Abstract
Surgically created arteriovenous fistulae (AVF) for hemodialysis can contribute to hemodynamic changes. We describe the cases of 2 male patients in whom new right ventricular enlargement developed after an AVF was created for hemodialysis. Patient 1 sustained high-output heart failure solely attributable to the AVF. After AVF banding and subsequent ligation, his heart failure and right ventricular enlargement resolved. In Patient 2, the AVF contributed to new-onset right ventricular enlargement, heart failure, and ascites. His severe pulmonary hypertension was caused by diastolic heart failure, diabetes mellitus, and obstructive sleep apnea. His right ventricular enlargement and heart failure symptoms did not improve after AVF ligation.
We think that our report is the first to specifically correlate the echocardiographic finding of right ventricular enlargement with AVF sequelae. Clinicians who treat end-stage renal disease patients should be aware of this potential sequela of AVF creation, particularly in the upper arm. We recommend obtaining preoperative echocardiograms in all patients who will undergo upper-arm AVF creation, so that comparisons can be made postoperatively. Alternative consideration should be given to creating the AVF in the radial artery, because of less shunting and therefore less potential for right-sided heart failure and pulmonary hypertension. A multidisciplinary approach is optimal when selecting patients for AVF banding or ligation.
Keywords: Arteriovenous fistula/complications/surgery; arteriovenous shunt, surgical/adverse effects; cardiac output; heart failure/etiology; hypertension, pulmonary/etiology/physiopathology; ligation/adverse effects/methods; renal dialysis/adverse effects; renal insufficiency/complications; severity of illness index; ventricular dysfunction, right/physiopathology
Patients who have chronic kidney disease often die of cardiovascular disease.1 The preferred vascular-access site for hemodialysis patients is the surgically created arteriovenous fistula (AVF).2 The AVF is associated with hemodynamic changes, including increased cardiac output, decreased systemic vascular resistance, increased sympathetic activity, and increased pulmonary flow and pressure.3 Authors have described overt heart failure caused by increased cardiac output after AVF creation.4 We report the cases of 2 patients in whom right ventricular (RV) enlargement developed after the creation of an AVF, and we discuss our decisions to ligate each AVF.
Case Reports
Patient 1
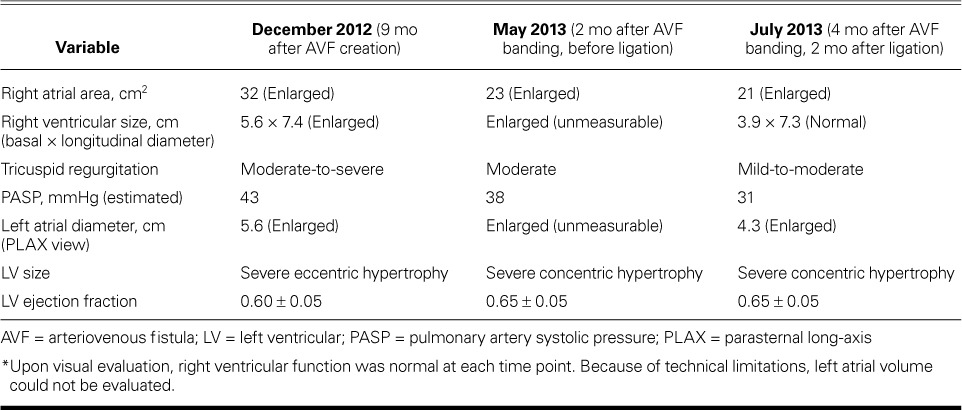
In March 2012, a 54-year-old man with end-stage renal disease who was undergoing hemodialysis via central venous access had a brachiocephalic AVF created in his left upper arm. His medical history was notable for alcoholic cirrhosis and consequent liver transplantation in 2004, and pancreas, small-bowel, large-bowel, and partial stomach transplantation because of mesenteric-vasculature thrombus. The sequelae of acute renal failure and sepsis eventually necessitated hemodialysis. An echocardiogram from 2003 showed normal ventricular sizes and function; however, no echocardiogram was obtained immediately before AVF creation. In the months after AVF creation, the patient experienced new exertional dyspnea and palpitations and had hypotensive episodes during dialysis. He was referred for cardiologic evaluation in December 2012, 9 months after AVF creation. At this time, his blood pressure was 155/82 mmHg in the right arm. Findings during the comprehensive examination included a well-matured, enlarged, serpentine, cephalic venous outflow tract with a prominent thrill and bruit in the left arm, jugular venous distention with large V waves, a systolic crescendo murmur, a diastolic decrescendo murmur, and a pulsatile liver. An echocardiogram showed RV enlargement (Fig. 1A). Cardiac catheterization revealed nonobstructive coronary artery disease with high-output heart failure: right atrial pressure, 10 mmHg; pulmonary artery pressure, 32/10 mmHg (mean pressure, 21 mmHg); pulmonary capillary wedge pressure, 16 mmHg; and cardiac index, 5.35 L/min/m2. Ultrasonograms of the AVF showed a markedly elevated flow volume (average, 8 L/min) and a diameter of 1.9 cm. (Table I lists various echocardiographic findings.)
Fig. 1.
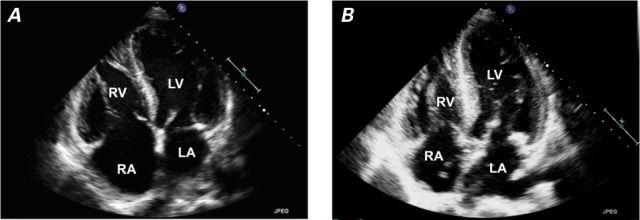
Patient 1. Transthoracic echocardiograms (4-chamber views) show A) right atrial and right ventricular enlargement 9 months after arteriovenous fistula creation, and B) near-normal right atrial and ventricular sizes 4 months after banding of the fistula and 2 months after its ligation.
LA = left atrium; LV = left ventricle; RA = right atrium; RV = right ventricle
TABLE I.
Echocardiographic Characteristics of Patient 1 over Time *
Because of the high-output heart failure and elevated flow volumes, we decided to surgically correct the AVF. We planned a staged approach: initial reduction of the AVF's size via banding, and later ligation. Staging, we thought, would minimize sudden hemodynamic changes and enable us to evaluate symptomatic improvement after the banding.
The patient underwent AVF banding in March 2013, 12 months after AVF creation. His cephalic venous outflow tract was reduced by 25%, which he tolerated well. Although his heart failure symptoms improved, they did not completely resolve, nor did the echocardiographic findings. The AVF flow volume was reduced to an average of 4.3 L/min.
In May 2013, the patient underwent AVF ligation and placement of a tunneled venous dialysis catheter. Two months later, his symptoms had improved dramatically, the size of his RV had returned to normal, and the degree of tricuspid regurgitation on echocardiography was reduced (Fig. 1B). The patient's condition remained stable, and he later underwent kidney transplantation.
Patient 2
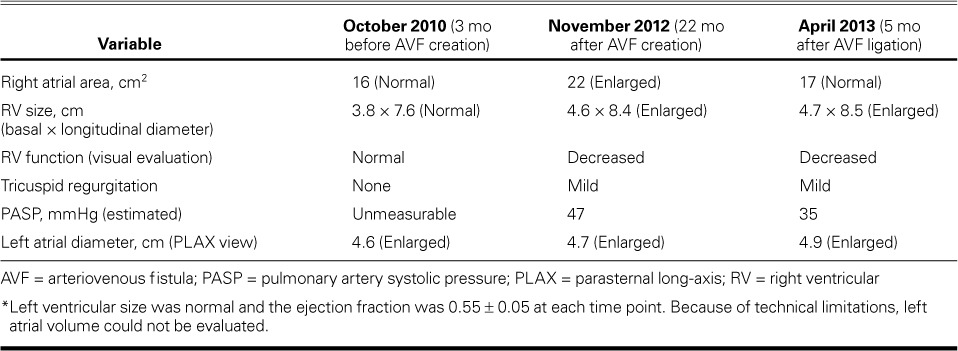
In January 2011, a 35-year-old man with end-stage renal disease underwent the creation of a left brachiobasilic AVF. His medical history included mitral valve endocarditis, treated with bioprosthetic mitral valve replacement in 2008; morbid obesity; diabetes mellitus requiring insulin; hypertension; deep vein thrombosis; coronary artery and peripheral vascular disease; osteomyelitis and consequent amputation above the right knee, and atrial flutter requiring cardioversion. A routine echocardiogram in October 2010 had shown normal RV size (Fig. 2A), but no echocardiogram was obtained just before AVF creation in July 2011. (Table II shows various echocardiographic findings over time.)
Fig. 2.
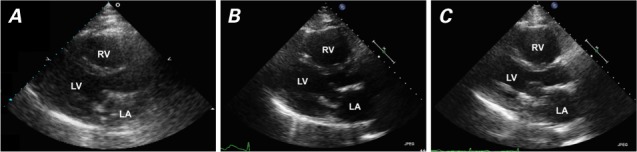
Patient 2. Transthoracic echocardiograms (parasternal long-axis views) show A) normal right ventricular (RV) size 3 months before arteriovenous fistula creation (October 2010), B) RV enlargement 22 months after fistula creation (November 2012), and C) unchanged RV size 5 months after fistula ligation (April 2013).
LA = left atrium; LV = left ventricle
TABLE II.
Echocardiographic Characteristics of Patient 2 over Time *
Six months after operation, flow through the AVF was not adequate for dialysis. The patient's occluded left subclavian artery was treated with angioplasty. Six months later, he needed recanalization of the left subclavian vein with stent placement. In May 2012, 15 months after AVF creation, he was hospitalized with new-onset ascites, and echocardiograms showed new RV enlargement and dysfunction. The ascites was thought to be caused by right-sided heart failure (RHF).
The patient was hospitalized again for heart failure in September 2012. His blood pressure was 127/69 mmHg in the right arm, and physical examination revealed ascites and left-lower-extremity edema. Ultrasonograms of the AVF in the left upper arm showed flow rates ranging from 1.4 to 3.4 L/min (average rate, 2.1 L/min). Cardiac catheterization revealed biventricular heart failure and severe pulmonary hypertension (PH): right atrial pressure, 20 mmHg; pulmonary artery pressure, 80/40 mmHg (mean pressure, 52 mmHg); pulmonary capillary wedge pressure, 30 mmHg; and cardiac index, 3.19 L/min/m2. Figure 2B shows an echocardiogram from November 2012, 21 months after AVF creation. A transesophageal echocardiogram showed a well-seated mitral bioprosthesis with no mitral regurgitation or stenosis to explain the left-sided heart failure and severe PH.
The patient's cardiologist, nephrologist, and vascular surgeon discussed whether the AVF was contributing to the worsening RV enlargement, heart failure, and resultant ascites and liver failure. The patient's competing left-sided heart failure was presumed to be caused by diastolic heart failure, diabetes mellitus, and obstructive sleep apnea, with a likely fixed component of severe PH; therefore, it was decided not to ligate the AVF and instead to administer aggressive dialysis to treat the patient's left-sided heart failure. However, bleeding at the AVF site necessitated AVF ligation in November 2012. The ligation had not substantially improved the chronic RHF symptoms or the RV enlargement as of April 2013 (Fig. 2C). As expected, the patient continued to need frequent paracenteses for ascites. He was diagnosed formally with cirrhosis of the liver (determined to be caused by chronic RHF), and he continued to deteriorate clinically. He died in July 2013 of a large embolic stroke.
Discussion
These cases highlight the medical complexities in 2 hemodialysis patients in whom RV enlargement developed after AVF creation. We think that our report is the first to specifically correlate the echocardiographic finding of RV enlargement with AVF sequelae. Patient 1's AVF was ligated because of clinical suspicion that the AVF was causing new RV enlargement and high-output heart failure, and ligation indeed yielded the reversal of symptoms and the improved RV echocardiographic findings. Patient 2's AVF was ligated because of bleeding; other contributory factors led to no clinical or echocardiographic improvement in his RHF and RV enlargement.
An association between AVF creation and symptomatic RHF has been reported twice previously. Clarkson and colleagues5 described the case of a 52-year-old man whose PH and RHF developed 6 years after AVF creation for hemodialysis and rapidly resolved after surgical ligation of the AVF. That patient had PH, but not ascites. Rao and associates3 described the case of a 69-year-old man whose symptomatic RHF developed 4 years after AVF creation and rapidly improved after ligation. That patient had a pleural effusion, hepatomegaly, and ascites, but not PH. The heart failure in these authors' patients developed chronically over a period of years; conversely, in both of our patients, RV enlargement and heart failure developed within months of AVF creation.
The estimated prevalence of PH among hemodialysis patients (17%–50%) is higher than that in the general population. Increases in cardiac output alone are insufficient to explain this phenomenon. Proposed mechanisms include elevated pulmonary vascular resistance, vascular calcification, chronic hypoxia, and uremia. Pulmonary hypertension has resolved after renal transplantation and banding or closure of AVFs.6 An association between AVF flow and elevated pulmonary artery pressures was reported after an observational study of 34 patients. Those investigators7 found lower flow rates in radial AVFs than in brachial AVFs, which suggests that distally placed AVFs decrease the risk of RHF and PH. In addition, dialysis patients with AVFs have higher right-sided flow volumes than do patients who have central venous catheters. This is probably caused by excess venous return to the right side of the heart after AVF creation.8 Both of our cases strongly support the association between upper-arm AVF creation and RV enlargement.
The National Kidney Foundation–Kidney Disease Outcomes Quality Initiative recommends weekly monitoring of hemodialysis access by physical examination, and monthly measurements of access blood flow. Whether monthly measurements provide sufficient data for clinical decisions about changes to vascular access is unclear, and more study on this topic is warranted.2 It is prudent that the cardiologist, nephrologist, and vascular surgeon consider AVF-induced RV enlargement and high-output or right-sided heart failure in the hemodialysis patient who develops symptoms or signs of heart failure that are independent of the presence of PH. The echocardiographic finding of RV enlargement after AVF creation can be the first clue to this underappreciated complication. Obtaining preoperative echocardiograms in patients who will undergo upper-arm AVF creation can enable meaningful comparisons later. Alternatively, arm AVFs can be preferentially created at the radial artery site, which appears to be less prone to high degrees of shunting and resultant RHF and PH. The decision between AVF banding and ligation requires a multidisciplinary approach and, often, performing right-sided heart catheterization.
Footnotes
From: Jefferson Heart Institute, Thomas Jefferson University Hospital, Philadelphia, Pennsylvania 19107
References
- 1.Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13(3):745–53. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 2.Vascular Access Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(Suppl 1):S248–73. doi: 10.1053/j.ajkd.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 3.Rao N, Worthley M, Disney P, Faull R. Dramatic improvement in decompensated right heart failure due to severe tricuspid regurgitation following ligation of arteriovenous fistula in a renal transplant recipient. Semin Dial. 2014;27(2):E24–6. doi: 10.1111/sdi.12145. [DOI] [PubMed] [Google Scholar]
- 4.Stern AB, Klemmer PJ. High-output heart failure secondary to arteriovenous fistula. Hemodial Int. 2011;15(1):104–7. doi: 10.1111/j.1542-4758.2010.00518.x. [DOI] [PubMed] [Google Scholar]
- 5.Clarkson MR, Giblin L, Brown A, Little D, Donohoe J. Reversal of pulmonary hypertension after ligation of a brachiocephalic arteriovenous fistula. Am J Kidney Dis. 2002;40(3):E8. doi: 10.1053/ajkd.2002.34932. [DOI] [PubMed] [Google Scholar]
- 6.Kosmadakis G, Aguilera D, Carceles O, Da Costa Correia E, Boletis I. Pulmonary hypertension in dialysis patients. Ren Fail. 2013;35(4):514–20. doi: 10.3109/0886022X.2013.766559. [DOI] [PubMed] [Google Scholar]
- 7.Beigi AA, Sadeghi AM, Khosravi AR, Karami M, Masoudpour H. Effects of the arteriovenous fistula on pulmonary artery pressure and cardiac output in patients with chronic renal failure. J Vasc Access. 2009;10(3):160–6. doi: 10.1177/112972980901000305. [DOI] [PubMed] [Google Scholar]
- 8.Di Lullo L, Floccari F, Polito P. Right ventricular diastolic function in dialysis patients could be affected by vascular access. Nephron Clin Pract. 2011;118(3):c257–61. doi: 10.1159/000321867. [DOI] [PubMed] [Google Scholar]


