Abstract
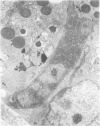
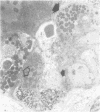
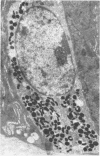
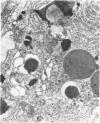
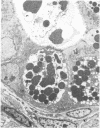
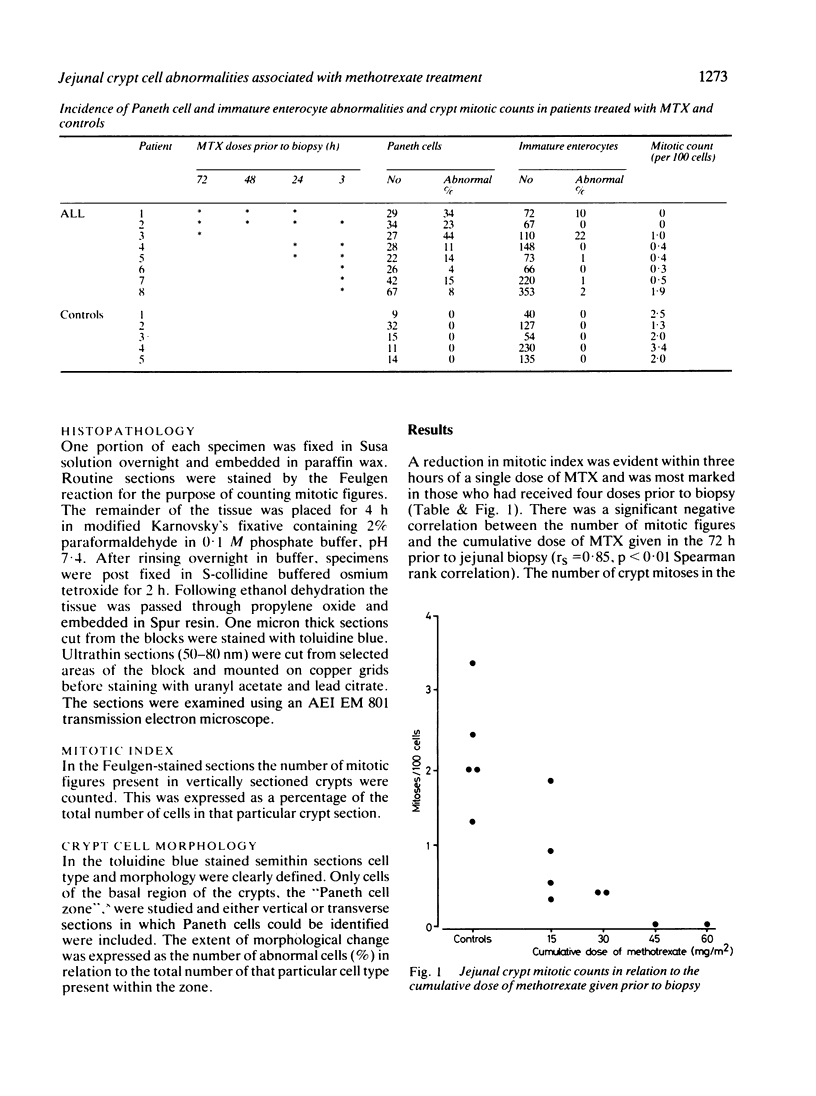
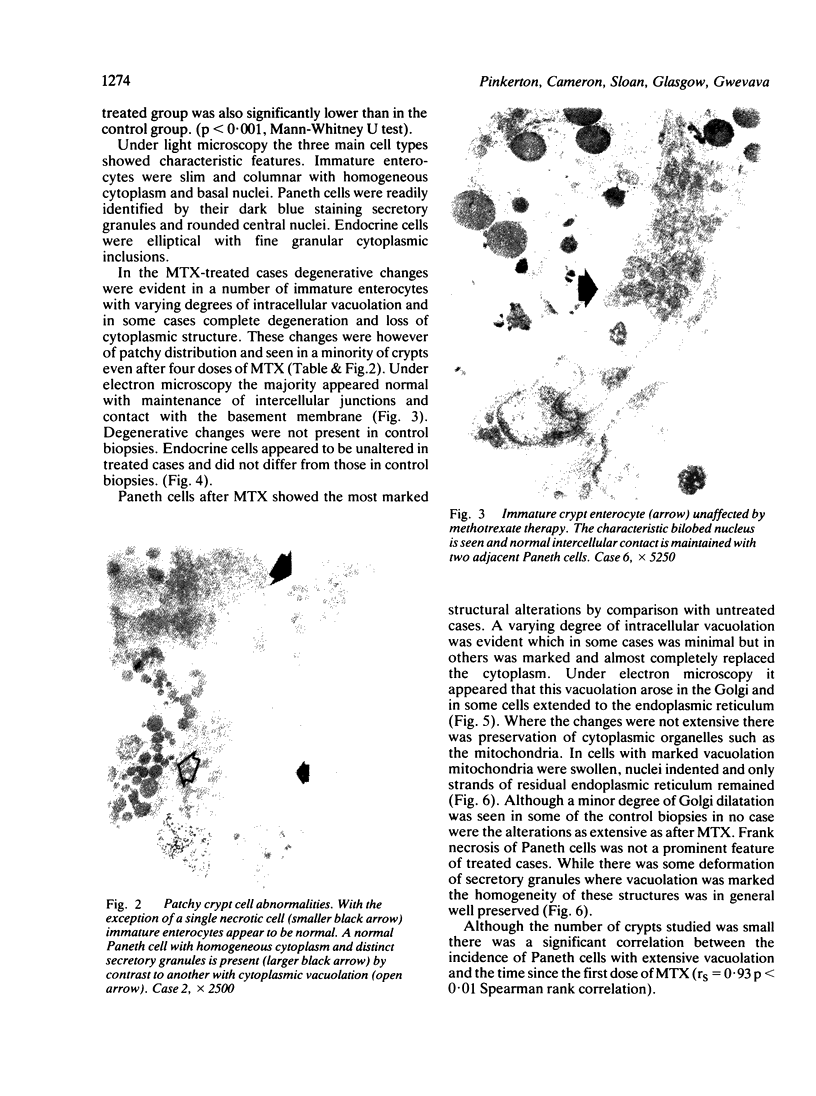
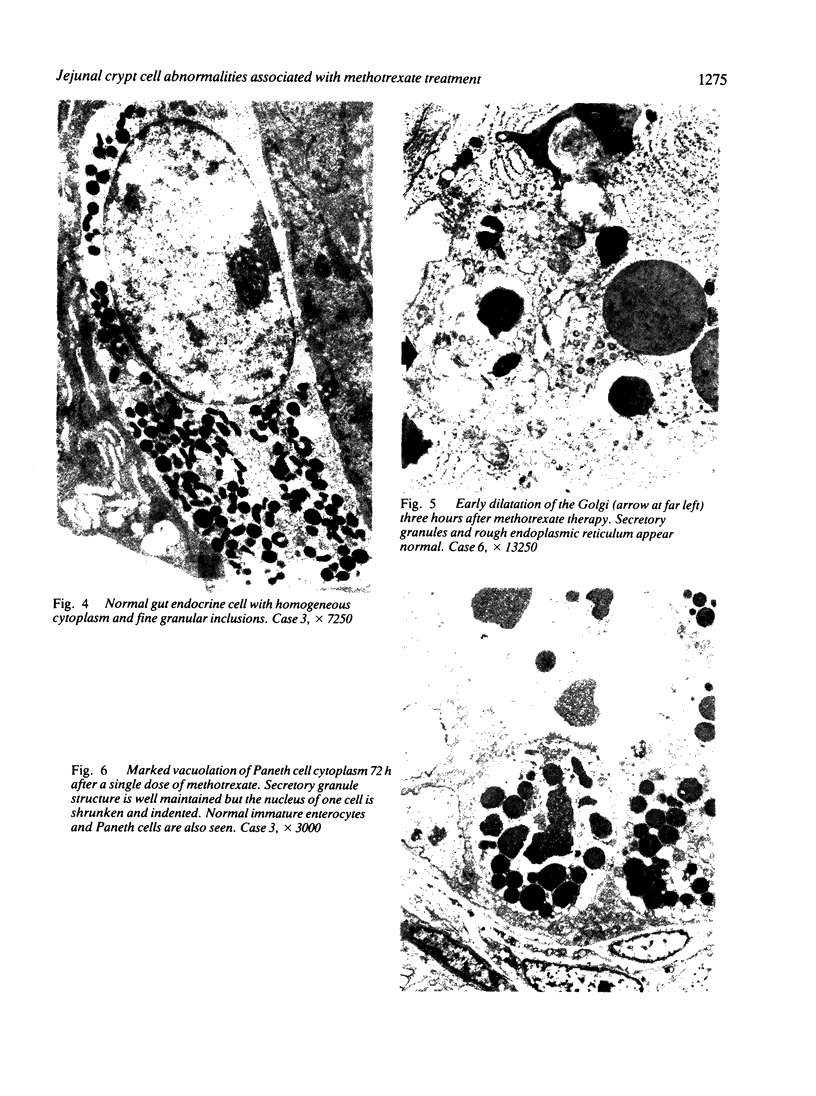
Jejunal mucosal crypts were examined in jejunal biopsies from eight children with acute lymphoblastic leukaemia who had recently received methotrexate treatment. By comparison with biopsies from children under investigation for suspected malabsorption crypt mitosis was significantly reduced and showed a negative correlation with the dose of methotrexate given prior to biopsy. The three major cell types were studied under light and transmission electron microscopy. Gut endocrine cells were unaffected by therapy and immature crypt enterocytes showed only patchy degenerative abnormalities. By contrast a number of Paneth cells showed striking structural alterations with vacuolar dilatation of the cytoplasm. The extent of this correlated with the time since methotrexate treatment rather than its dose and may have been a functional response rather than of a toxic nature.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baskerville A., Batter-Hatton D. Intestinal lesions induced experimentally by methotrexate. Br J Exp Pathol. 1977 Dec;58(6):663–669. [PMC free article] [PubMed] [Google Scholar]
- Bleyer W. A. The clinical pharmacology of methotrexate: new applications of an old drug. Cancer. 1978 Jan;41(1):36–51. doi: 10.1002/1097-0142(197801)41:1<36::aid-cncr2820410108>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Bohane T. D., Cutz E., Hamilton J. R., Gall D. G. Acrodermatitis enteropathica, zinc, and the Paneth cell. A case report with family studies. Gastroenterology. 1977 Sep;73(3):587–592. [PubMed] [Google Scholar]
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III. Entero-endocrine cells. Am J Anat. 1974 Dec;141(4):503–519. doi: 10.1002/aja.1001410405. [DOI] [PubMed] [Google Scholar]
- Creamer B., Pink I. J. Paneth-cell deficiency. Lancet. 1967 Feb 11;1(7485):304–306. doi: 10.1016/s0140-6736(67)91239-1. [DOI] [PubMed] [Google Scholar]
- Cutler E. A., Palmer J., Kontras S. B. Chemotherapy and possible zinc deficiency. N Engl J Med. 1977 Jul 21;297(3):168–169. doi: 10.1056/NEJM197707212970314. [DOI] [PubMed] [Google Scholar]
- Galjaard H., van der Meer-Fieggen W., Giesen J. Feedback control by functional villus cells on cell proliferation and maturation in intestinal epithelium. Exp Cell Res. 1972 Jul;73(1):197–207. doi: 10.1016/0014-4827(72)90120-6. [DOI] [PubMed] [Google Scholar]
- Gent A. E., Creamer B. Paneth cell secretion. Digestion. 1972;7(1):1–12. doi: 10.1159/000197258. [DOI] [PubMed] [Google Scholar]
- Glasgow J. F., Corkey C. W., Molla A. Critical assessment of small bowel biopsy in children. Arch Dis Child. 1979 Aug;54(8):604–608. doi: 10.1136/adc.54.8.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwavava N. J., Pinkerton C. R., Glasgow J. F., Sloan J. M., Bridges J. M. Small bowel enterocyte abnormalities caused by methotrexate treatment in acute lymphoblastic leukaemia of childhood. J Clin Pathol. 1981 Jul;34(7):790–795. doi: 10.1136/jcp.34.7.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson R. W., 2nd, Jervis H. R., Kingry R. L., Wallace J. R. Small bowel changes associated with vincristine sulfate treatment: an experimental study in the guinea pig. Cancer. 1974 Dec;34(6):1888–1896. doi: 10.1002/1097-0142(197412)34:6<1888::aid-cncr2820340606>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Jeynes B. J., Altmann G. G. Light and scanning electron microscopic observations of the effects of sublethal doses of methotrexate on the rat small intestine. Anat Rec. 1978 May;191(1):1–17. doi: 10.1002/ar.1091910102. [DOI] [PubMed] [Google Scholar]
- Margolis S., Philips F. S., Sternberg S. S. The cytotoxicity of methotrexate in mouse small intestine in relation to inhibition of folic acid reductase and of DNA synthesis. Cancer Res. 1971 Dec;31(12):2037–2046. [PubMed] [Google Scholar]
- Muggia A. L., Wagman E., Milles S. S., Spiro H. M. Response of the Gastrointestinal Tract of the Mouse to 5-Fluorouracil. Am J Pathol. 1963 Apr;42(4):407–414. [PMC free article] [PubMed] [Google Scholar]
- PHILIPS F. S., THIERSCH J. B., FERGUSON F. C. Studies of the action of 4-aminopteroylglutamic acid and its congeners in mammals. Ann N Y Acad Sci. 1950 Jul 7;52(8):1349–1359. doi: 10.1111/j.1749-6632.1950.tb54036.x. [DOI] [PubMed] [Google Scholar]
- PHILIPS F. S., THIERSCH J. B., FERGUSON F. C. Studies of the action of 4-aminopteroylglutamic acid and its congeners in mammals. Ann N Y Acad Sci. 1950 Jul 7;52(8):1349–1359. doi: 10.1111/j.1749-6632.1950.tb54036.x. [DOI] [PubMed] [Google Scholar]
- Pinkerton C. R., Welshman S. G., Dempsey S. I., Bridges J. M., Glasgow J. F. Absorption of methotrexate under standardized conditions in children with acute lymphoblastic leukaemia. Br J Cancer. 1980 Oct;42(4):613–615. doi: 10.1038/bjc.1980.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecken E. O., Pearse A. G. Histochemical study on the Paneth cell in the rat. Gut. 1966 Feb;7(1):86–93. doi: 10.1136/gut.7.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. W., Antonioli J. A., Vannotti A. The effect of oral methotrexate on the rat intestine. Biochem Pharmacol. 1966 Oct;15(10):1479–1489. doi: 10.1016/0006-2952(66)90193-6. [DOI] [PubMed] [Google Scholar]
- Rodning C. B., Wilson I. D., Erlandsen S. L. Immunoglobulins within human small-intestinal Paneth cells. Lancet. 1976 May 8;1(7967):984–987. doi: 10.1016/s0140-6736(76)91860-2. [DOI] [PubMed] [Google Scholar]
- Shaw M. T., Spector M. H., Ladman A. J. Effects of cancer, radiotherapy and cytotoxic drugs on intestinal structure and function. Cancer Treat Rev. 1979 Sep;6(3):141–151. doi: 10.1016/s0305-7372(79)80066-3. [DOI] [PubMed] [Google Scholar]
- Skipper H. E., Schabel F. M., Jr, Mellett L. B., Montgomery J. A., Wilkoff L. J., Lloyd H. H., Brockman R. W. Implications of biochemical, cytokinetic, pharmacologic, and toxicologic relationships in the design of optimal therapeutic schedules. Cancer Chemother Rep. 1970 Dec;54(6):431–450. [PubMed] [Google Scholar]
- Slavin R. E., Dias M. A., Saral R. Cytosine arabinoside induced gastrointestinal toxic alterations in sequential chemotherapeutic protocols: a clinical-pathologic study of 33 patients. Cancer. 1978 Oct;42(4):1747–1759. doi: 10.1002/1097-0142(197810)42:4<1747::aid-cncr2820420413>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- TAYLOR S. G., HASS G. M., CRUMRINE J. L., SLAUGHTER D. P. Toxic reactions of 4-amino-pteroylglutamic acid (aminopterin) in patients with far-advanced neoplastic disease. Cancer. 1950 May;3(3):493–503. doi: 10.1002/1097-0142(1950)3:3<493::aid-cncr2820030310>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- TRIER J. S. Morphologic alterations induced by methotrexate in the mucosa of human proximal intestine. II. Electron microscopic observations. Gastroenterology. 1962 Oct;43:407–424. [PubMed] [Google Scholar]
- Trier J. S., Browning T. H. Morphologic response of the mucosa of human small intestine to x-ray exposure. J Clin Invest. 1966 Feb;45(2):194–204. doi: 10.1172/JCI105332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERKHEISER W. C. The relation of folic acid reductase to aminopterin toxicity. J Pharmacol Exp Ther. 1962 Aug;137:167–172. [PubMed] [Google Scholar]
- WOLL E., OLESON J. J. The effects of a folic acid antagonist (aminopterin) on albino rats; a study in the pathogenesis of sprue. Br J Exp Pathol. 1951 Oct;32(5):458–461. [PMC free article] [PubMed] [Google Scholar]