulation (e.g., murine ARDS [7]) limit our extrapolation to the human condition, but the complexity and variable expression of the obese phenotype and even its interaction with the mouse model itself lead to numerous pitfalls and the need to keep in mind several caveats to the approach.
Prime among these pitfalls is the assumption of “simplicity” in a model. This occurs when studies purporting to examine discrete elements of the metabolic syndrome (e.g., diabetes) do not examine these in isolation of obesity and the other facets of the syndrome (and perhaps changes in the microbiome [67]) or, at the very least, take them into account (e.g., Table 1). For instance, the db/db mouse model has been used variously to “specifically” examine leptin resistance, diabetes, or obesity in reported studies. Yet this model represents a milieu of all of these and is also noted to be dyslipidemic. Hence, it remains unclear which elements of obesity and the metabolic syndrome may be operative in the majority of reported findings in the db/db model. Furthermore, as has been noted by Lu and colleagues (32), the db/db mouse, although lacking the long form of the leptin receptor (ObRb), which mediates leptin-driven signal transducer and activator of transcription signaling, is otherwise replete with the short forms of this receptor, which may have signaling effects on both leukocytes and the lung epithelium (50, 68). Thus, whether the db/db mouse represents an appropriate model of leptin resistance remains an open question. This caveat does not diminish the db/db mouse as an important model of obesity, but rather, highlights its relevance in examining florid metabolic syndrome in its entirety and its potential role in determining the effects of the short forms of the leptin receptor, particularly though comparison with other models such as the aleptinemic ob/ob mouse (32).
Another important pitfall of obese mouse modeling is highlighted by the above-cited study by Shah (34), in which the effects of obesity on LPS-induced acute lung injury were shown to vary by mouse strain, apparently because of strain-dependent susceptibility to glucose intolerance with weight gain. A growing literature has been examining these interactions (69, 70) and has yielded important insights for those working in lung disease models. Among them, it appears that BALB/c mice, the most commonly used strain in allergic airways disease modeling, are extremely resistant to the metabolic syndrome altogether (70), making these animals suboptimal for the examination of obesity’s effects on allergic asthma. In contrast, the C57Bl/6 mouse strain, the most common background for transgenic and genetically deleted mice, appears to be susceptible to the development of metabolic syndrome but delayed in its onset compared with other obesity-prone strains (70), making the duration of high-fat diet important in this strain, as suggested in studies of AHR by Shore (62), and bringing age into the equation, as well. Thus, the interaction between obesity and mouse background genetics is critically important and must be evaluated for each model.
Lastly, the definition of obesity itself presents perhaps the greatest pitfall of all in mouse modeling. What defines obesity in mice? Although obesity in humans is typically (and somewhat inadequately) defined by BMI, there is no useful equivalent for mice. Furthermore, much of what is sought in a mouse model of obesity is instead the metabolic syndrome, as defined in humans by central obesity and two of the following: elevated triglyceride levels, reduced high-density lipoproteins, increased blood pressure, or increased fasting glucose. Glucose intolerance is a common (if variable) occurrence in mouse models of obesity, and, although in contrast to humans, mice usually demonstrate elevated high-density lipoprotein levels, the other manifestations of dyslipidemia (elevated triglycerides and low-density lipoproteins) are typically replicated (11, 71). Because the role of systemic hypertension is not often a consideration in modeling lung diseases, its presence or absence may be less relevant for many researchers. Yet for those examining pulmonary vascular disease (and possibly ARDS), the effects of cardiac remodeling and even systemic vascular injury may be critical to the model.
As stated above, in any given obese mouse model, the manifestations of obesity are likely to be unique on the basis of not only the model chosen (e.g., DIO, db/db, etc.) but also the genetic background of the mice, the composition and duration of the diet, and the age of the mice. Thus, particularly in diet-induced models, an arbitrary definition of “obesity” on the basis of a duration of diet or even percent weight gain compared with lean control subjects is ultimately uninformative in understanding which facets of obesity and the metabolic syndrome are being examined or in comparing one report’s findings to another’s. In the end, “obesity” should be defined clearly by the investigator for the model used, and, where possible, the manifestations of obesity and the metabolic syndrome should be characterized, unless this information has been published previously and the current model is identical. A parallel approach using models that only manifest individual elements of obesity and the metabolic syndrome, such as diabetes or hypercholesterolemia, has also been exploited to isolate and dissect the roles these conditions may play in the pathogenesis of lung disease (e.g., pneumonia and ARDS [37]), and many more such models have been reported but have yet to be examined in the context of lung disease (71). Moving forward, such models represent a powerful approach to understanding the complex underpinning of obesity’s effects on the lung.
Taken together, these pitfalls and caveats highlight the need for not only a comprehensive understanding of the varying metabolic aspects of each model and the limitations of the approach, but also the use of more standardized approaches among investigators and the development of models isolating individual aspects of obesity and the metabolic syndrome.
Conclusions
Mouse models combining obesity and pulmonary diseases have already contributed significantly to our understanding of the interactions between the obese state and diseases of the lung, and moving forward, such models are likely to provide even greater insight. As this field expands, it will be critically important for researchers to recognize and address the pitfalls that accompany such work.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health grants R03 AI117069 and P30 GM103532.
Originally Published in Press as DOI: 10.1165/rcmb.2016-0063PS on May 10, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Wollstein M, Meltzer SJ. The character of the pneumonic lesions produced by intrabronchial insufflation of virulent streptococci. J Exp Med. 1913;18:548–555. doi: 10.1084/jem.18.5.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenthal N, Brown S. The mouse ascending: perspectives for human-disease models. Nat Cell Biol. 2007;9:993–999. doi: 10.1038/ncb437. [DOI] [PubMed] [Google Scholar]
- 5.Moore BB, Lawson WE, Oury TD, Sisson TH, Raghavendran K, Hogaboam CM. Animal models of fibrotic lung disease. Am J Respir Cell Mol Biol. 2013;49:167–179. doi: 10.1165/rcmb.2013-0094TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates JH, Rincon M, Irvin CG. Animal models of asthma. Am J Physiol Lung Cell Mol Physiol. 2009;297:L401–L410. doi: 10.1152/ajplung.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM Acute Lung Injury in Animals Study Group. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44:725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meuwissen R, Berns A. Mouse models for human lung cancer. Genes Dev. 2005;19:643–664. doi: 10.1101/gad.1284505. [DOI] [PubMed] [Google Scholar]
- 9.Mizgerd JP, Skerrett SJ. Animal models of human pneumonia. Am J Physiol Lung Cell Mol Physiol. 2008;294:L387–L398. doi: 10.1152/ajplung.00330.2007. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro SD. Animal models for chronic obstructive pulmonary disease: age of klotho and marlboro mice. Am J Respir Cell Mol Biol. 2000;22:4–7. doi: 10.1165/ajrcmb.22.1.f173. [DOI] [PubMed] [Google Scholar]
- 11.Lutz TA, Woods SC.Overview of animal models of obesity. Curr Protoc Pharmacol 2012. Chapter 5:Unit 5.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell JC, Proctor SD. Small animal models of cardiovascular disease: tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc Pathol. 2006;15:318–330. doi: 10.1016/j.carpath.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41:317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 14.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153:1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 15.Naggert JK, Fricker LD, Varlamov O, Nishina PM, Rouille Y, Steiner DF, Carroll RJ, Paigen BJ, Leiter EH. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet. 1995;10:135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- 16.West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol. 1992;262:R1025–R1032. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- 17.Moss M, Guidot DM, Steinberg KP, Duhon GF, Treece P, Wolken R, Hudson LD, Parsons PE. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med. 2000;28:2187–2192. doi: 10.1097/00003246-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Yu S, Christiani DC, Thompson BT, Bajwa EK, Gong MN. Role of diabetes in the development of acute respiratory distress syndrome. Crit Care Med. 2013;41:2720–2732. doi: 10.1097/CCM.0b013e318298a2eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anzueto A, Frutos-Vivar F, Esteban A, Bensalami N, Marks D, Raymondos K, Apezteguía C, Arabi Y, Hurtado J, González M, et al. Ventila group. Influence of body mass index on outcome of the mechanically ventilated patients. Thorax. 2011;66:66–73. doi: 10.1136/thx.2010.145086. [DOI] [PubMed] [Google Scholar]
- 20.Gong MN, Bajwa EK, Thompson BT, Christiani DC. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010;65:44–50. doi: 10.1136/thx.2009.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karnatovskaia LV, Lee AS, Bender SP, Talmor D, Festic E US Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG–LIPS) Obstructive sleep apnea, obesity, and the development of acute respiratory distress syndrome. J Clin Sleep Med. 2014;10:657–662. doi: 10.5664/jcsm.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien JM, Jr, Phillips GS, Ali NA, Lucarelli M, Marsh CB, Lemeshow S. Body mass index is independently associated with hospital mortality in mechanically ventilated adults with acute lung injury. Crit Care Med. 2006;34:738–744. doi: 10.1097/01.CCM.0000202207.87891.FC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martino JL, Stapleton RD, Wang M, Day AG, Cahill NE, Dixon AE, Suratt BT, Heyland DK. Extreme obesity and outcomes in critically ill patients. Chest. 2011;140:1198–1206. doi: 10.1378/chest.10-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickkers P, de Keizer N, Dusseljee J, Weerheijm D, van der Hoeven JG, Peek N. Body mass index is associated with hospital mortality in critically ill patients: an observational cohort study. Crit Care Med. 2013;41:1878–1883. doi: 10.1097/CCM.0b013e31828a2aa1. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien JM, Jr, Philips GS, Ali NA, Aberegg SK, Marsh CB, Lemeshow S. The association between body mass index, processes of care, and outcomes from mechanical ventilation: a prospective cohort study. Crit Care Med. 2012;40:1456–1463. doi: 10.1097/CCM.0b013e31823e9a80. [DOI] [PubMed] [Google Scholar]
- 26.Prescott HC, Chang VW, O’Brien JM, Jr, Langa KM, Iwashyna TJ. Obesity and 1-year outcomes in older Americans with severe sepsis. Crit Care Med. 2014;42:1766–1774. doi: 10.1097/CCM.0000000000000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stapleton RD, Dixon AE, Parsons PE, Ware LB, Suratt BT NHLBI Acute Respiratory Distress Syndrome Network. The association between BMI and plasma cytokine levels in patients with acute lung injury. Chest. 2010;138:568–577. doi: 10.1378/chest.10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kordonowy LL, Burg E, Lenox CC, Gauthier LM, Petty JM, Antkowiak M, Palvinskaya T, Ubags N, Rincón M, Dixon AE, et al. Obesity is associated with neutrophil dysfunction and attenuation of murine acute lung injury. Am J Respir Cell Mol Biol. 2012;47:120–127. doi: 10.1165/rcmb.2011-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellmeyer A, Martino JM, Chandel NS, Scott Budinger GR, Dean DA, Mutlu GM. Leptin resistance protects mice from hyperoxia-induced acute lung injury. Am J Respir Crit Care Med. 2007;175:587–594. doi: 10.1164/rccm.200603-312OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shore SA, Lang JE, Kasahara DI, Lu FL, Verbout NG, Si H, Williams ES, Terry RD, Lee A, Johnston RA. Pulmonary responses to subacute ozone exposure in obese vs. lean mice. J Appl Physiol (1985) 2009;107:1445–1452. doi: 10.1152/japplphysiol.00456.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Götz AA, Rozman J, Rödel HG, Fuchs H, Gailus-Durner V, Hrabě de Angelis M, Klingenspor M, Stoeger T. Comparison of particle-exposure triggered pulmonary and systemic inflammation in mice fed with three different diets. Part Fibre Toxicol. 2011;8:30. doi: 10.1186/1743-8977-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, Lee A, Shore SA. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol. 2006;290:L856–L865. doi: 10.1152/ajplung.00386.2005. [DOI] [PubMed] [Google Scholar]
- 33.Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol (1985) 2008;104:1727–1735. doi: 10.1152/japplphysiol.00075.2008. [DOI] [PubMed] [Google Scholar]
- 34.Shah D, Romero F, Duong M, Wang N, Paudyal B, Suratt BT, Kallen CB, Sun J, Zhu Y, Walsh K, et al. Obesity-induced adipokine imbalance impairs mouse pulmonary vascular endothelial function and primes the lung for injury. Sci Rep. 2015;5:11362. doi: 10.1038/srep11362. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Pothen JJ, Poynter ME, Bates JH. The inflammatory twitch as a general strategy for controlling the host response. J Immunol. 2013;190:3510–3516. doi: 10.4049/jimmunol.1202595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ubags ND, Burg E, Antkowiak M, Wallace AM, Dilli E, Bement J, Wargo MJ, Poynter ME, Wouters EF, Suratt BT.A comparative study of lung host defense in murine obesity models: insights into neutrophil function Am J Respir Cell Mol Biol(In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madenspacher JH, Draper DW, Smoak KA, Li H, Griffiths GL, Suratt BT, Wilson MD, Rudel LL, Fessler MB. Dyslipidemia induces opposing effects on intrapulmonary and extrapulmonary host defense through divergent TLR response phenotypes. J Immunol. 2010;185:1660–1669. doi: 10.4049/jimmunol.0903501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konter JM, Parker JL, Baez E, Li SZ, Ranscht B, Denzel M, Little FF, Nakamura K, Ouchi N, Fine A, et al. Adiponectin attenuates lipopolysaccharide-induced acute lung injury through suppression of endothelial cell activation. J Immunol. 2012;188:854–863. doi: 10.4049/jimmunol.1100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ubags ND, Stapleton RD, Vernooy JH, Burg E, Bement J, Hayes CM, Ventrone S, Zabeau L, Tavernier J, Poynter ME, et al. Hyperleptinemia is associated with impaired pulmonary host defense. J Clin Invest Insight. 2016;1:e82101. doi: 10.1172/jci.insight.82101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phung DT, Wang Z, Rutherford S, Huang C, Chu C. Body mass index and risk of pneumonia: a systematic review and meta-analysis. Obes Rev. 2013;14:839–857. doi: 10.1111/obr.12055. [DOI] [PubMed] [Google Scholar]
- 41.Fezeu L, Julia C, Henegar A, Bitu J, Hu FB, Grobbee DE, Kengne AP, Hercberg S, Czernichow S. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis. Obes Rev. 2011;12:653–659. doi: 10.1111/j.1467-789X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 42.Mertz D, Kim TH, Johnstone J, Lam PP, Science M, Kuster SP, Fadel SA, Tran D, Fernandez E, Bhatnagar N, et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347:f5061. doi: 10.1136/bmj.f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. People at high risk of developing flu-related complications. 2015[updated 2015 Jan 8]. Available from: http://www.cdc.gov/flu/about/disease/high_risk.htm
- 44.Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137:1236–1243. doi: 10.1093/jn/137.5.1236. [DOI] [PubMed] [Google Scholar]
- 45.O’Brien KB, Vogel P, Duan S, Govorkova EA, Webby RJ, McCullers JA, Schultz-Cherry S. Impaired wound healing predisposes obese mice to severe influenza virus infection. J Infect Dis. 2012;205:252–261. doi: 10.1093/infdis/jir729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milner JJ, Rebeles J, Dhungana S, Stewart DA, Sumner SC, Meyers MH, Mancuso P, Beck MA. Obesity increases mortality and modulates the lung metabolome during pandemic H1N1 influenza virus infection in mice. J Immunol. 2015;194:4846–4859. doi: 10.4049/jimmunol.1402295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim YH, Kim JK, Kim DJ, Nam JH, Shim SM, Choi YK, Lee CH, Poo H. Diet-induced obesity dramatically reduces the efficacy of a 2009 pandemic H1N1 vaccine in a mouse model. J Infect Dis. 2012;205:244–251. doi: 10.1093/infdis/jir731. [DOI] [PubMed] [Google Scholar]
- 48.Mancuso P. Obesity and respiratory infections: does excess adiposity weigh down host defense? Pulm Pharmacol Ther. 2013;26:412–419. doi: 10.1016/j.pupt.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol. 2010;184:3127–3133. doi: 10.4049/jimmunol.0903220. [DOI] [PubMed] [Google Scholar]
- 50.Ubags ND, Vernooy JH, Burg E, Hayes C, Bement J, Dilli E, Zabeau L, Abraham E, Poch KR, Nick JA, et al. The role of leptin in the development of pulmonary neutrophilia in infection and acute lung injury. Crit Care Med. 2014;42:e143–e151. doi: 10.1097/CCM.0000000000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore SI, Huffnagle GB, Chen GH, White ES, Mancuso P. Leptin modulates neutrophil phagocytosis of Klebsiella pneumoniae. Infect Immun. 2003;71:4182–4185. doi: 10.1128/IAI.71.7.4182-4185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang AJ, To KK, Li C, Lau CC, Poon VK, Chan CC, Zheng BJ, Hung IF, Lam KS, Xu A, et al. Leptin mediates the pathogenesis of severe 2009 pandemic influenza A(H1N1) infection associated with cytokine dysregulation in mice with diet-induced obesity. J Infect Dis. 2013;207:1270–1280. doi: 10.1093/infdis/jit031. [DOI] [PubMed] [Google Scholar]
- 53.Hunt WR, Zughaier SM, Guentert DE, Shenep MA, Koval M, McCarty NA, Hansen JM. Hyperglycemia impedes lung bacterial clearance in a murine model of cystic fibrosis-related diabetes. Am J Physiol Lung Cell Mol Physiol. 2014;306:L43–L49. doi: 10.1152/ajplung.00224.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bates JH. Physiological mechanisms of airway hyperresponsiveness in obese asthma. Am J Respir Cell Mol Biol. 2016;54:618–623. doi: 10.1165/rcmb.2016-0019PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dixon AE, Poynter ME. Mechanisms of asthma in obesity. Pleiotropic aspects of obesity produce distinct asthma phenotypes. Am J Respir Cell Mol Biol. 2016;54:601–608. doi: 10.1165/rcmb.2016-0017PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schatz M, Zeiger RS, Yang SJ, Chen W, Sajjan S, Allen-Ramey F, Camargo CA., Jr Prospective study on the relationship of obesity to asthma impairment and risk. J Allergy Clin Immunol Pract. 2015;3:560–5.e1. doi: 10.1016/j.jaip.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 57.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159:2582–2588. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 58.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128:508–15.e1–2. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dixon JB, Chapman L, O’Brien P. Marked improvement in asthma after lap-band surgery for morbid obesity. Obes Surg. 1999;9:385–389. doi: 10.1381/096089299765552981. [DOI] [PubMed] [Google Scholar]
- 60.Chapman DG, Irvin CG, Kaminsky DA, Forgione PM, Bates JH, Dixon AE. Influence of distinct asthma phenotypes on lung function following weight loss in the obese. Respirology. 2014;19:1170–1177. doi: 10.1111/resp.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rastogi D, Fraser S, Oh J, Huber AM, Schulman Y, Bhagtani RH, Khan ZS, Tesfa L, Hall CB, Macian F. Inflammation, metabolic dysregulation, and pulmonary function among obese urban adolescents with asthma. Am J Respir Crit Care Med. 2015;191:149–160. doi: 10.1164/rccm.201409-1587OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shore SA. Obesity and asthma: lessons from animal models. J Appl Physiol (1985) 2007;102:516–528. doi: 10.1152/japplphysiol.00847.2006. [DOI] [PubMed] [Google Scholar]
- 63.Medoff BD, Okamoto Y, Leyton P, Weng M, Sandall BP, Raher MJ, Kihara S, Bloch KD, Libby P, Luster AD. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol. 2009;41:397–406. doi: 10.1165/rcmb.2008-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calixto MC, Lintomen L, Schenka A, Saad MJ, Zanesco A, Antunes E. Obesity enhances eosinophilic inflammation in a murine model of allergic asthma. Br J Pharmacol. 2010;159:617–625. doi: 10.1111/j.1476-5381.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, Iwakura Y, Israel E, Bolger K, Faul J, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20:54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leiria LO, Arantes-Costa FM, Calixto MC, Alexandre EC, Moura RF, Folli F, Prado CM, Prado MA, Prado VF, Velloso LA, et al. Increased airway reactivity and hyperinsulinemia in obese mice are linked by ERK signaling in brain stem cholinergic neurons. Cell Reports. 2015;11:934–943. doi: 10.1016/j.celrep.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 67.Shore SA, Cho Y. Obesity and asthma: microbiome-metabolome interactions. Am J Respir Cell Mol Biol. 2016;54:609–617. doi: 10.1165/rcmb.2016-0052PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Löllmann B, Grüninger S, Stricker-Krongrad A, Chiesi M. Detection and quantification of the leptin receptor splice variants Ob-Ra, b, and, e in different mouse tissues. Biochem Biophys Res Commun. 1997;238:648–652. doi: 10.1006/bbrc.1997.7205. [DOI] [PubMed] [Google Scholar]
- 69.Fearnside JF, Dumas ME, Rothwell AR, Wilder SP, Cloarec O, Toye A, Blancher C, Holmes E, Tatoud R, Barton RH, et al. Phylometabonomic patterns of adaptation to high fat diet feeding in inbred mice. PLoS One. 2008;3:e1668. doi: 10.1371/journal.pone.0001668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alexander J, Chang GQ, Dourmashkin JT, Leibowitz SF. Distinct phenotypes of obesity-prone AKR/J, DBA2J and C57BL/6J mice compared to control strains. Int J Obes. 2006;30:50–59. doi: 10.1038/sj.ijo.0803110. [DOI] [PubMed] [Google Scholar]
- 71.Kennedy AJ, Ellacott KL, King VL, Hasty AH. Mouse models of the metabolic syndrome. Dis Model Mech. 2010;3:156–166. doi: 10.1242/dmm.003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.