Abstract
Obese asthma presents with inherent hyperresponsiveness to methacholine or augmented allergen-driven allergic asthma, with an even greater magnitude of methacholine hyperresponsiveness. These physiologic parameters and accompanying obese asthma symptoms can be reduced by successful weight loss, yet the underlying mechanisms remain incompletely understood. We implemented mouse models of diet-induced obesity, dietary and surgical weight loss, and environmental allergen exposure to examine the mechanisms and mediators of inherent and allergic obese asthma. We report that the methacholine hyperresponsiveness in these models of inherent obese asthma and obese allergic asthma manifests in distinct anatomical compartments but that both are amenable to interventions that induce substantial weight loss. The inherent obese asthma phenotype, with characteristic increases in distal airspace tissue resistance and tissue elastance, is associated with elevated proinflammatory cytokines that are reduced with dietary weight loss. Surprisingly, bariatric surgery–induced weight loss further elevates these cytokines while reducing methacholine responsiveness to levels similar to those in lean mice or in formerly obese mice rendered lean through dietary intervention. In contrast, the obese allergic asthma phenotype, with characteristic increases in central airway resistance, is not associated with increased adaptive immune responses, yet diet-induced weight loss reduces methacholine hyperresponsiveness without altering immunological variables. Diet-induced weight loss is effective in models of both inherent and allergic obese asthma, and our examination of the fecal microbiome revealed that the obesogenic Firmicutes/Bacteroidetes ratio was normalized after diet-induced weight loss. Our results suggest that structural, immunological, and microbiological factors contribute to the manifold presentations of obese asthma.
Keywords: asthma, obesity, airway hyperresponsiveness, weight loss, bariatric surgery
Clinical Relevance
This article demonstrates that mouse models of diet-induced obesity can be useful for modeling the inherent and allergen-induced methacholine hyperresponsiveness of obesity, and that weight-loss regimens elicit benefit in mouse models of obese asthma.
Asthma is a common chronic disorder of the airways that typically involves a complex interaction between airway reactivity and inflammation. About 300 million people suffer from asthma worldwide (1), and the U.S. asthma prevalence is ∼1 in 12 adults and 1 in 11 children (2). The epidemic of obesity poses a substantial threat to public health and takes a great toll on health care costs (3, 4). Asthma is an important comorbidity of obesity, and ∼250,000 new cases of asthma related to obesity are reported in the United States each year (5, 6). Furthermore, many individuals with obese asthma are allergic to environmental allergens (7). Individuals with obese asthma do not respond well to standard asthma controller therapy (8) and exhibit increased hospitalization rates (9). Although most individuals with severe asthma are obese (10), we have little understanding of the mechanisms linking obesity to asthma and have no treatments specifically targeting asthma in obesity.
Weight loss imparts a health benefit to the many comorbidities associated with obesity, but diet, exercise, and even pharmacologically induced weight loss exhibit poor long-term efficacy. Bariatric surgery, on the other hand, has emerged as a promising intervention to reduce weight and the comorbidities associated with obesity (3, 4, 11), including asthma (12–14). Morbidly obese patients who undergo bariatric surgery demonstrate significant improvements in asthma control, asthma quality of life, and airway responsiveness (15). Furthermore, bariatric surgery has led investigators to discover two distinct phenotypes of asthma in obesity. One is an early-onset phenotype characterized by high IgE that appears to be conventional allergic asthma exacerbated by obesity (12–14), although this interpretation is complicated by the fact that after bariatric surgery, patients with asthma have been shown to have higher levels of inflammatory mediators (CD4+ lymphocytes and cytokine production from these cells) despite improved asthma control (15). The other phenotype of obese asthma is a late-onset form with low IgE that we have postulated arises in individuals with particularly compliant airways (16). Again, however, there are likely to be additional contributing factors because these individuals also exhibit increased mediators in their visceral adipose tissue (17), suggesting the involvement of metabolic factors.
It thus remains unclear as to exactly how weight loss improves symptoms and control in both the early-onset allergic and the late-onset nonallergic forms of obese asthma. Accordingly, in this study, we built on prior work in mouse models of allergic asthma (18) and obese asthma (19) to develop an animal model in which these questions could be addressed. To make this model as relevant as possible to the human population, we induced obesity via feeding a high-fat diet, and we achieved weight loss both through the ad libitum feeding of a low-fat diet and by sleeve gastrectomy, the variant of bariatric surgery that is now emerging as the gold standard in obese human patients. In addition, because obesity is associated with alterations in the intestinal commensal bacterial microbiota (20, 21), with possible consequences for asthma (22), we examined how the gut microbiome is affected in these mice as a consequence of weight loss.
Materials and Methods
A complete description of Materials and Methods is provided in the online supplement.
Mice
Mice purchased from The Jackson Laboratory were maintained on a 12-hour light/dark cycle and were provided food and water ad libitum at the University of Vermont. Low-fat diets (D12450B) and high-fat diets (D12492) were purchased from Research Diets (New Brunswick, NJ) and stored frozen. Food in cages was replaced twice each week. Protocols were approved by the University of Vermont Institutional Animal Care and Use Committee.
Model of Obese Inherent Asthma
Twenty-week-old male C57BL/6J mice were maintained on food containing 10% fat (low-fat diet; LFD) or 60% fat (high-fat diet; HFD) from 6 weeks of age until analysis at 22 weeks of age.
House Dust Mite Extract Model of Allergic Asthma
Sixteen-week old male C57BL/6J mice maintained on LFD or HFD from 6 weeks of age were intranasally instilled with 1 μg house dust mite (HDM) extract (Greer, Lenoir, NC) twice a week for 4 weeks while remaining on their respective diets. The mice were then maintained on their same diet or were subjected to the dietary weight-loss regimen for 3 weeks, during which time they received no intranasal HDM exposures. To elicit an allergic asthma–like exacerbation, ∼ 23-week-old mice were challenged intranasally 4 days in a row with 10 µg HDM extract, and were analyzed 1 day later.
Mouse Models of Weight Loss
To model diet-induced weight loss, the obese mice were switched from a HFD to an LFD. To model bariatric surgery–induced weight loss, the obese mice were subjected to sleeve gastrectomy and maintained on HFD.
Mouse Phenotyping
Responsiveness to inhaled methacholine was assessed in closed-chested mice using a flexiVent (SCIREQ, Montréal, QC, Canada) with the mice ventilated at 200 breaths per minute with a 0.25 ml tidal volume and a positive end-expiratory pressure of 3 cm H2O (23–25). Respiratory input impedance data were fit to the single-compartment and constant-phase models.
Immunophenotyping
After euthanasia at the completion of flexiVent analysis, blood was collected and serum was prepared, from which Luminex assays were performed and HDM-specific immunoglobulins were measured. Lungs were then lavaged with 1 ml Dulbecco's phosphate-buffered saline (DPBS), the bronchoalveolar lavage (BAL) fluid was centrifuged, and the supernatant was collected. Total and differential cell counts were conducted, and immunoassays were performed on the BAL fluid. Lungs were dissected and ground to a fine powder under liquid nitrogen, protein was extracted, and cytokines were enumerated by ELISA. Splenocytes were then isolated (26) and cultured with 15 μg/ml HDM extract for 96 hours, and cytokines were measured from cell supernatants by ELISA.
Measurement of Fecal Bacterial Diversity
From fecal pellets collected on the day of the final HDM challenge from the mice exposed to the allergic asthma model, bacterial DNA was extracted (QIAGEN, Valencia, CA) and subjected to quantitative polymerase chain reaction analysis of total eubacterial, Bacteroidetes-, Firmicutes-, and Proteobacteria-specific 16S ribosomal RNA (27).
Statistical Analyses
Data were analyzed using GraphPad Prism 6 for Windows (GraphPad Software, Inc., La Jolla, CA). Data are presented as mean ± SEM. A P value <0.05 was considered statistically significant.
Results
Dietary- or Surgically Induced Weight Loss Decreases Inherent Methacholine Hyperresponsiveness in a Mouse Model of Obesity
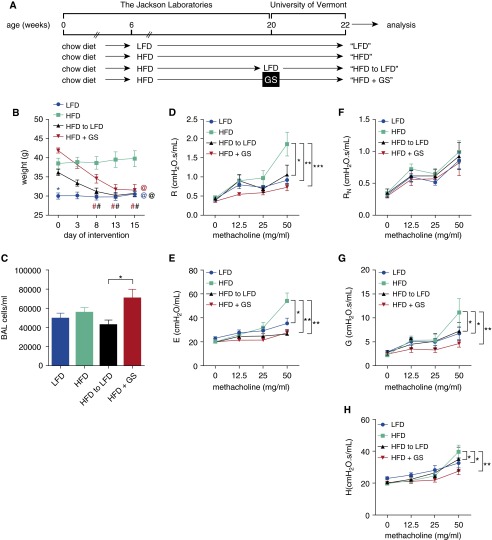
To model the inherent methacholine hyperresponsiveness associated with obese asthma, the mice were maintained on a high-fat diet (HFD) from 6 weeks of age until 20 weeks of age and lean control mice were maintained on a low-fat diet (LFD) over the same time period (Figure 1A). At 20 weeks of age, when the lean mice weighed 30.0 ± 0.8 g and the obese mice weighed 38.7 ± 0.7 g (P ≤ 0.0001), two subgroups of obese mice were established; one was switched from the HFD to a LFD, whereas another was maintained on the HFD but subjected to gastric sleeve bariatric surgery. Within 15 days, the HFD to LFD mice and the gastric sleeve mice lost substantial body weight such that their mass was insignificantly different from that of the LFD mice, whereas the mice maintained on the HFD and not subjected to surgery remained obese (Figure 1B). At Day 15, the cellularity of the BAL revealed a significant difference only between the two weight-loss regimens, whereas there were no differences between the lean and the obese mice (Figure 1C). All BAL cells were identified as alveolar macrophages, with those from the HFD group having a lipid-laden appearance (data not shown).
Figure 1.
(A) Bariatric surgery or a low-fat diet decrease body weight and inherent methacholine responsiveness in a model of obese asthma. C57BL/6J male mice were maintained on a 10% fat diet (low-fat diet, LFD) or a 60% fat diet (high-fat diet, HFD) from 6 to 20 weeks of age, at which time they were maintained on their same diets, or HFD mice were placed on an LFD or maintained on the HFD and subjected to gastric sleeve (GS) surgery. (B) Mouse weights were recorded after interventions. Blue *, compared with HFD on Day 0; @, compared with HFD on Day 15; and #, compared with Day 0 within treatment (by color). (C) On Day 15, the total cellularity of the bronchoalveolar lavage (BAL) was assessed. (D and E) Responsiveness to inhaled methacholine was calculated using the single-compartment model, which measures resistance (R) and elastance (E), respectively. (F–H) The mice were also assessed for methacholine responsiveness using the constant-phase model, which measures Newtonian resistance (RN), tissue resistance (G), and tissue elastance (H), respectively. n = 8 mice per group. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
We next assessed the responsiveness of the mice to inhaled methacholine. Whereas there were no differences in the baseline measurements among any of the groups (data not shown) or after challenge with phosphate-buffered saline and methacholine doses of up to 25 mg/ml, the HFD mice displayed significantly elevated airway resistance (R) and elastance (E) at the 50 mg/ml dose of methacholine, compared with each of the other groups (Figures 1D and 1E) when assessed using the single-compartment model. When these mechanical effects were further broken down in terms of the parameters of the constant-phase model, the HFD mice displayed significantly elevated tissue resistance (G) and tissue elastance (H), but no difference in Newtonian resistance (RN, reflective of central airway resistance), at the 50 mg/ml dose of methacholine, compared with each of the other groups (Figures 1F–1H). These data suggest that the inherent methacholine hyperresponsiveness of obesity manifests primarily in the distal airway and is reversible, with substantial weight loss achieved through either surgery or diet.
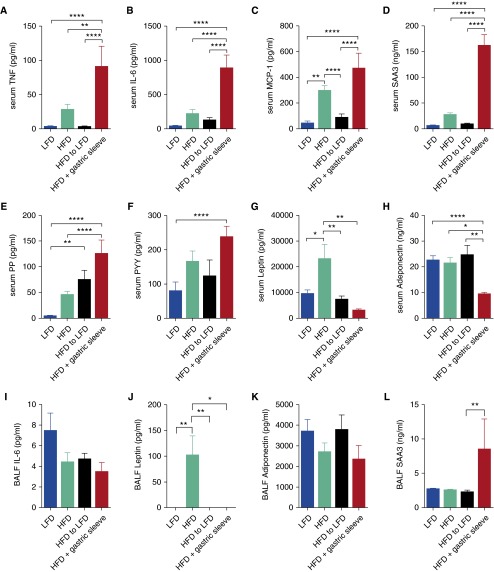
Because obesity is considered a chronic proinflammatory disease state (28), we measured a number of proinflammatory cytokines, acute-phase reactants, pancreatic peptides, and adipokines that have been reported to be dysregulated in obesity. There were significant and substantial elevations in serum levels of tumor necrosis factor (TNF), interleukin (IL)-6, monocyte chemoattractant protein (MCP)-1, serum amyloid A3 (SAA3), pancreatic polypeptide (PP), and peptide YY (PYY) in the HFD mice subjected to sleeve gastrectomy, compared with the other groups (Figures 2A–2H). These same proteins displayed trends toward or significant elevations in the serum of the HFD mice compared with that of the LFD mice, and in the case of TNF, IL-6, MCP-1, and SAA3, a decrease as a consequence of LFD-induced weight loss. Leptin levels were elevated in the serum of HFD mice but returned to the lower levels observed in the LFD mice as a consequence of surgical or diet-induced weight loss. Adiponectin was decreased in the circulation of the mice subjected to sleeve gastrectomy. Amylin, glucagon-like peptide (GLP)-1, and ghrelin were too low to quantitate reliably in the serum of any of the mice (data not shown). In the BAL fluid, TNF, amylin, PP, GLP-1, PYY, ghrelin, and MCP-1 were too low to quantitate reliably (data not shown), whereas IL-6 and adiponectin were no different among any of the groups, leptin was elevated in the HFD mice and reduced as a consequence of either weight-loss regimen, and SAA3 was elevated only in the mice subjected to sleeve gastrectomy (Figures 2I–2L). The inherent airway responsiveness of obesity in our mice is thus dissociated from many of the systemic inflammatory cytokines elevated in the obese state, but it is associated with increased serum leptin.
Figure 2.
Bariatric surgery or LFD affect proinflammatory cytokine, pancreatic hormone, and adipokine levels in the circulation and lavageable airspaces in a model of obese asthma. On Day 15, after dietary or surgical interventions (as described in Figure 1), (A–H) serum and (I–L) BAL fluid (BALF) levels of cytokines were measured by Luminex assay. n = 8–12 mice per group. MCP, monocyte chemoattractant protein; PP, pancreatic polypeptide; PYY, peptide YY; SAA3, serum amyloid A3. *P ≤ 0.05; **P ≤ 0.01; ****P ≤ 0.0001.
Dietary Weight Loss Decreases Methacholine Hyperresponsiveness in a Mouse Model of Obese Allergic Asthma
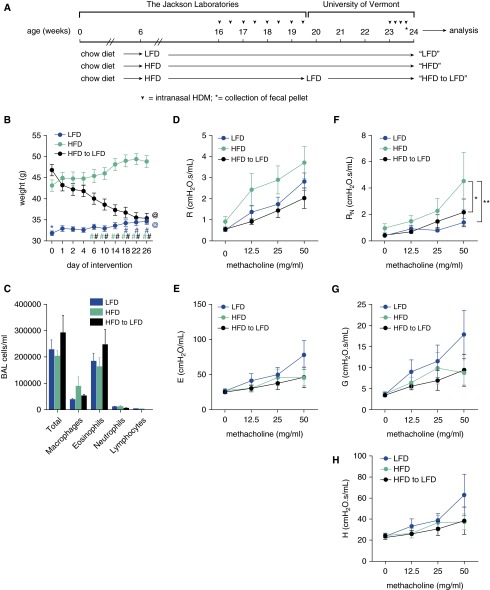
To model obese allergic asthma, the mice were maintained on an HFD from 6 weeks of age until 16 weeks of age, whereas the lean control mice were maintained on a LFD during this same time period. To simulate low-dose allergen exposure, the mice in these two groups were allergen sensitized by the intranasal instillation of 1 µg HDM extract twice a week from Week 17 to Week 20 (eight total instillations), during which time they remained on their respective diets (Figure 3A). At 20 weeks of age, the lean mice weighed 31.8 ± 0.6 g, whereas the obese mice weighed 45.2 ± 1.0 g (P ≤ 0.0001). The mice were then maintained on their same diet or were subjected to dietary weight loss (by feeding the LFD) for 3 weeks, during which time they received no intranasal HDM exposures. To induce an allergic asthma–like exacerbation, the mice were challenged intranasally 4 days in a row with 10 µg HDM extract and analyzed 1 day after the fourth challenge. By Day 26 of LFD feeding, the formerly obese mice lost substantial body weight, such that their mass was insignificantly different from that of the LFD mice, whereas the mice maintained on the HFD remained obese (Figure 3B). The BAL fluid at Day 26 revealed a substantial influx of eosinophils, consistent with previously reported HDM models of allergic airway disease (29), but there were no significant differences in the total cells, macrophages, eosinophils, neutrophils, or lymphocytes among the LFD, HFD, or dietary weight-loss groups (Figure 3C). Allergen exposure elicited methacholine hyperresponsiveness, as indicated by the elevated values of R, E, RN, G, and H (Figures 3D–3H) relative to those from the nonallergic mice (Figures 1D–1H, respectively). However, unlike in the nonallergic mice, the only significant change in lung mechanics in the allergic mice was a significantly elevated RN at the 50 mg/ml dose of methacholine in the HFD mice, compared with the LFD mice and the mice subjected to diet-induced weight loss (Figure 3F). The response to 50 mg/ml methacholine in the LFD mice also appears to be limited to roughly equivalent increases in G and H, with little effect on RN (Figures 3F–3H), which is compatible with an increase in peripheral airway closure caused by epithelial thickening and increased airway secretions, as we have seen previously in allergic inflammation in mice (30). Interestingly, the allergic HFD mice seemed to manifest hyperresponsiveness only in the central airways (Figures 3F–3H), which is reverse of their nonallergic counterparts (Figures 1F–1H). In other words, obesity in these mice appears to have prevented allergic inflammation from affecting the distal airways, and even more curiously, this effect seems to be preserved when the obese animals go on to lose weight.
Figure 3.
A low-fat diet decreases body weight and central airway methacholine hyperresponsiveness in a model of obese allergic asthma. C57BL/6J male mice that had been maintained on a 10% fat diet (LFD) or a 60% fat diet (HFD) since 6 weeks of age were administered two doses per week of 1 μg house dust mite (HDM) extract intranasally beginning at 16 weeks of age. The mice were maintained on their respective diets until 20 weeks of age, at which time a group of HFD mice were placed on an LFD. Groups of mice were then maintained on their same diet or were subjected to the dietary weight-loss regimen for 3 weeks (beginning on Day 0), during which time they received no intranasal HDM exposures. (A) To induce an allergic asthma–like exacerbation, 23-week-old mice were intranasally challenged 4 days in a row with 10 µg HDM extract and analyzed 1 day after the fourth challenge. (B) Body weights were recorded relative to the day of dietary intervention. Blue *, compared with HFD on Day 0; @, compared with HFD on Day 26; and #, compared with Day 0 within treatment (by color). (C) On Day 26, the total cellularity of the BAL was assessed. (D and E) Responsiveness to inhaled methacholine was calculated using the single-compartment model, which measures resistance (R) and elastance (E), respectively. (F–H) The mice were also assessed for methacholine responsiveness using the constant-phase model, which measures RN, G, and H, respectively. n = 8–10 mice per group. *P ≤ 0.05; **P ≤ 0.01.
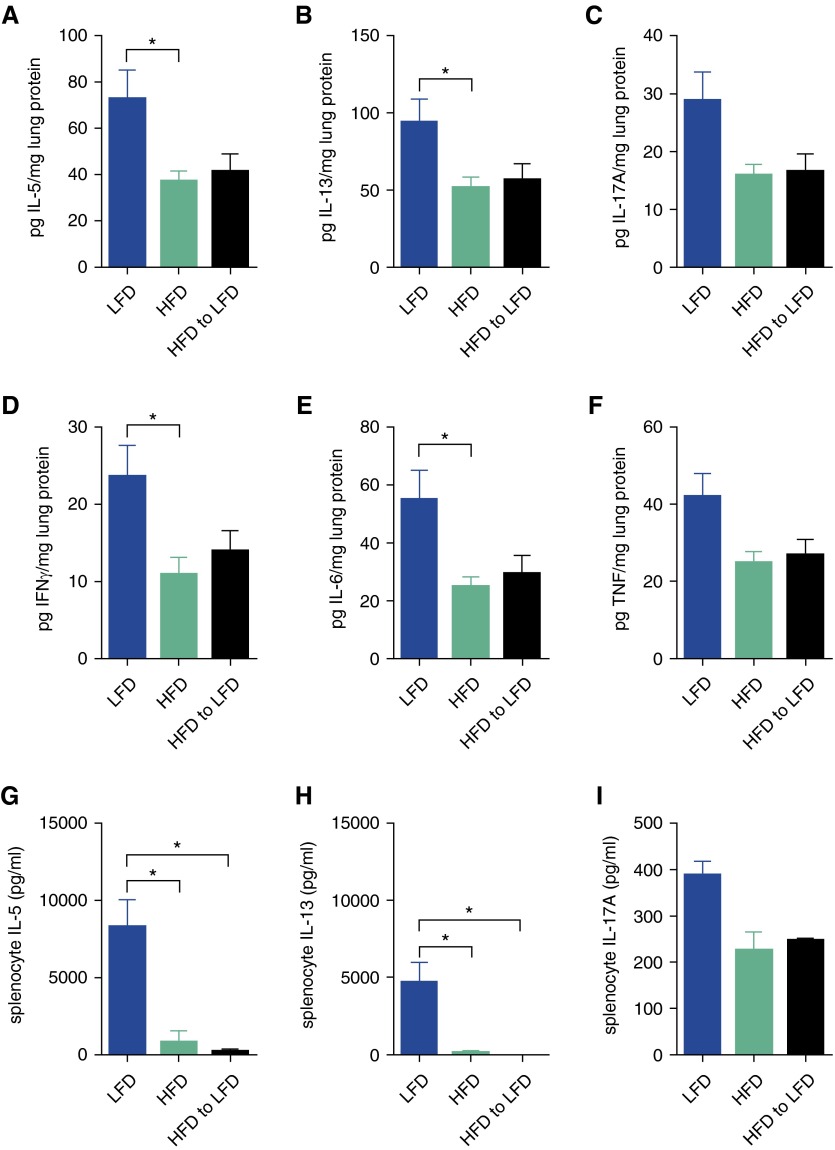
We measured trends toward or significantly reduced levels of lung lysate IL-5, IL-13, IL-17A, IFNγ, IL-6, and TNF in the HFD mice and the diet-induced weight loss mice compared with the LFD mice (Figures 4A–4F). To ascertain whether these responses in the lung after in vivo allergen challenge were reflective of the induced adaptive immune response, we stimulated splenocytes with HDM in vitro and observed significantly reduced levels of IL-5 and IL-13, with a trend toward reduced levels of IL-17A, in the HFD mice and the diet-induced weight loss mice, compared with the LFD mice (Figures 4G–4I). IFNγ, indicative of a Th1 response, was not detected in the culture supernatants from any of the groups. There was robust production of HDM-specific IgG1 and measurable production of HDM-specific IgG2c in all three groups of mice, but there were no significant differences among the groups (data not shown). Furthermore, we measured no differences (data not shown) among any of the groups in lung expression of the genes encoding mucin 5 subtypes A and C (Muc5ac), chloride channel accessory 1 (Clca1, Gob5), α smooth muscle actin (Acta2), myosin heavy chain (Myh11), IL-6 (Il6), chemokine (C-C motif) ligand 2 (Ccl2), neurotrophic tyrosine kinase receptor type 1 (Ntrk), complement factor D/adipsin (Cdf), tumor necrosis factor receptor superfamily member 1b (Tnfrsf1b), or serum amyloid A3 (Saa3). These data show that cytokines indicative of Th1, Th2, and Th17 activity, as well as splenocyte responses to antigen restimulation, are highest in animals maintained on a low-fat diet and seem to be suppressed in obese allergic asthma.
Figure 4.
Obesity-associated decreases in allergen-driven cytokine production are not corrected by weight loss in a model of obese allergic asthma. On Day 26 after dietary intervention (as described in Figure 3), (A–F) lung tissue and (G–I) HDM-restimulated splenocyte culture supernatant levels of cytokines were measured by ELISA; n = 8–10 mice per group. *P ≤ 0.05.
Dietary Weight Loss Is Associated with Normalization of an Obesogenic Intestinal Commensal Bacterial Composition
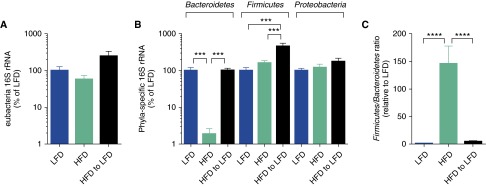
It is well recognized that the intestinal commensal bacterial composition affects weight gain and weight loss and that dietary manipulation affects the bacterial commensal composition (21, 31). To examine the composition of intestinal bacteria in our mouse model of obese allergic asthma and weight loss, we measured the abundance of total eubacterial and phyla-specific DNA encoding 16S ribosomal RNA. There were no differences in the abundance of total eubacterial 16S in any of the groups (Figure 5A). However, there were significant and substantial decreases in the abundance of Bacteroidetes phyla in the HFD mice compared with the LFD mice and the diet-induced weight loss mice, as well as small but significant increases in the abundance of Firmicutes phyla in the diet-induced weight loss mice compared with the LFD and the HFD mice (Figure 5B). There were no differences in the abundance of Proteobacteria in any of the groups. An elevated Firmicutes/Bacteroidetes ratio has been demonstrated to promote weight gain (20); therefore, the presence of an elevated Firmicutes/Bacteroidetes ratio in the HFD mice compared the LFD mice was not surprising, but the diet-induced weight-loss regimen had a rapid capacity to dramatically re-establish a lean-like Firmicutes/Bacteroidetes ratio (Figure 5C).
Figure 5.
Weight loss corrects the elevated gut commensal Firmicutes/Bacteroidetes ratio associated with obesity. On Day 26 after dietary intervention (as described in Figure 3), fecal pellet total eubacterial 16S (A) ribosomal RNA (rRNA) and (B) phlya-specific 16S rRNA DNA content was quantitated, and (C) the Firmicutes/Bacteroidetes ratio, relative to that found in the LFD mice, was calculated; n = 8–10 mice per group. ***P ≤ 0.001; ****P ≤ 0.0001.
Discussion
We report herein the development of mouse diet-induced obese inherent and allergic asthma model systems with which we have assessed the impact of weight loss on physiological, immunological, and microbiological end points. As reported previously, the mice made obese by feeding an HFD exhibit inherent methacholine responsiveness consistent with that of obese asthma (32, 33). We use the term “obese inherent asthma” to describe the phenomenon of augmented methacholine reactivity that occurs in obesity despite the absence of overt inflammation. Importantly, individuals who are obese and individuals who are obese with (nonallergic) asthma have distinct physiology (16, 34). A new finding from our mouse model of obese inherent asthma is that weight loss profoundly mitigates obesity-associated inherent methacholine responsiveness, as reported in human subjects (7, 8, 12–15, 17, 19, 34). A potential confounding factor in drawing these conclusions is that we have used measurements of respiratory system mechanics as surrogates for lung mechanics, which presumes negligible contribution from the chest wall. Whereas the compliant chest wall of the mouse makes this a reasonable assumption in animals of normal weight (30), it does not automatically follow when the animals become obese. However, our estimates of the tissue parameters G and H at baseline are no different among the various groups of mice, regardless of their obesity status (Figures 1G, 1H, 3G, and 3H), from which we can conclude that it is reasonable to continue to ignore the effects of the chest wall in these animals.
The increased tissue resistance (G) and tissue elastance (H) in the obese HFD mice as compared with the lean LFD, diet-induced weight loss mice and the sleeve gastrectomy mice thus indicates that the inherent methacholine hyperresponsiveness of the obese mice likely developed as a consequence of decreased lung volume leading to progressive derecruitment of distal airways and airway closure. A possible consequence of this that must be borne in mind is that distal airway closure might have interfered with the delivery of the aerosols of allergen and agonist to the lung periphery. This could have reduced the amount of bronchoconstriction elicited at the higher methacholine doses in the obese animals. On the other hand, this cannot account for all our observations because the tissue parameters G and H were the same in all groups of mice at baseline (Figures 1G, 1H, 3G, and 3H); therefore, the delivery of methacholine would also have been the same until a differential bronchoconstrictive response had developed. Genetically obese mice also have reduced lung volumes (35), and data from humans with obese asthma support this notion, because there is more distal lung collapse in obesity, which is reversed by weight loss (16). However, a decreased lung volume is unlikely to completely explain the obese asthma phenotype, because improvements in airway reactivity did not correlate with an improvement in functional residual capacity in obese bariatric surgery patients with asthma; instead, individuals with obese asthma also have changes in airway wall compliance, thickness, or perhaps surfactant dysfunction (34).
Obesity is fundamentally a state of altered adipose tissue homeostasis (28), and the adipose tissue from individuals with obese asthma is more proinflammatory than is adipose tissue from obese individuals who do not have asthma (17). Neutralization of these proinflammatory proteins, including TNF (36), IL-1, or IL-17A (32), can prevent methacholine hyperresponsiveness in obesity. In our studies, the mice rendered obese by consuming a high-fat diet demonstrated significantly higher levels of serum MCP-1 and leptin, together with trends toward elevated levels of TNF, IL-6, and SAA3, than did the mice on a low-fat diet or those subjected to diet-induced weight loss. Activated adipose tissue macrophages are implicated as the principal sources of these cytokines in the setting of obesity (37). Obese adipose tissue is prone to ischemia, which causes focal adipocyte necrosis, which is cleared subsequently by macrophages (38) that become activated and induce chronic, background systemic inflammation (17). Interestingly, macrophage depletion in obesity can restore methacholine responsiveness to the levels seen in lean mice (36).
The adipokine leptin was also increased in the HFD mice compared with the LFD mice and the mice subjected to surgical or diet-induced weight loss. Elevated levels of leptin are associated with increased airway reactivity in individuals with obese asthma (17). Adiponectin is an antiinflammatory adipokine, levels of which in the serum were diminished only as a consequence of sleeve gastrectomy. Interestingly, adiponectin-deficient mice exhibited worsened allergic airway inflammation and exacerbated decrements in dynamic lung compliance (indicative of effects in the small airways) in a model of chronic mild disease (39), whereas increasing serum adiponectin levels decreased allergic airway inflammation and methacholine hyperresponsiveness (40). Thus, the lean state appears to be associated with a profile of reduced systemic proinflammatory cytokines and leptin, together with an abundance of circulating antiinflammatory adiponectin. Interestingly, the airway epithelium expresses receptors for both leptin and adiponectin (17), making these cells potential targets for modulation by adipokines in obese asthma (41).
Sleeve gastrectomy is the most commonly performed surgical weight-loss procedure in the United States, accounting for ∼ 50% of all weight-loss procedures and surpassing both the bypass and the lap band (42). Interestingly, in our mouse model of sleeve gastrectomy, in which weight loss proceeded with similar kinetics and to a similar magnitude as diet-induced weight loss, inherent methacholine hyperresponsiveness was diminished, but TNF, IL-6, MCP-1, and SAA3, as well as the pancreatic peptides PP and PYY, were elevated significantly. In human patients, sleeve gastrectomy reduces circulating levels of ghrelin, amylin, and leptin, while increasing PP, PYY, and GLP-1 (43). Whereas we were unable to detect ghrelin, amylin, and GLP-1 in the serum of any of the mice, the reduction in leptin and the increase in PP and PYY in the mice subjected to sleeve gastrectomy suggest that our bariatric surgery model induced neurohormonal changes consistent with those observed in humans.
We do not believe surgical complications account for the elevation of proinflammatory cytokines in the mice subjected to sleeve gastrectomy because there was no indication of overt inflammation (in the BAL fluid), the mice were ambulatory and explorative, they were passing both feces and urine, and their body weights never dropped below those of the lean mice. In addition, the elevated proinflammatory cytokines in the surgical mice could be a transient response of the adipose tissue macrophages to the altered adipocyte homeostasis that accompanies such substantial and rapid weight loss (44). In this case, it raises an interesting paradox about the roles of these proteins, whether they function as mediators or simply serve as biomarkers. It remains a possibility that the surgery per se elicited some of the protection from methacholine responsiveness observed in the formerly obese mice, which could come about as a consequence of stress, changes to the microbiome, or other factors.
Obese women with adult-onset asthma who underwent bariatric surgery developed BAL CD4+ cells that secreted significantly more inflammatory cytokines in response to in vitro CD3 and CD28 stimulation, yet their methacholine responsiveness was improved significantly (15). In a separate study of obese women with adult-onset asthma who underwent bariatric surgery, a nonsignificant improvement in methacholine responsiveness was accompanied by an increase in BAL IL-8 and MCP-1 (17). These results indicate that changes in addition to reducing proinflammatory mediators lead to normalization of methacholine responsiveness after weight loss.
Reduced leptin levels may help explain the decreased methacholine responsiveness in the surgical and diet-induced weight loss mice. The levels of serum and visceral adipose tissue leptin are highly correlated, and we have reported that visceral adipose leptin correlates significantly with airway reactivity in obese women with nonallergic asthma (17). The mechanisms by which leptin might affect airway responsiveness could be related to effects on remodeling or surfactant function (17) or to inhibition of the parasympathetic drive, causing bronchodilation (45).
Since diet-induced weight loss was as effective as surgery in the mice in eliciting weight loss and reducing the inherent methacholine hyperresponsiveness of obesity, and because there was substantial mortality in the HDM sensitized and challenged mice subjected to sleeve gastrectomy (data not shown), we elected to use the diet-induced weight-loss regimen in the allergic obese asthma model. The increased Newtonian resistance (RN) in the obese allergic HFD mice as compared with the lean LFD and the diet-induced weight loss mice indicate that the obese mice had a form of allergen-induced methacholine hyperresponsiveness affecting the central airways, whereas the inherent methacholine hyperresponsiveness of obesity affects the more distal airway. It should be recognized that the cohorts of mice used in the inherent and allergic asthma experiments were analyzed at different times (separated by months) and were of different ages (by 2 wk). Furthermore, the obese mice in the allergic asthma group were heavier than the obese mice in the inherent asthma group and, in contrast to the mice exposed to HDM, the inherent asthma mice were not instilled intranasally with a liquid. Therefore, there are limitations to comparing these two models and ascribing the augmented and altered pattern of methacholine responsiveness in the allergic mice exclusively to the consequences of allergen exposure. Nonetheless, our findings are consistent with the distinct physiological responses we have described in obese individuals with nonallergic late-onset asthma, and those obese individuals with earlier-onset allergic disease (46). We have no evidence of augmented inflammation or remodeling in these mice, because there were no differences in the expressions of proinflammatory genes (Il6, Ccl2, Cdf, Tnfrs1b, Ntrk2, and Saa3), mucus-associated genes (Muc5ac and Clca1/Gob5), smooth muscle actin (Acta2), or myosin (Myh11). However, it is possible that there are alterations in the airways at levels that would not be reflected by altered gene expression in whole-lung samples. The low lung volumes could manifest as alterations to the contractile properties of the smooth muscle (47), which could affect methacholine responsiveness by exacerbating existing smooth muscle dysfunction in obese allergic asthma.
Given that allergic asthma is driven frequently by ILC2s, Th2 cells, and their secreted cytokines, we examined whether the increased methacholine hyperresponsiveness of obese allergic asthma would correlate with augmented markers of the atopic response. Indeed, all the HDM-sensitized mice demonstrated a marked abundance of eosinophils in BAL. However, the HDM-sensitized mice with the most marked response to the methacholine challenge, the HFD group, did not show signs of an increased Th2 response relative to the lean mice, nor was there an alteration in the degree or type of response in the diet-induced weight loss mice relative to the HFD mice. In fact, IL-5, IL-13, IL-17A, IL-6, and TNF were lower in the obese or diet-induced weight loss mice compared with the LFD mice, which demonstrated the lowest methacholine reactivity. Our findings are perhaps not surprising, because serum IL-5 and IL-13 levels in humans with obese allergic asthma have been reported to be no different from those in lean humans with allergic asthma (48). As we have recently reported (19), investigators using mouse models of high-fat diet–induced allergic asthma have reported inconsistent findings regarding the magnitude of the adaptive (Th2) immune response, airway eosinophilia, and methacholine hyperresponsiveness relative to mice fed a low-fat diet. The reasons for these discrepancies include mouse strain, weights, and ages; the antigen used; the antigen delivery; and the duration of antigen exposure. Furthermore, all these models, including ours, used antigen exposures after the mice had become obese, which does not model the early-onset obese allergic asthma phenotype more prevalent in the human population.
Alterations in the intestinal microbial composition can affect and be affected by body weight (21). Characteristically obesogenic gut bacterial commensal profiles include an increased Firmicutes/Bacteroidetes ratio (20, 49) that can be reversed by preventing weight gain or promoting weight loss (50, 51). Interestingly, several forms of weight loss in humans (52), including bariatric surgery (53), also reduce the Firmicutes/Bacteroidetes ratio. The impact that the commensal microbiota could have on the complex pathophysiologic manifestations of obese asthma are manifold; they include the absorption of micronutrients and the generation of circulating metabolites that could affect structure and function, as have been reviewed recently (22). How microbiome changes are participating in the resolution of obesity-associated asthma remains to be determined.
Although human diet and exercise studies indicate that a minimum of 5–10% weight loss is required to influence asthma control (54, 55), we obtained more substantial weight loss in our mouse models (15–25%). How much weight loss is required for the beneficial effects we observed is unknown, and nor is it known how sustained the impacts of weight loss are in our mouse models. In individuals with obese asthma undergoing a behavioral weight-reduction program, significant improvements in spirometry, methacholine responsiveness, asthma control, and asthma quality of life were seen within 3 months (56). In individuals with obese asthma subjected to bariatric surgery, the decreased risk of a severe exacerbation remained substantially reduced for as long as this has been studied (up to 24 mo) (57), whereas asthma symptoms have been reduced and spirometry has been improved for as long as 5 years (58). That obesity-associated asthma manifestations are amenable to improvement through weight loss both in our system and in human studies reinforces the value of these mouse models for the study of disease pathogenesis and treatment.
Supplementary Material
Footnotes
This work was supported by a Pilot Project grant and Core support through the Vermont Lung Center CoBRE program, by National Institutes of Health grant P30 GM103532, and by Core support through the Vermont Center for Immunobiology and Infectious Diseases CoBRE program, P20 GM103496.
Author Contributions: Conception and design: J.L.A., B.T.S., J.H.T.B., C.G.I., S.R.R., P.M.F., A.E.D., and M.E.P.; analysis and interpretation: J.L.A., M.C., L.R.H., M.J.R., A.G., N.A.D., M.I.A., B.T.S., J.H.T.B., C.G.I., S.R.R., P.M.F., A.E.D., and M.E.P.; and drafting the manuscript and reviewing for important intellectual content: J.L.A., M.C., B.T.S., J.H.T.B., C.G.I., S.R.R., P.M.F., A.E.D., and M.E.P.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0070OC on April 11, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2015[accessed 2016 Mar 21]Available from: http://www.ginasthma.org/
- 2.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. Hyattville, MD: National Center for Health Statistics; 2012. [PubMed] [Google Scholar]
- 3.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 4.Switzer NJ. Current trends in obesity: body composition assessment, weight regulation, and emerging techniques in managing severe obesity. J Interv Gastroenterol. 2013;3:34. [Google Scholar]
- 5.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 7.Ather JL, Poynter ME, Dixon AE. Immunological characteristics and management considerations in obese patients with asthma. Expert Rev Clin Immunol. 2015;11:793–803. doi: 10.1586/1744666X.2015.1040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon A. The treatment of asthma in obesity. Expert Rev Respir Med. 2012;6:331–340. doi: 10.1586/ers.12.22. [DOI] [PubMed] [Google Scholar]
- 9.Vortmann M, Eisner MD. BMI and health status among adults with asthma. Obesity (Silver Spring) 2008;16:146–152. doi: 10.1038/oby.2007.7. [DOI] [PubMed] [Google Scholar]
- 10.Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, Haselkorn T. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2014;133:1549–1556. doi: 10.1016/j.jaci.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Saber AA, Elgamal MH, McLeod MK. Bariatric surgery: the past, present, and future. Obes Surg. 2008;18:121–128. doi: 10.1007/s11695-007-9308-7. [DOI] [PubMed] [Google Scholar]
- 12.Pradeepan S, Garrison G, Dixon AE. Obesity in asthma: approaches to treatment. Curr Allergy Asthma Rep. 2013;13:434–442. doi: 10.1007/s11882-013-0354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sideleva O, Black K, Dixon AE. Effects of obesity and weight loss on airway physiology and inflammation in asthma. Pulm Pharmacol Ther. 2013;26:455–458. doi: 10.1016/j.pupt.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sideleva O, Dixon AE. The many faces of asthma in obesity. J Cell Biochem. 2014;115:421–426. doi: 10.1002/jcb.24678. [DOI] [PubMed] [Google Scholar]
- 15.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128:508–515.e501–502. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Alwan A, Bates JH, Chapman DG, Kaminsky DA, DeSarno MJ, Irvin CG, Dixon AE. The nonallergic asthma of obesity. A matter of distal lung compliance. Am J Respir Crit Care Med. 2014;189:1494–1502. doi: 10.1164/rccm.201401-0178OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sideleva O, Suratt BT, Black KE, Tharp WG, Pratley RE, Forgione P, Dienz O, Irvin CG, Dixon AE. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med. 2012;186:598–605. doi: 10.1164/rccm.201203-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin RA, Hodgkins SR, Dixon AE, Poynter ME. Aligning mouse models of asthma to human endotypes of disease. Respirology. 2014;19:823–833. doi: 10.1111/resp.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon AE, Poynter ME. Mechanisms of asthma in obesity: pleiotropic aspects of obesity produce distinct asthma phenotypes. Am J Respir Cell Mol Biol. 2016;54:601–608. doi: 10.1165/rcmb.2016-0017PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587:4153–4158. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shore SA, Cho Y. Obesity and asthma: microbiome-metabolome interactions. Am J Respir Cell Mol Biol. 2016;54:609–617. doi: 10.1165/rcmb.2016-0052PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundblad LK, Thompson-Figueroa J, Allen GB, Rinaldi L, Norton RJ, Irvin CG, Bates JH. Airway hyperresponsiveness in allergically inflamed mice: the role of airway closure. Am J Respir Crit Care Med. 2007;175:768–774. doi: 10.1164/rccm.200610-1410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundblad LK, Rinaldi LM, Poynter ME, Riesenfeld EP, Wu M, Aimi S, Barone LM, Bates JH, Irvin CG. Detrimental effects of albuterol on airway responsiveness requires airway inflammation and is independent of β-receptor affinity in murine models of asthma. Respir Res. 2011;12:27. doi: 10.1186/1465-9921-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundblad LK, Irvin CG, Adler A, Bates JH. A reevaluation of the validity of unrestrained plethysmography in mice. J Appl Physiol (1985) 2002;93:1198–1207. doi: 10.1152/japplphysiol.00080.2002. [DOI] [PubMed] [Google Scholar]
- 26.Paveglio SA, Allard J, Foster Hodgkins SR, Ather JL, Bevelander M, Campbell JM, Whittaker LeClair LA, McCarthy SM, van der Vliet A, Suratt BT, et al. Airway epithelial indoleamine 2,3-dioxygenase inhibits CD4+ T cells during Aspergillus fumigatus antigen exposure. Am J Respir Cell Mol Biol. 2011;44:11–23. doi: 10.1165/rcmb.2009-0167OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barman M, Unold D, Shifley K, Amir E, Hung K, Bos N, Salzman N. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun. 2008;76:907–915. doi: 10.1128/IAI.01432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman SM, Tully JE, Nolin JD, Lahue KG, Goldman DH, Daphtary N, Aliyeva M, Irvin CG, Dixon AE, Poynter ME, et al. Endoplasmic reticulum stress mediates house dust mite-induced airway epithelial apoptosis and fibrosis. Respir Res. 2013;14:141. doi: 10.1186/1465-9921-14-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagers S, Lundblad LK, Ekman M, Irvin CG, Bates JH. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J Appl Physiol (1985) 2004;96:2019–2027. doi: 10.1152/japplphysiol.00924.2003. [DOI] [PubMed] [Google Scholar]
- 31.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, Iwakura Y, Israel E, Bolger K, Faul J, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20:54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathews JA, Wurmbrand AP, Ribeiro L, Neto FL, Shore SA. Induction of IL-17A precedes development of airway hyperresponsiveness during diet-induced obesity and correlates with complement factor D. Front Immunol. 2014;5:440. doi: 10.3389/fimmu.2014.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bates JH, Dixon AE. Potential role of the airway wall in the asthma of obesity. J Appl Physiol (1985) 2015;118:36–41. doi: 10.1152/japplphysiol.00684.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shore SA. Obesity and asthma: lessons from animal models. J Appl Physiol (1985) 2007;102:516–528. doi: 10.1152/japplphysiol.00847.2006. [DOI] [PubMed] [Google Scholar]
- 36.Kim JY, Sohn JH, Lee JH, Park JW. Obesity increases airway hyperresponsiveness via the TNF-α pathway and treating obesity induces recovery. PLoS One. 2015;10:e0116540. doi: 10.1371/journal.pone.0116540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Medoff BD, Okamoto Y, Leyton P, Weng M, Sandall BP, Raher MJ, Kihara S, Bloch KD, Libby P, Luster AD. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol. 2009;41:397–406. doi: 10.1165/rcmb.2008-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006;118:389–395. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 41.Sood A, Shore SA. Adiponectin, leptin, and resistin in asthma: basic mechanisms through population studies. J Allergy (Cairo) 2013;2013:785835. doi: 10.1155/2013/785835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spaniolas K, Kasten KR, Brinkley J, Sippey ME, Mozer A, Chapman WH, Pories WJ. The changing bariatric surgery landscape in the USA. Obes Surg. 2015;25:1544–1546. doi: 10.1007/s11695-015-1764-x. [DOI] [PubMed] [Google Scholar]
- 43.Dimitriadis E, Daskalakis M, Kampa M, Peppe A, Papadakis JA, Melissas J. Alterations in gut hormones after laparoscopic sleeve gastrectomy: a prospective clinical and laboratory investigational study. Ann Surg. 2013;257:647–654. doi: 10.1097/SLA.0b013e31826e1846. [DOI] [PubMed] [Google Scholar]
- 44.Mardinoglu A, Heiker JT, Gärtner D, Björnson E, Schön MR, Flehmig G, Klöting N, Krohn K, Fasshauer M, Stumvoll M, et al. Extensive weight loss reveals distinct gene expression changes in human subcutaneous and visceral adipose tissue. Sci Rep. 2015;5:14841. doi: 10.1038/srep14841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arteaga-Solis E, Zee T, Emala CW, Vinson C, Wess J, Karsenty G. Inhibition of leptin regulation of parasympathetic signaling as a cause of extreme body weight-associated asthma. Cell Metab. 2013;17:35–48. doi: 10.1016/j.cmet.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chapman DG, Irvin CG, Kaminsky DA, Forgione PM, Bates JH, Dixon AE. Influence of distinct asthma phenotypes on lung function following weight loss in the obese. Respirology. 2014;19:1170–1177. doi: 10.1111/resp.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McClean MA, Matheson MJ, McKay K, Johnson PR, Rynell AC, Ammit AJ, Black JL, Berend N. Low lung volume alters contractile properties of airway smooth muscle in sheep. Eur Respir J. 2003;22:50–56. doi: 10.1183/09031936.03.00099802. [DOI] [PubMed] [Google Scholar]
- 48.Sutherland TJ, Cowan JO, Young S, Goulding A, Grant AM, Williamson A, Brassett K, Herbison GP, Taylor DR. The association between obesity and asthma: interactions between systemic and airway inflammation. Am J Respir Crit Care Med. 2008;178:469–475. doi: 10.1164/rccm.200802-301OC. [DOI] [PubMed] [Google Scholar]
- 49.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 50.Ravussin Y, Koren O, Spor A, LeDuc C, Gutman R, Stombaugh J, Knight R, Ley RE, Leibel RL. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity (Silver Spring) 2012;20:738–747. doi: 10.1038/oby.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 53.Tremaroli V, Karlsson F, Werling M, Ståhlman M, Kovatcheva-Datchary P, Olbers T, Fändriks L, le Roux CW, Nielsen J, Bäckhed F. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22:228–238. doi: 10.1016/j.cmet.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma J, Strub P, Xiao L, Lavori PW, Camargo CA, Jr, Wilson SR, Gardner CD, Buist AS, Haskell WL, Lv N. Behavioral weight loss and physical activity intervention in obese adults with asthma. A randomized trial. Ann Am Thorac Soc. 2015;12:1–11. doi: 10.1513/AnnalsATS.201406-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scott HA, Gibson PG, Garg ML, Pretto JJ, Morgan PJ, Callister R, Wood LG. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: a randomized trial. Clin Exp Allergy. 2013;43:36–49. doi: 10.1111/cea.12004. [DOI] [PubMed] [Google Scholar]
- 56.Pakhale S, Baron J, Dent R, Vandemheen K, Aaron SD. Effects of weight loss on airway responsiveness in obese adults with asthma: does weight loss lead to reversibility of asthma? Chest. 2015;147:1582–1590. doi: 10.1378/chest.14-3105. [DOI] [PubMed] [Google Scholar]
- 57.Hasegawa K, Tsugawa Y, Chang Y, Camargo CA., Jr Risk of an asthma exacerbation after bariatric surgery in adults. J Allergy Clin Immunol. 2015;136:288–294.e8. doi: 10.1016/j.jaci.2014.12.1931. [DOI] [PubMed] [Google Scholar]
- 58.Hewitt S, Humerfelt S, Søvik TT, Aasheim ET, Risstad H, Kristinsson J, Mala T. Long-term improvements in pulmonary function 5 years after bariatric surgery. Obes Surg. 2014;24:705–711. doi: 10.1007/s11695-013-1159-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.