Abstract
The major feature of leukemic cells is an arrest of differentiation accompanied by highly active proliferation. In many subtypes of acute myeloid leukemia, these features are mediated by the aberrant Wnt/β-Catenin pathway. In our study, we established the lectin LecB as inducer of the differentiation of the acute myeloid leukemia cell line THP-1 and used it for the investigation of the involved processes. During differentiation, functional autophagy and low β-Catenin levels were essential. Corresponding to this, a high β-Catenin level stabilized proliferation and inhibited autophagy, resulting in low differentiation ability. Initiated by LecB, β-Catenin was degraded, autophagy became active and differentiation took place within hours. Remarkably, the reduction of β-Catenin sensitized THP-1 cells to the autophagy-stimulating mTOR inhibitors. As downmodulation of E-Cadherin was sufficient to significantly reduce LecB-mediated differentiation, we propose E-Cadherin as a crucial interaction partner in this signaling pathway. Upon LecB treatment, E-Cadherin colocalized with β-Catenin and thereby prevented the induction of β-Catenin target protein expression and proliferation. That way, our study provides for the first time a link between E-Cadherin, the aberrant Wnt/β-Catenin signaling, autophagy and differentiation in acute myeloid leukemia. Importantly, LecB was a valuable tool to elucidate the underlying molecular mechanisms of acute myeloid leukemia pathogenesis and may help to identify novel therapy approaches.
Introduction
Acute myeloid leukemia (AML) is the most common type of leukemia comprising 80% of the cases in adults and 15–20% in children. It is a disease of the hematopoietic system and is characterized by a massive accumulation of immature and nonfunctional myeloid precursor cells in the blood and the bone marrow.1 This arrest of differentiation and infinite proliferation of the myeloid cells are the major problems in the pathophysiology of AML. The initial therapeutic option is still classical chemotherapy with cytarabin and anthracylines that exhibit undesirable side effects especially in older patients. Because of this, the 5-year survival rate is between 30 and 40%, and even more unfavorable in patients >65 years of age.1,2 However, AML is quite a heterogeneous disease with variable prognosis, thus classified in subtypes according to morphology and cytogenetics.3,4 A high degree of AML cases involve chromosomal translocations that generate chimeric oncoproteins stimulating abnormal proliferation and a block of myeloid differentiation. Common fusion proteins in AML are AML1-ETO, PML-RARα or the MLL translocation.1,5–7 Interestingly, all these fusion proteins and the Flt3-internal tandem duplication mutation are associated with aberrant Wnt signaling inducing proliferation and stem cell self-renewal.8–11 The induction of Wnt signaling is accompanied by increased β-Catenin levels and initiates in turn the expression of tumorigenic Wnt target genes like Cyclin D1 or c-myc. Indeed, high β-Catenin levels are also found in primary AML samples.12 The better understanding of these cytogenetic modifications involves a great potential for the development of personalized and targeted therapy, as the classical chemotherapeutics only tend to highly proliferating cells. The issue of the differentiation arrest is not addressed at all. Recently, impressive successes of a new differentiation therapy concept have been achieved in acute promyelocytic leukemia (APL). In APL, the PML-RARα fusion protein represses – insusceptible to the natural ligand retinoic acid – the transcription of genes important for the myeloid differentiation. Pharmacological doses of all-trans retinoic acid (ATRA) or arsen trioxide target specifically the PML-RARα fusion protein and induce its degradation and thereby the transcription and the terminal differentiation of the immature precursor cells. Using this concept, striking remission rates and long-term survival between 80 and 90% could be achieved.13,14 In addition, various therapy attempts of ATRA in combination with other chemotherapeutics are in clinical trials to improve the outcome of the classical therapy.5,15,16 Unfortunately, ATRA and arsen trioxide are only clinically successful in the rare APL subtype (5–10%) of AML. Thereby, other substances that initiate the differentiation of other AML subtypes are needed and are currently investigated.17–21
In our study, we focused on LecB, a fucose-binding lectin of P. aeruginosa. Lectins are common carbohydrate-binding proteins produced by animals, plants or microorganisms contributing to cell adhesion and recognition.22,23 They are implicated in the innate immune system or act as toxins, for example, Shiga or cholera toxins.24–26 The specificity of lectins to a distinct sugar moiety can be used for the recognition of tumor cells as these often exhibit substantial changes in the glycosylation pattern of their surface proteins compared with healthy cells.27,28 As fucosylation is often affected in tumors, fucose-specific lectins are of interest as biomarkers and for targeted therapy.29,30 Furthermore, it has been shown that LecB is cytotoxic to tumor cells and provokes strong agglutination and an attenuated tumor growth rate.31,32
Results
LecB induces differentiation and apoptosis of acute monocytic leukemia cells
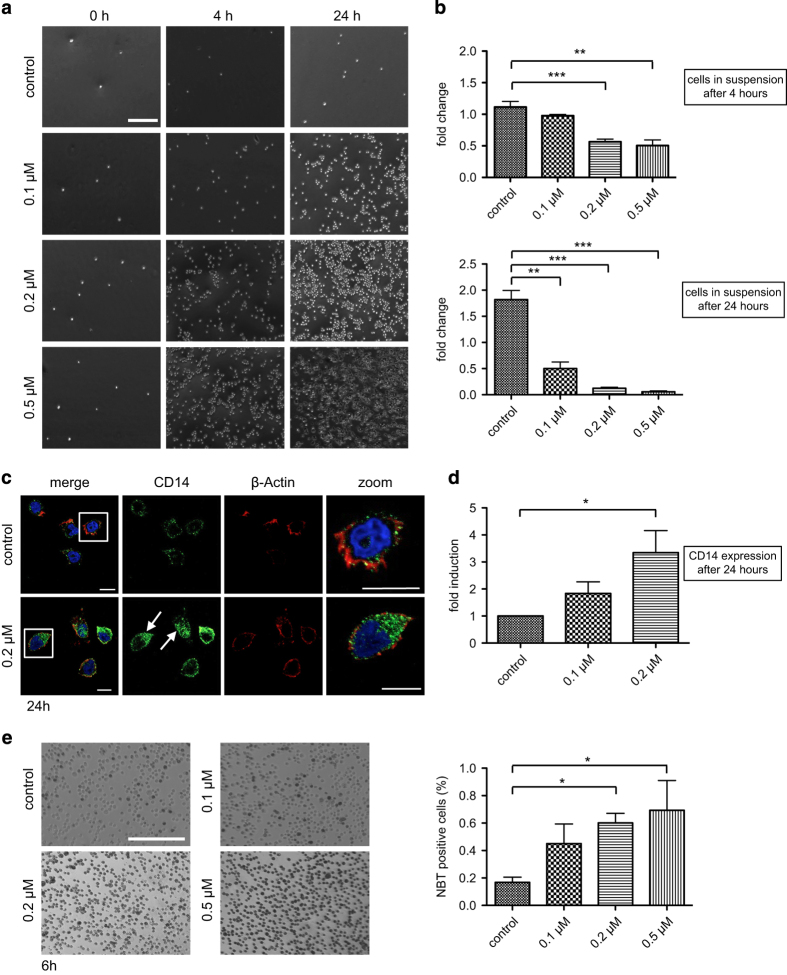
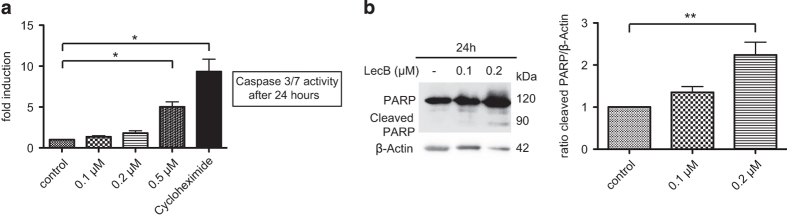
First, we examined the role of LecB in acute myeloid leukemia as some lectins have been shown to act as growth inhibiting and cytotoxic on tumor cells.22 We administered the lectin at different concentrations (from 0.1 to 0.5 μM) to the monocytic cell line THP-1. Interestingly, LecB was able to induce the differentiation of THP-1 cells as shown by attachment assays, immunophenotyping and the nitrobluetetrazolium (NBT) reduction. When treated with LecB, monocytic THP-1 cells adhered to the cell culture plate in a concentration- and time-dependent manner. After 4 h, cells treated with 0.2 and 0.5 μM LecB attached to a great extent compared with no attachment of nontreated cells (Figure 1a). After 24 h, strong attachment was confirmed for all used concentrations of LecB. In order to quantify the adherence, the amount of cells remaining in suspension upon LecB treatment was determined (Figure 1b). Approximately 56% and 50% of the cells treated with 0.2 and 0.5 μM, respectively, were left in suspension after 4 h. This trend was further validated after 24 h, revealing only 11 and 5% of cells in suspension. Nontreated cells continued to proliferate as usual that was confirmed by an almost doubled cell number after 24 h. Immunophenotyping of CD14 expression, which is upregulated during monocytic differentiation, confirmed the results of the attachment assay. A strong increase in CD14 expression was obvious after 24 h of treatment with 0.2 μM LecB by immunofluorescence (Figure 1c, arrows and zoom). According to this observation, flow cytometry analysis revealed a significant induction (approximately threefold) of CD14 expression (Figure 1d). The differentiation-inducing activity of LecB was also assessed by the NBT reduction assay, an established test that measures the ability of differentiated macrophages to induce a respiratory burst. In the presence of reactive oxygen species, the yellow-colored NBT is converted to a blue insoluble compound inside the cells (Figure 1e). The 6-h incubation with LecB yielded a significant induction of the respiratory burst as 51% (0.2 μM) and 58% (0.5 μM) of the THP-1 cells were NBT positive compared with 15% in the nontreated control (Figure 1e, quantification). When blocking the binding sites of LecB with the soluble ligand L-fucose before the treatment, the cells displayed no differentiation as shown in attachment and NBT reduction assays (Supplementary Figure S1A and B). As it is well established that differentiated cells undergo cell death after a certain lifetime, we assessed the induction of apoptosis. We already observed a hint for apoptosis as the cells treated with 0.5 μM LecB for 24 h seem to change their morphology. The attached cells exhibited membrane blebs and in the following detached again from the cell culture plate after 24 h compared with the 4- h treatment. The cells apparently began to die after they had differentiated. Correspondingly, after 24 h of LecB treatment, the activity of the effector caspases 3 and 7 revealed a significant induction (fivefold) of apoptosis at a concentration of 0.5 μM compared with nontreated cells (Figure 2a). As positive control we used Cycloheximide (100 μg/ml) that is highly toxic for cells because of its inhibitory effect on protein synthesis. Lower LecB concentrations did not induce apoptosis to a high extent. Most probably the apoptosis is delayed corresponding to the slower differentiation. To further elucidate the involvement of apoptosis, we also analyzed poly-(ADP-ribose)-polymerase (PARP) cleavage during LecB treatment. PARP is implicated in the repair of damaged DNA and exhibits a cleavage domain by which caspases can degrade the protein.33 In accordance with the caspase results, we observed a twofold increase of the cleaved PARP protein upon stimulation with 0.2 μM LecB for 24 h (Figure 2b). Taken together, LecB induces a very fast differentiation of monocytic THP-1 cells followed by apoptosis of the differentiated cells.
Figure 1.
LecB induces differentiation of AML cells. (a) Light microscopy images of THP-1 cells treated with the indicated concentrations of LecB. After distinct time points, the cells remaining in suspension were removed and the adhered ones were illustrated. Scale bar, 250 μm. (b) The number of cells remaining in suspension upon LecB treatment was determined at indicated time points. Results are expressed as fold change of the cell amount present in the samples relative to the amount at time point 0. (c) Immunofluorescence images of THP-1 cells treated with 0.2 μM LecB for 24 h and subsequently stained with a CD14 specific antibody (green, arrows), Phalloidin-Atto-647 (red) and DAPI (blue). Scale bars, 10 μm. (d) Flow cytometry analysis of THP-1 cells treated with the indicated LecB concentrations for 24 h revealed a significant induction of CD14 expression. Results are expressed as fold change relative to the nontreated control sample. (e) Light microscopy images of THP-1 cells treated with the indicated concentrations of LecB and NBT substrate. After 6 h, the amount of cells bearing the insoluble blue intracellular accumulations were defined as NBT positive and quantified by ImageJ. The values are expressed relative to the total amount of cells. Scale bar, 200 μm. *P<0.05; **P<0.01; ***P<0.001.
Figure 2.
LecB induces apoptosis of AML cells. (a) THP-1 cells were treated with indicated concentrations of LecB for 24 h and the induction of Caspase 3/7 activity was determined. Cycloheximide (100 μg/ml) was used as positive control. Results are expressed as fold change relative to the nontreated control sample. (b) PARP cleavage was detected by western blot analysis upon LecB treatment for 24 h. The quantification illustrates the fold change of the PARP cleavage normalized to β-Actin. Values represent the mean±S.E.M. of at least three independent experiments. *P<0.05; **P<0.01.
LecB induces increased autophagy and a reduction of the β-Catenin level
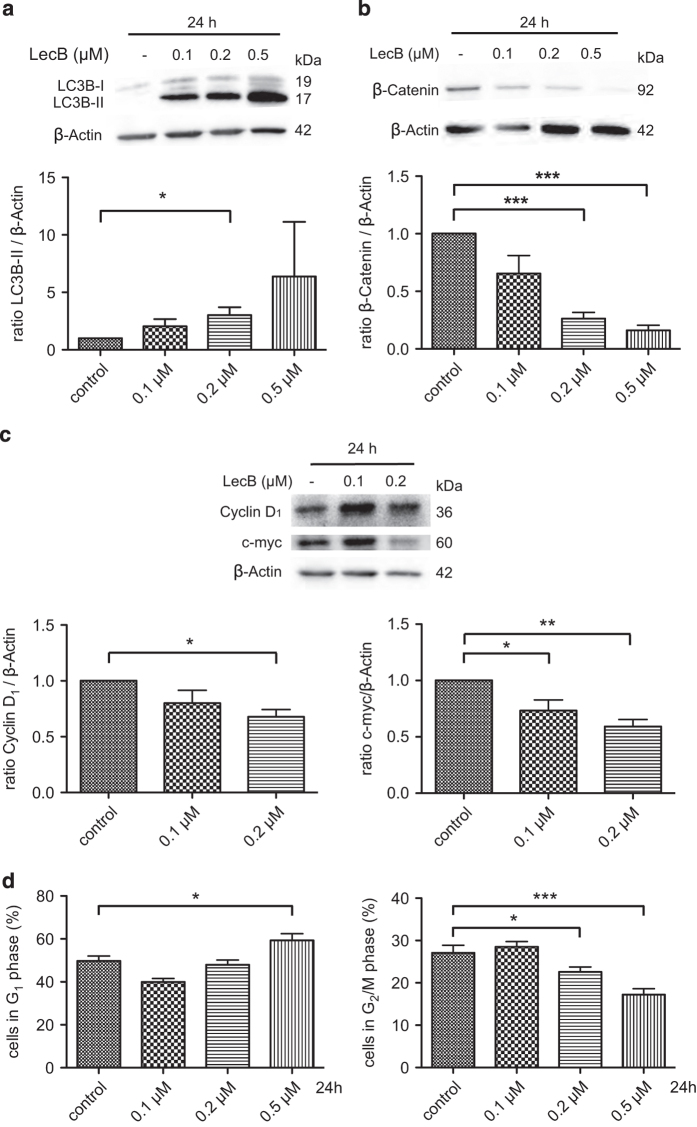
Next, we investigated the mode of action of LecB-mediated differentiation. As the enhanced degradation of redundant proteins is highly important during development, the regular differentiation in the monocyte–macrophage lineage is also accompanied by autophagy.34 In addition, the degradation of the oncogenic fusion proteins by autophagy plays a major role in ATRA-induced differentiation5,13,35 and in the differentiation process triggered by other substances.36–39 During the initiation of autophagy, LC3B gets lipidated and inserts into the autophagosomal membrane (LC3B-II), and for this reason it is considered as a common marker of autophagy. Therefore, we assessed the influence of LecB on autophagy by analyzing the conversion of LC3B-I to LC3B-II in western blot analysis. Indeed, we found a strong increase of LC3B-II in response to rising concentrations of LecB after 24 h (Figure 3a). As a further aspect, we analyzed the role of LecB in β-Catenin signaling that is often dysregulated in AML and contributes to stem cell self-renewal, proliferation and also autophagy.8,12,40–43 Interestingly, LecB treatment led to a striking concentration-dependent reduction of β-Catenin protein levels as depicted in the western blot in Figure 3b. Only 26 and 16% of β-Catenin expression was detected after 24 h using 0.2 and 0.5 μM LecB, respectively. Comparable results were observed after 6 h of LecB treatment as the β-Catenin level decreased and a conversion of LC3-I to LC3-II was determined (Supplementary Figure S2). In order to check whether β-Catenin target genes are affected by LecB treatment, we investigated the expression of Cyclin D1 and c-myc. Western blot experiments after 24 h of LecB treatment (0.2 μM) actually confirmed reduced protein levels of Cyclin D1 and c-myc by 30 and 40%, respectively (Figure 3c). As differentiated cells lose their capacity to divide and our results point toward a reduction of proliferation-related proteins, we performed cell cycle analysis to gain further insights into the status of proliferation upon LecB treatment. The 24-h LecB treatment revealed G1 accumulation of 59% of the cells (0.5 μM) compared with 49% of the cells in the control (Figure 3d, left). In addition, we determined a significant reduction of cells in G2/M phase with 22% (0.2 μM) and 17% (0.5 μM) compared with 27% in the nontreated control (Figure 3d, right). The amount of cells in S phase remained stable (data not shown). In summary, LecB treatment causes an induction of autophagy and a decrease in the level of β-Catenin and its target proteins accompanied by a clear reduction of proliferative-active THP-1 cells.
Figure 3.
LecB induces autophagy and reduction of β-Catenin in AML cells. (a) THP-1 cells were stimulated with the indicated concentrations of LecB for 24 h and the conversion of LC3B-I to the autophagosome marker LC3B-II (arrows) was analyzed in western blot experiments. Representative western blot images are illustrated. The quantification shows the fold increase of the LC3B-II normalized to β-Actin. (b) THP-1 cells were stimulated with LecB for 24 h and the β-Catenin protein level was determined in western blot experiments. Representative western blot images and their quantification are illustrated. The results illustrate the fold change of the β-Catenin level normalized to β-Actin. (c) Cells were stimulated with the indicated concentrations of LecB for 24 h and the expression of β-Catenin target genes Cyclin D1 and c-myc was analyzed in western blot experiments. Representative western blot images are illustrated. The quantification shows the fold change of the protein level normalized to β-Actin. (d) Treatment with LecB for 24 h affected the proliferation of THP-1 cells shown by cell cycle analysis with propidium iodide and measured by flow cytometry. *P<0.05; **P<0.01; ***P<0.001.
Autophagic activity and the β-Catenin level regulate the extent of differentiation
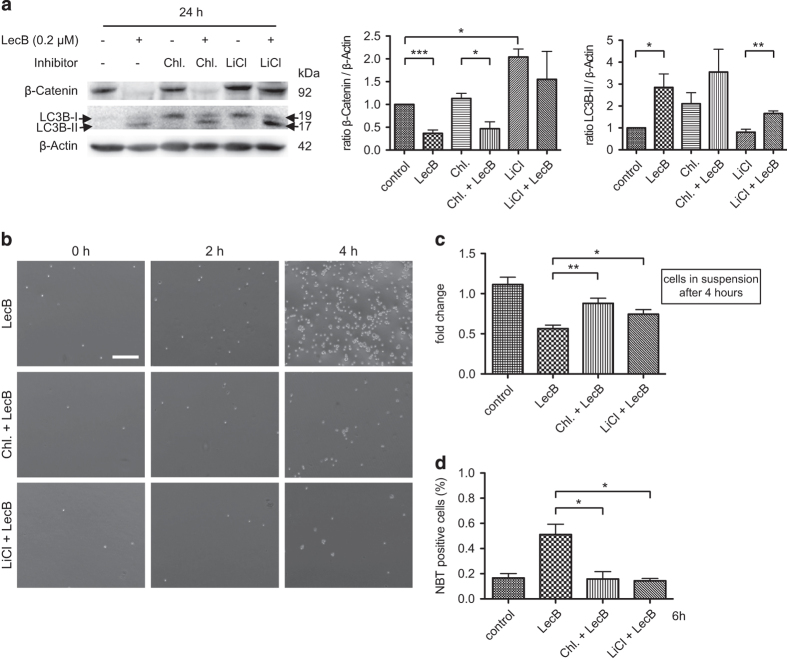
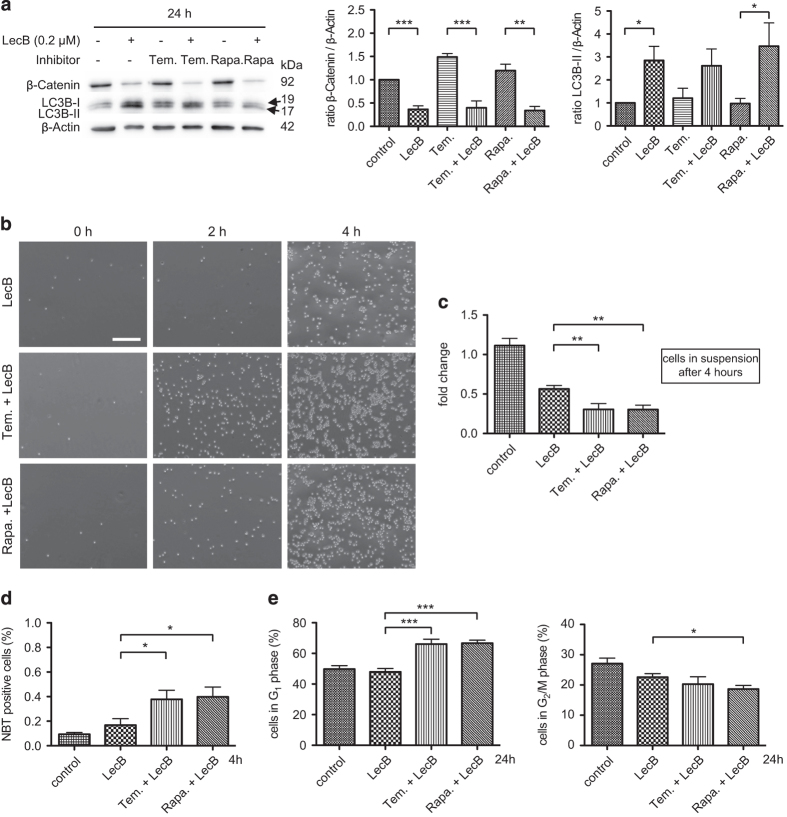
In the following, we aimed at elucidating whether degradation of β-Catenin or autophagic activity is the crucial factor for differentiation. Hence, we performed further experiments with chloroquine (10 μM) that inhibits autophagy by preventing the fusion of autophagosomes and lysosomes and the subsequent degradation of autophagic substrates, and lithium chloride (LiCl, 20 mM) that stabilizes the β-Catenin protein level by inhibiting the GSK-3β kinase activity. We therefore performed western blot analysis after treating the cells with appropriate LecB/inhibitor combinations for 24 h. Using chloroquine alone, the analysis revealed a slight conversion of LC3B-I to LC3B-II and a stable β-Catenin level. In combination with LecB (0.2 μM), the β-Catenin level significantly decreased to a level comparable to the LecB-treated sample and LC3B-II increased. Even if there was a measurable conversion to LC3B-II in cells treated with LecB/chloroquine, autophagy is supposed to be not functional as only the first steps of autophagy take place whereas the fusion with the lysosome is blocked. In contrast, LiCl provided a stable β-Catenin level – even in combination with LecB – and the induction of autophagy was diminished compared with the control samples (Figure 4a). This indicates that a high β-Catenin level correlates with low autophagy and vice versa. To verify the functional influence of the inhibitors, we treated cells with LecB in the presence or absence of the inhibitors and performed attachment assays. Cells treated only with chloroquine or LiCl behaved like nontreated cells and proliferated normally (Supplementary Figure S3). Remarkably, LecB-mediated differentiation was attenuated in the presence of chloroquine and LiCl, so that almost no cells attached to the cell culture plate after 4 h of treatment compared with cells treated only with LecB (Figure 4b). The quantification analysis confirmed that inhibition of functional autophagy (chloroquine) and a highly stable β-Catenin level (LiCl) significantly diminished the differentiation-inducing effect of LecB as 88% (chloroquine) and 74% (LiCl) of the cells were still in solution compared with 56% in only LecB-treated cells after 4 h (Figure 4c). Furthermore, only 10 and 14% of the cells treated with LecB (0.2 μM) in combination with chloroquine or LiCl were NBT positive compared with 51% of only LecB-treated cells after 6 h (Figure 4d). With regard to proliferation, post 24 h of treatment, there was only mild influence of chloroquine and LiCl on LecB-induced outcomes (data not shown). Summarizing, we showed that both a functional autophagy and a low β-Catenin level are crucial for the induction of LecB-mediated differentiation of THP-1 cells.
Figure 4.
Autophagy and the β-Catenin level regulate differentiation. THP-1 cells were treated for 24 h with 0.2 μM LecB±the autophagy inhibitor chloroquine (Chl.: 10 μM) or the GSK-3 inhibitor lithium chloride (LiCl: 20 mM) that stabilized the β-Catenin level. (a) Western blot analysis and quantification. The shown results illustrate the fold increase of LC3B-II (arrows) and the β-Catenin expression normalized to β-Actin. (b) The graph depicts light microscopy images of THP-1 cells treated with LecB (0.2 μM)±inhibitor for the indicated time points. The inhibitors were capable to counteract LecB-induced attachment to a cell culture plate. Scale bar, 250 μM. (c) The number of cells remaining in suspension upon treatment was determined at indicated time points. Results are expressed as fold change of the cell amount present in the samples relative to the amount at time point 0. (d) Quantification of an NBT assay of cells treated with the appropriate combinations for 6 h compared with only LecB-treated cells. Values represent the amount of NBT-positive cells relative to the total amount of cells. The results represent the mean±S.E.M. of at least three independent experiments. *P<0.05; **P<0.01; ***P<0.001.
The LecB-triggered decrease of β-Catenin sensitizes AML cells to mTOR inhibitors
We observed that increased autophagy is essential during differentiation, and thus we investigated the impact of the autophagy-stimulating mTOR inhibitors Temsirolimus (10 μM) and Rapamycin (100 nM). Notably, the treatment with the mTOR inhibitors alone for 24 h did not cause a β-Catenin decrease or generation of LC3B-II (Figure 5a). In addition, the mTOR inhibitors did not influence autophagic flux as we observed no larger changes in the level of the autophagic substrate p62 over time (Supplementary Figure S4A). Contrarily, Rapamycin and Temsirolimus were sufficient to largely reduce phosphorylation of the S6-kinase (S6K), another downstream target of the mTOR kinase and implicated in protein synthesis (Supplementary Figure S4B). Thereby, we proved the functionality of the inhibitors at the concentrations used in our study and a specific block of the mTOR-mediated autophagy induction. This finding once more indicates that a stable β-Catenin level inhibits autophagy, even if stimulators of autophagy are applied. According to this, mTOR inhibitors were not sufficient to induce any differentiating effect in THP-1 cells (Supplementary Figure S3). Remarkably, by using mTOR inhibitors in combination with LecB (0.2 μM) for 24 h, β-Catenin was significantly decreased whereas LC3B-II increased (Figure 5a). Correspondingly, the attachment of cells treated with mTOR inhibitors and LecB was further accelerated compared with only LecB-treated cells (Figure 5b). In particular, only 30% of cells remained in suspension upon treatment with the combination of mTOR inhibitors/LecB for 4 h compared with 56% of the cells treated with LecB only (Figure 5c). The high extent and the speed of differentiation were further approved by NBT reduction as 38% (Temsirolimus/LecB) and 40% (Rapamycin/LecB) of the cells were positive already after 4 h of treatment compared with 17% of cells treated with LecB alone (Figure 5d). Fitting to the remarkable differentiation activity, proliferation was significantly blocked by the mTOR inhibitor/LecB combinations after 24 h. This was shown by a significantly high G1 accumulation of ∼67% of the cells compared with 48% of LecB-treated cells and a reduction of cells in the G2/M phase with 18% (Rapamycin/LecB) and 20% (Temsirolimus/LecB) compared with 22% in the control (Figure 5e). Notably, the reduction of β-Catenin possesses a great potential to sensitize cells to mTOR inhibitors and stimulate autophagy and differentiation.
Figure 5.
The LecB-triggered decrease of β-Catenin sensitizes AML cells to mTOR inhibitors. THP-1 cells were treated with 0.2 μM LecB±mTOR inhibitors Temsirolimus (Tem.: 10 μM) and Rapamycin (Rapa.: 100 nM) for 24 h. (a) Western blot analysis and quantification. The shown results illustrate the fold change of the LC3B and the β-Catenin expression normalized to β-Actin. (b) The graph depicts light microscopy images of THP-1 cells treated with LecB±inhibitor. Treatment of THP-1 cells with the mTOR inhibitors and LecB significantly enhanced cell attachment to the culture plate compared with only LecB-treated cells. Scale bar, 250 μm. (c) The number of cells remaining in suspension upon treatment was determined at indicated time points. Results are expressed as fold change of the cell amount present in the samples relative to the amount at time point 0. (d) Quantification of an NBT assay treated with the appropriate combination for 4 h compared with only LecB-treated cells. Values represent the amount of NBT-positive cells relative to the total amount of cells. (e) Cells were treated with LecB in combination with mTOR inhibitors for 24 h and the cell cycle activity was analyzed with propidium iodide and measured by flow cytometry. Values represent the mean±S.E.M. of at least three independent experiments. *P<0.05; **P<0.01; ***P<0.001.
LecB strengthens the formation of E-Cadherin/β-Catenin complexes
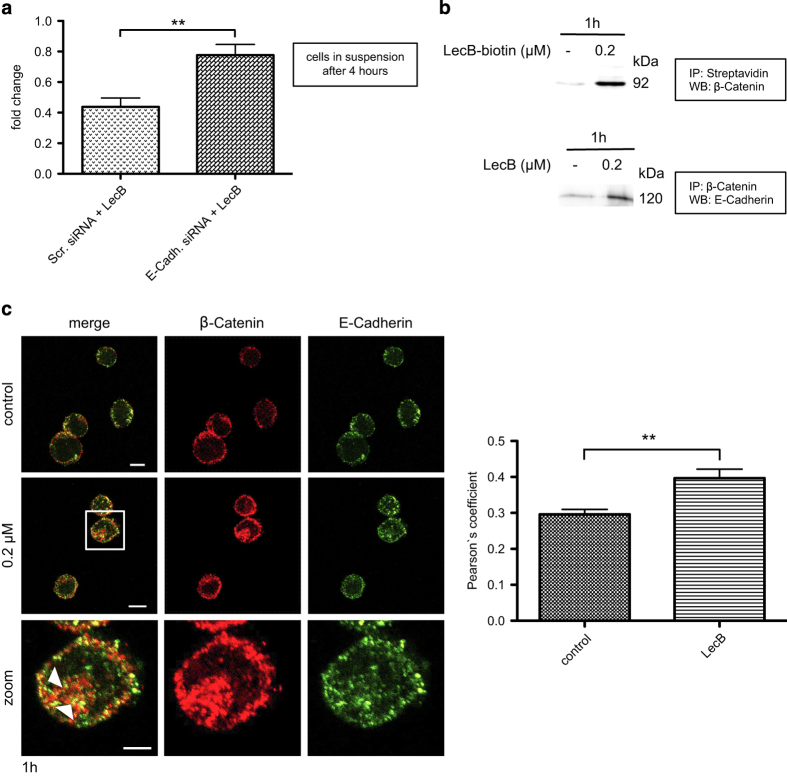
In order to determine how the effect of LecB is mediated at the plasma membrane, we analyzed the binding to potential receptor candidates. As E-Cadherin is an important regulator of β-Catenin signaling in cancer44 and it was reported that it contributes to monocytic differentiation,45 we performed immunofluorescence analysis to check for the LecB/E-Cadherin interaction. We observed that LecB binds to the plasma membrane of THP-1 cells and is in close contact with E-Cadherin 30 min after addition of the lectin (Supplementary Figure S5). Next, we investigated whether E-Cadherin has a functional role during LecB-induced differentiation of THP-1 cells. Here, the E-Cadherin protein level was downmodulated to 65% using a specific E-Cadherin siRNA compared with a scrambled control siRNA (Supplementary Figure S6). Interestingly, a reduction of E-Cadherin expression led to significantly diminished LecB-induced differentiation. After 4 h of LecB treatment, 77% of cells transfected with siRNA against E-Cadherin remained in solution compared with only 44% of cells transfected with a scrambled control siRNA (Figure 6a). In addition, we confirmed the interaction between LecB and β-Catenin and between β-Catenin and E-Cadherin in response to 1 h of LecB treatment by immunoprecipitation (Figure 6b). This was further approved by immunofluorescence images showing pronounced accumulations of E-Cadherin and β-Catenin at the plasma membrane and intracellularly (see arrowheads) compared with nontreated cells (Figure 6c). The analysis of these images revealed a significantly increased colocalization of E-Cadherin and β-Catenin determined by Pearson’s coefficient. These findings suggest that LecB strengthens the formation of E-Cadherin/β-Catenin clusters at the plasma membrane followed by cellular uptake of these complexes, leading to a cell fate different to that of Wnt-induced β-Catenin signaling.
Figure 6.
LecB strengthens the formation of E-Cadherin/β-Catenin complexes. (a) THP-1 cells were first transfected with the indicated siRNAs for 72 h and then treated with 0.2 μM LecB for 4 h. The number of cells remaining in suspension upon the treatment was determined. Results are expressed as fold change of the cell amount present in the samples relative to the amount at time point 0. (b) Western blot analysis of THP-1 cells stimulated with 0.2 μM LecB or biotinylated LecB for 1 h. Immunoprecipitations for streptavidin and β-Catenin were performed and the precipitated protein complexes were stained for β-Catenin and E-Cadherin, respectively. (c) Immunofluorescence images of THP-1 cells treated with 0.2 μM LecB for 1 h and subsequently stained with a β-Catenin- (green) and an E-Cadherin-specific antibody (red). Colocalizations are indicated with arrowheads. Scale bars, 10 μm (overview) and 5 μm (zoom). Colocalization was determined by Pearson’s coefficient, and the values represent the mean±S.E.M. of 10 analyzed cells per condition. **P<0.01.
Discussion
The major feature of leukemia cells is a differentiation arrest accompanied by highly active proliferation. A specific and successful differentiation therapy using ATRA and arsen trioxide was established for a small subset of patients carrying the oncogenic PML-RARα fusion protein apparent in APL.17,46 As the classical chemotherapeutics exhibit high toxicity and side effects and did not undergo striking changes in the past decades, it is highly important to identify further therapeutics addressing the leukemia cell maturation.
In our study, we identified the fucose-binding lectin LecB as a potent differentiation agent in the AML cell line THP-1. LecB treatment caused a very fast and profound differentiation – even at low concentrations – as shown by adherence assay, immunophenotyping and NBT reduction (Figures 1a–e). These effects were specifically mediated by LecB binding to glycosylated host cell receptors as blocking of the LecB-binding sites with L-fucose prevented the induction of differentiation (Supplementary Figure S1). In addition, the deregulated proliferation was disrupted shown by a remarkable decrease of cells in the G2/M phase of the cell cycle and G1 accumulation upon lectin treatment (Figure 3d). As differentiated cells usually exhibit a limited lifespan, we confirmed that the cells underwent apoptosis subsequent to their LecB-induced differentiation (Figure 2).
The mechanism of action of LecB involved an induction of autophagy and a decrease of β-Catenin (Figures 3 and 4). Actually, an aberrant Wnt/β-Catenin signaling, modulated by oncogenic fusion proteins, is frequently implicated in the pathogenesis of leukemia and contributes to stem cell self-renewal, reduced differentiation and apoptosis.8,47,48 We found that the β-Catenin level decreased whereas the conversion of LC3B-I to the lipidated form LC3B-II increased in response to LecB treatment (Figure 3a). The LecB-induced reduction of β-Catenin diminished the expression of proliferation-inducing target genes like Cyclin D1 and c-myc and the extent of proliferation (Figures 3b–d). Interestingly, a functional autophagy was essential for the differentiation of AML cells as well, even if the β-Catenin level was low, as it was seen for samples treated with chloroquine in the presence and absence of LecB. Hence, the inhibition of autophagy attenuated the LecB-mediated effects on differentiation (Figure 4). Accordingly, mTOR inhibitors, which stimulate autophagy, are already in preclinical and clinical studies. However, the clinical benefit of these dugs either alone or in combination with chemotherapeutics has been relatively low and disappointing.49–51 Interestingly, our results indicate that β-Catenin stabilization by LiCl attenuates the conversion to LC3B-II regardless of LecB addition (Figure 4). In addition, the application of the autophagy-stimulating mTOR inhibitors Temsirolimus and Rapamycin to THP-1 cells did not induce any differentiation as long as the β-Catenin level remained stable (Figure 5). This notably indicates that a high β-Catenin level inhibits functional autophagy and compensates the effect of mTOR inhibitors. The inhibitory effect might illustrate an explanation for the resistance of AML patients to mTOR inhibitors.11,49
We found two important effects of the high β-Catenin expression that might mediate the block of differentiation, namely the induction of target protein expression and the inhibition of autophagy. However, the distinct mechanism by which β-Catenin maintains the stem cell-like character of the cancer cells is still controversially discussed and the genetic program is not fully understood. First, it is suggested that β-Catenin is able to block differentiation by modulating differentiation- and proliferation-specific genes.52 Exemplarily, β-Catenin repressed C/EBPα expression in adipogenesis.53 In addition, the regulators of hematopoietic stem cell self-renewal, Hoxb4 and Bmi1, are target genes of the Wnt/β-Catenin signaling.54 Second, it was reported in recent literature that β-Catenin inhibits autophagy in several cancer cells. Overexpression of β-Catenin decreases drug-mediated cytotoxicity and autophagy in breast cancer stem-like cells.43 This is fitting to our findings using mTOR inhibitors as the high expression of β-Catenin comprised the induction of autophagy as well. It is further proposed that the inhibition is mediated via a negative correlation between β-Catenin and Beclin that is part of the autophagy initiation complex.55,56
Finally, we observed a remarkable influence of E-Cadherin during the differentiation of THP-1 cells. E-Cadherin is capable to compete with Wnt signaling by sequestering β-Catenin at the plasma membrane, mediating cell–cell adhesion and antagonizing the Wnt signaling. Interestingly, E-Cadherin expression is often epigenetically silenced by aberrant hypermethylation, leading to enhanced migration in AML, and is correlated with poor prognosis.57 In our study, we confirmed the importance of E-Cadherin for the inhibition of tumor progression as downmodulation of E-Cadherin efficiently attenuated LecB-induced differentiation (Figure 6a). Indeed, we could prove that LecB is in a complex with β-Catenin and increased colocalization of E-Cadherin and β-Catenin (Figures 6b and c). This argues that LecB interacts with and further promotes the formation of E-Cadherin clusters that stabilize β-Catenin firmly in the complex and thereby prevent nuclear translocation and target gene expression as already suggested.58,59
Thus, our study provides for the first time a link between E-Cadherin, β-Catenin, autophagy and differentiation in AML. Moreover, the lectin LecB was a valuable tool to elucidate the underlying molecular mechanisms of the aberrant Wnt/β-Catenin signaling in AML and to gain further insights into possible points of application and new therapeutic approaches.
Materials and methods
Cell culture
THP-1 cells were obtained from the BIOSS Toolbox facility at the University of Freiburg (Freiburg, Germany). Cells were maintained in RPMI-1640 supplemented with 10% FBS, 2 mM L-glutamine and 1% Penicillin Streptomycin (Life Technologies, Gibco, Darmstadt, Germany) at 37 °C and 5% CO2.
Pharmacological inhibitors, siRNA and antibodies
Treatment with inhibitors was performed as follows: we used the autophagy inhibitor chloroquine (10 μM), the mTOR inhibitors Temsirolimus (10 μM) and Rapamycin (100 nM) and the GSK-3 inhibitor LiCl (20 mM). Where indicated, cells were preincubated with the inhibitors for 20 min at 37 °C and inhibitors were maintained in the medium during lectin incubation. All inhibitors were purchased from Sigma-Aldrich (Munich, Germany). Preblocking of LecB with 0.3 M L-fucose (Sigma-Aldrich) was performed for 30 min at room temperature, and afterwards the preblocked LecB was added to the cells. E-Cadherin siRNA and scrambled siRNA were purchased from Santa Cruz Biotechnology (Heidelberg, Germany). Antibodies used in this study were purchased from Cell Signaling Technology (Leiden, The Netherlands) and Santa Cruz Biotechnology.
Differentiation
Cells were treated with the indicated concentrations of LecB and the differentiation was assessed by the attachment to a cell culture plate. Therefore, cells in suspension were removed, attached cells were washed once with PBS and images were acquired using an EVOS microscope (Peqlab Biotechnologie GmbH, Erlangen, Germany). For the quantification, cells remaining in suspension were counted with a hemocytometer and illustrated as fold change compared with the cell amount at the starting time point. The NBT assay is based on the ability of functionally differentiated myelomonocytic cells to induce a respiratory burst. Cells were incubated with indicated concentrations of LecB±inhibitors and NBT for distinct time points. Afterwards, cells containing the blue insoluble dye were counted using ImageJ A 1.45b (NIH, Bethesda, MD, USA). NBT was purchased from Sigma-Aldrich. Differentiation was additionally assessed by immunophenotyping with the monocyte/macrophage marker CD14 in immunofluorescence and FACS experiments. FACS measurements were performed with FACS Gallios (Beckman Coulter, Krefeld, Germany); results were analyzed using FlowJo 8.2 (Treestar Ashland, OR, USA).
Cell cycle analysis
Cells were treated with indicated concentrations of LecB±inhibitors for 24 h, and then cell nuclei were isolated and stained using the CycleTEST PLUS DNA Reagent Kit (BD Biosciences, Heidelberg, Germany) according to the manufacturer’s protocol and FACS measurements were performed.
Apoptosis analysis
In order to detect the apoptotic activity of cells upon LecB treatment, we treated the cells with indicated concentrations of LecB for 24 h and assessed the Caspase 3/7 activities with the Caspase-Glo 3/7 assay (Promega, Mannheim, Germany) according to the manufacturer’s instructions. In addition, we analyzed PARP cleavage that is typically induced by caspases during extensive DNA damage. Cycloheximide (100 μg/ml) was used as positive control.
Transfection
Cells were transfected with specific siRNA using Lipofectamine 2000 (Life Technologies, Invitrogen, Darmstadt, Germany) according to the manufacturer’s protocol prepared in OptiMem medium (Life Technologies, Gibco, Darmstadt, Germany). Cells were incubated at 37 °C, washed after 6 h of transfection and incubated with supplemented medium for another 66 h. Afterwards, lectin treatment was performed.
Western blot analysis
Cells were treated with LecB for indicated time points, washed with PBS and lysed in RIPA buffer supplemented with protease and phosphatase inhibitors. Protein concentration was analyzed using the Pierce BCA protein assay kit (Thermo Scientific, Schwerte, Germany) and measured with Tecan Safire microplate reader (Tecan, Crailsheim, Germany) using Magellan software. Next, 5–20 μg of the protein lysates were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was blocked with 3% BSA for 1 h and incubated with primary and HRP-linked secondary antibodies for 1 h each. Detection was performed by a chemiluminescence reaction using the Fusion-FX7 Advance imaging system (Vilber-Lourmat, Eberhardzell, Germany).
Immunofluorescence and FACS analysis
Cells for immunofluorescence and FACS analysis were incubated±0.2 μM lectin at 37 °C for 24 h and fixed afterwards with 4% PFA for 15 min at room temperature. All following steps were performed at room temperature. For antibody staining, the samples were treated with ammonium chloride solution (50 mM) and saponin/BSA solution for 15 min each. Next, the cells were incubated for 30 min with the primary (1 : 100) and the labeled secondary antibody (1 : 200), respectively, and FACS analysis was performed as described above. Immunofluorescence samples were additionally washed, stained with DAPI (Roth, Karlsruhe, Germany) and Phalloidin-Atto-647 (Sigma-Aldrich) for 10 min and mounted in Mowiol 4-88 (Roth). Fluorescence images were recorded at room temperature using a laser-scanning confocal fluorescence microscope system (Nikon Eclipse Ti-E inverted microscope equipped with a Nikon A1R confocal laser scanning system, 60× oil immersion objective, NA=1,49, laser lines: 405, 488, 561 and 640 nm, Nikon GmbH, Düsseldorf, Germany). Image acquisition and analysis was performed with NIS-Elements (Nikon, version 4.10.04), and for quantification of colocalizations, Pearson’s coefficient was determined.
Statistical analysis
Data of at least three independent experiments are presented as mean±S.E.M. and were compared by unpaired t-test or the one-sample t-test using GraphPad Prism 5.0 (La Jolla, CA, USA). A P-value of ≤0.05 was considered statistically significant (*P<0.05; **P<0.01; ***P<0.001). Values represent the mean±S.E.M. of at least three independent experiments.
Acknowledgments
WR acknowledges the support by the Excellence Initiative of the German Research Foundation (EXC 294), by a grant from the Ministry of Science, Research and the Arts of Baden-Württemberg (Az: 33-7532.20) and by a starting grant of the European Research Council (Programme ‘Ideas’ – call identifier: ERC-2011-StG 282105). SA is grateful for the support by the International Max Planck Research School for Molecular and Cellular Biology (IMPRS-MCB). SZ thanks the China Scholarship Council (CSC) for a PhD fellowship.
Glossary
- AML
acute myeloid leukemia
- APL
acute promyelocytic leukemia
- ATRA
all-trans retinoic acid
- LiCl
lithium chloride
- NBT
nitrobluetetrazolium
- PARP
poly-(ADP-ribose)-polymerase.
The authors declare no conflict of interest.
Footnotes
Supplemental Information accompanies the paper on the Cell Death Discovery website (http://www.nature.com/cddiscovery)
References
- Löwenberg B , Downing J , Burnett A . Acute myeloid leukemia. N Engl J Med 1999; 341: 1051–1062. [DOI] [PubMed] [Google Scholar]
- Vardiman JW , Harris NL , Brunning RD . The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 2002; 100: 2292–2302. [DOI] [PubMed] [Google Scholar]
- Dombret H . Gene mutation and AML pathogenesis. Blood 2011; 118: 5366–5367. [DOI] [PubMed] [Google Scholar]
- Weinberg OK , Seetharam M , Ren L , Seo K , Ma L , Merker JD et al. Clinical characterization of acute myeloid leukemia with myelodysplasia-related changes as defined by the 2008 WHO classification system. Blood 2009; 113: 1906–1908. [DOI] [PubMed] [Google Scholar]
- Orfali N , McKenna SL , Cahill MR , Gudas LJ , Mongan NP . Retinoid receptor signaling and autophagy in acute promyelocytic leukemia. Exp Cell Res 2014; 324: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi F , Annibali O , Cerchiara E , Tirindelli MC , Avvisati G . Cytogenetic abnormalities in adult non-promyelocytic acute myeloid leukemia: a concise review. Crit Rev Oncol Hematol 2011; 80: 331–346. [DOI] [PubMed] [Google Scholar]
- Martelli MP , Sportoletti P , Tiacci E , Martelli MF , Falini B . Mutational landscape of AML with normal cytogenetics: biological and clinical implications. Blood Rev 2013; 27: 13–22. [DOI] [PubMed] [Google Scholar]
- Mikesch J-H , Steffen B , Berdel WE , Serve H , Müller-Tidow C . The emerging role of Wnt signaling in the pathogenesis of acute myeloid leukemia. Leukemia 2007; 21: 1638–1647. [DOI] [PubMed] [Google Scholar]
- Müller-Tidow C , Steffen B , Cauvet T , Ji P , Diederichs S , Sargin B et al. Translocation products in acute myeloid leukemia activate the Wnt signaling pathway in hematopoietic cells. Mol Cell Biol 2004; 24: 2890–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskus W , Sharma S , Saha S , Shah B , Devaraj SGT , Sun B et al. Pre-clinical efficacy of combined therapy with novel β-catenin antagonist BC2059 and histone deacetylase inhibitor against AML cells. Leukemia 2014; 29: 1267–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung J , Esposito MT , Gandillet A , Zeisig BB , Griessinger E , Bonnet D et al. β-Catenin mediates the establishment and drug resistance of MLL leukemic stem cells. Cancer Cell 2010; 18: 606–618. [DOI] [PubMed] [Google Scholar]
- Simon M , Grandage VL , Linch DC , Khwaja A . Constitutive activation of the Wnt/beta-catenin signalling pathway in acute myeloid leukaemia. Oncogene 2005; 24: 2410–2420. [DOI] [PubMed] [Google Scholar]
- Gocek E , Marcinkowska E . Differentiation therapy of acute myeloid leukemia. Cancers (Basel) 2011; 3: 2402–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo-Coco F , Avvisati G , Vignetti M , Thiede C , Orlando SM , Iacobelli S et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 2013; 369: 111–121. [DOI] [PubMed] [Google Scholar]
- Nichol JN , Garnier N , Miller WH . Triple A therapy: the molecular underpinnings of the unique sensitivity of leukemic promyelocytes to anthracyclines, all-trans-retinoic acid and arsenic trioxide. Best Pract Res Clin Haematol 2014; 27: 19–31. [DOI] [PubMed] [Google Scholar]
- Montalban-Bravo G , Garcia-Manero G . Novel drugs for older patients with acute myeloid leukemia. Leukemia 2014; 29: 760–769 1–10. [DOI] [PubMed] [Google Scholar]
- Bruserud O . Induction of differentiation and apoptosis- a possible strategy in the treatment of adult acute myelogenous leukemia. Oncologist 2000; 5: 454–462. [DOI] [PubMed] [Google Scholar]
- Gupta K , Chakrabarti A , Rana S , Ramdeo R , Roth BL , Agarwal ML et al. Securinine, a myeloid differentiation agent with therapeutic potential for AML. PLoS One 2011; 6: e21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N , Solari ML , Becker H , Pantic M , Gärtner F , Maul-Pavicic A et al. Robust in vivo differentiation of t(8;21)-positive acute myeloid leukemia blasts to neutrophilic granulocytes induced by treatment with dasatinib. Leukemia 2010; 24: 1779–1781. [DOI] [PubMed] [Google Scholar]
- Wald DN , Vermaat HM , Zang S , Lavik A , Kang Z , Peleg G et al. Identification of 6-benzylthioinosine as a myeloid leukemia differentiation-inducing compound. Cancer Res 2008; 68: 4369–4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo S-K , Noh E-K , Yoon D-J , Jo J-C , Koh S , Baek JH et al. Rosmarinic acid potentiates ATRA-induced macrophage differentiation in acute promyelocytic leukemia NB4 cells. Eur J Pharmacol 2015; 747: 36–44. [DOI] [PubMed] [Google Scholar]
- Gilboa-Garber N , Zinger-Yosovich KD , Sudakevitz D , Lerrer B , Imberty A , Wimmerova M et al. The five bacterial lectins (PA-IL, PA-IIL, RSL, RS-IIL and CV-II): interactions with diverse animal cells and glycoproteins. In: The Molecular Immunology of Complex Carbohydrates-3, WU AM (ed). Springer: New York, NY, USA, 2011, pp 155–211. [DOI] [PubMed] [Google Scholar]
- Rutishauser U , Sachs L . Cell-to-cell binding induced by different lectins. J Cell Biol 1975; 65: 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eierhoff T , Bastian B , Thuenauer R , Madl J , Audfray A , Aigal S et al. A lipid zipper triggers bacterial invasion. Proc Natl Acad Sci USA 2014; 111: 12895–12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes L , Römer W . Shiga toxins–from cell biology to biomedical applications. Nat Rev Microbiol 2010; 8: 105–116. [DOI] [PubMed] [Google Scholar]
- Eierhoff T , Stechmann B , Römer W , Ceresa B . Pathogen and toxin entry – how pathogens and toxins induce and harness endocytotic mechanisms. In: Molecular Regulation of Endocytosis, Brian Ceresa (ed), InTech: Rijeka, Croatia, 2012, pp 249–276. [Google Scholar]
- Häuselmann I , Borsig L . Altered tumor-cell glycosylation promotes metastasis. Front Oncol 2014; 4: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis JW , Granovsky M , Warren CE . Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta 1999; 1473: 21–34. [DOI] [PubMed] [Google Scholar]
- Macdougall SL , Schwarting GA , Parkinson D , Sullivan A . Increased fucosylation of glycolipids in a human leukaemia cell line (K562-Clone I) with decreased sensitivity to NK-mediated lysis. Immunology 1987; 62: 75–80. [PMC free article] [PubMed] [Google Scholar]
- Moriwaki K , Miyoshi E . Fucosylation and gastrointestinal cancer. World J Hepatol 2010; 2: 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovici J , Avichezer D , Gilboa-Garber N . Effect of lectins on tumorigenicity of AKR lymphoma cells of varying malignancy. Anticancer Res 1986; 6: 1411–1416. [PubMed] [Google Scholar]
- Avichezer D , Gilboa-Garber N . Antitumoral effects of Pseudomonas aeruginosa lectins on Lewis lung carcinoma cells cultured in vitro without and with murine splenocytes. Toxicon 1991; 29: 1305–1313. [DOI] [PubMed] [Google Scholar]
- Chaitanya GV , Steven AJ , Babu PP . PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun Signal 2010; 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y , Morgan MJ , Chen K , Choksi S , Liu Z . Induction of autophagy is essential for monocyte-macrophage differentiation. Blood 2012; 119: 2895–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchwicka A , Cebrat M , Sampath P , Snieżewski L , Marcinkowska E . Perspectives of differentiation therapies of acute myeloid leukemia: the search for the molecular basis of patients’ variable responses to 1,25-dihydroxyvitamin D and vitamin D analogs. Front Oncol 2014; 4: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie N , Zhong L , Liu L , Fang Y , Qi X , Cao J et al. Autophagy contributes to dasatinib-induced myeloid differentiation of human acute myeloid leukemia cells. Biochem Pharmacol 2014; 89: 74–85. [DOI] [PubMed] [Google Scholar]
- Huang H-L , Chen Y-C , Huang Y-C , Yang K-C , Pan HY , Shih S-P et al. Lapatinib induces autophagy, apoptosis and megakaryocytic differentiation in chronic myelogenous leukemia K562 cells. PLoS One 2011; 6: e29014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goussetis DJ , Altman JK , Glaser H , McNeer JL , Tallman MS , Platanias LC . Autophagy is a critical mechanism for the induction of the antileukemic effects of arsenic trioxide. J Biol Chem 2010; 285: 29989–29997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y , Zou Z , Becker N , Anderson M , Sumpter R , Xiao G et al. EGFR-mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell 2013; 154: 1269–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X , Beissert T , Kukoc-zivojnov N , Puccetti E , Altschmied J , Strolz C et al. γ-Catenin contributes to leukemogenesis induced by AML-associated translocation products by increasing the self-renewal of very primitive progenitor cells. Blood 2015; 103: 3535–3544. [DOI] [PubMed] [Google Scholar]
- Jia Z , Wang J , Wang W , Tian Y , XiangWei W , Chen P et al. Autophagy eliminates cytoplasmic β-catenin and NICD to promote the cardiac differentiation of P19CL6 cells. Cell Signal 2014; 26: 2299–2305. [DOI] [PubMed] [Google Scholar]
- Petherick KJ , Williams AC , Lane JD , Ordóñez-Morán P , Huelsken J , Collard TJ et al. Autolysosomal β-catenin degradation regulates Wnt-autophagy-p62 crosstalk. EMBO J 2013; 32: 1903–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y , Chang H , Peng X , Bai Q , Yi L , Zhou Y et al. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/β-catenin signaling pathway. PLoS One 2014; 9: e102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuberger J , Birchmeier W . Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol 2010; 2: a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayumi N , Watanabe E , Norose Y , Watari E , Kawana S , Geijtenbeek TBH et al. E-cadherin interactions are required for Langerhans cell differentiation. Eur J Immunol 2013; 43: 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ablain J , De The H . Revisiting the differentiation paradigm in acute promyelocytic leukemia. Blood 2011; 117: 5795–5802. [DOI] [PubMed] [Google Scholar]
- Scheller M , Huelsken J , Rosenbauer F , Taketo MM , Birchmeier W , Tenen DG et al. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol 2006; 7: 1037–1047. [DOI] [PubMed] [Google Scholar]
- Kirstetter P , Anderson K , Porse BT , Jacobsen SEW , Nerlov C . Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol 2006; 7: 1048–1056. [DOI] [PubMed] [Google Scholar]
- Chapuis N , Tamburini J , Green a S , Willems L , Bardet V , Park S et al. Perspectives on inhibiting mTOR as a future treatment strategy for hematological malignancies. Leukemia 2010; 24: 1686–1699. [DOI] [PubMed] [Google Scholar]
- Chiarini F , Lonetti A , Teti G , Orsini E , Bressanin D , Cappellini A et al. A combination of temsirolimus, an allosteric mTOR inhibitor, with clofarabine as a new therapeutic option for patients with acute myeloid leukemia. Oncotarget 2012; 3: 1615–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen ML , Simonsen A . Autophagy: friend or foe in the treatment of fusion protein-associated leukemias? Autophagy 2013; 9: 1–3. [DOI] [PubMed] [Google Scholar]
- Clevers H , Nusse R . Wnt/β-catenin signaling and disease. Cell 2012; 149: 1192–1205. [DOI] [PubMed] [Google Scholar]
- Kennell J , MacDougald O . Wnt signaling inhibits adipogenesis through β-catenin-dependent and -independent mechanisms. J Biol Chem 2005; 280: 24004–24010. [DOI] [PubMed] [Google Scholar]
- Staal FJT , Luis TC , Tiemessen MM . WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol 2008; 8: 581–593. [DOI] [PubMed] [Google Scholar]
- Nguyen TMB , Subramanian IV , Xiao X , Ghosh G , Nguyen P , Kelekar A et al. Endostatin induces autophagy in endothelial cells by modulating Beclin 1 and β-catenin levels. J Cell Mol Med 2009; 13: 3687–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R , Feng J , Dong S , Pan R , Zhuang H , Ding Z . Regulation of autophagy of prostate cancer cells by β-Catenin signaling. Cell Physiol Biochem 2015; 35: 926–932. [DOI] [PubMed] [Google Scholar]
- Shimamoto T , Ohyashiki JH , Ohyashiki K . Methylation of p15INK4b and E-cadherin genes is independently correlated with poor prognosis in acute myeloid leukemia. Leuk Res 2005; 29: 653–659. [DOI] [PubMed] [Google Scholar]
- Fagotto F . Looking beyond the Wnt pathway for the deep nature of β-catenin. EMBO Rep 2013; 14: 422–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W , Nusse R . Convergence of Wnt, β-catenin, and cadherin pathways. Science 2004; 303: 1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.