Abstract
Human peripheral blood lymphocytes (HPBLs) are one of the most sensitive cells to ionizing radiation (IR) in the human body, and IR-induced DNA damage and functional impairment of HPBLs are the adverse consequences of IR accidents and major side effects of radiotherapy. Phosphorylated H2AX (γH2AX) is a sensitive marker for DNA double-strand breaks, but the role and regulation of the pan-nuclear γH2AX response in HPBLs after IR remain unclear. We herein demonstrated that the pan-nuclear γH2AX signals were increased in a time- and dose-dependent manner, colocalized with >94% of TUNEL apoptotic staining, and displayed a typical apoptotic pattern in resting HPBLs after low LET X-ray IR. In addition, the X-irradiation-induced pan-nuclear p-ATM and p-DNA-PKcs responses also occurred in resting HPBLs, and were colocalized with 92–95% of TUNEL staining and 97–98% of the pan-nuclear γH2AX signals, respectively, with a maximum at 6 h post irradiation, but disappeared at 24 h post irradiation. Moreover, ATM/DNA-PKcs inhibitor KU55933, p53 inhibitor PFT-μ and pan-caspase inhibitor ZVAD-fmk significantly decreased X-irradiation-induced pan-nuclear γH2AX signals and TUNEL staining, protected HPBLs from apoptosis, but decreased the proliferative response to mitogen in X-irradiated HPBLs. Notably, whereas both KU55933 and PFT-μ increased the IR-induced chromosome breaks and mis-repair events through inhibiting the formation of p-ATM, p-DNA-PKcs and γH2AX foci in X-irradiated HPBLs, the ZVAD-fmk did not increase the IR-induced chromosomal instability. Taken together, our data indicate that pan-nuclear γH2AX response represents an apoptotic signal that is triggered by the transient pan-nuclear ATM and DNA-PKcs activation, and mediated by p53 and pan-caspases in X-irradiated HPBLs, and that caspase inhibitors are better than ATM/DNA-PKcs inhibitors and p53 inhibitors to block pan-nuclear γH2AX response/apoptosis and protect HPBLs from IR.
Introduction
In coping with ionizing radiation (IR)-induced double strand breaks (DSBs), mammalian cells promptly initiate the DNA damage response (DDR) to ensure efficient and accurate repair of damaged DNA and cell survival.1 It is commonly believed that a key step in the response to DSBs is histone variant H2AX phosphorylation at serine 139 (S139) in the megabase chromatin region flanking the break to provide a docking site for DNA repair factors, which form visible γH2AX immunofluorescent foci in mammals.2,3 Ataxia telangiectasia mutated (ATM) and DNA-dependent protein kinase catalytic subunit (DNA-PKcs) are two primary kinases that phosphorylate H2AX at S139 in response to IR-induced DSBs.4–6 Moreover, ATM also phosphorylates and activates the tumor suppressor p53 to induce cell cycle arrest, DNA repair or apoptosis by transactivating downstream target genes,7,8 and DNA-PKcs is dispensable for p53 activation.9–13 In addition to its role in transcription regulation, p53 also functions in a transcriptionally independent manner in DNA repair14 and apoptosis.15,16 The activation of the caspase family of cysteine proteases has been shown as an extremely important step in the execution of p53-mediated transcription-dependent and -independent apoptosis.17
Apart from IR-induced formation of γH2AX foci, the induction of pan-nuclear γH2AX signals has been demonstrated as a cellular reaction to IR and as a consequence of various regulation mechanisms.18–20 Cook et al.18 have reported that H2AX phosphorylation at tyrosine 142 (Y142) induces an apoptotic response instead of a damage repair response to genotoxic stress by preventing the binding of the damage repair factors to phospho-serine 139 of γH2AX and promoting the effective recruitment of pro-apoptotic factors to H2AX. Moreover, H2AX phosphorylation at Y142 produces visible whole-nuclear γH2AX immunofluorescence staining instead of foci in TUNEL-positive mouse embryonic fibroblasts exposed to 100 Gy of irradiation.18 However, the relationship between pan-nuclear γH2AX response and radiation quality remains unclear. Markova et al.19 observed the γ-irradiation-induced pan-nuclear γH2AX response in HPBLs, but it was unknown whether the pan-nuclear γH2AX response was related to apoptosis. In addition, it was demonstrated that localized heavy ion irradiation-induced clustered DNA damage in sub-nuclear regions induced the transient nuclear-wide H2AX phosphorylation in undamaged chromatin in mammalian cells, including human fibroblasts, the U2-OS tumor cells, mouse embryonic fibroblast cells and hamster ovary cells; however, none of the neighboring un-irradiated cells showed a nuclear-wide γH2AX response, and targeted cytoplasmic irradiation with particles also did not induce the nuclear-wide γH2AX response.20 It has been proposed that the particle-induced pan-nuclear γH2AX response is caused by ATM and DNA-PK activity distant to sites of DNA damage and is not related to either apoptosis or DSB repair.20 The nuclear-wide kinase activity of ATM and DNA-PK may be elicited by dense IR-induced small DNA fragments21 or by diffusion of active kinases from DNA DSBs. In addition, pan-nuclear γH2AX signals were induced by other stressors via various regulation mechanisms. It has been reported that tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), staurosporine, etoposide and ultraviolet irradiation induce the pan-nuclear γH2AX response associated with apoptosis in human cancer cells,22 mouse fibroblasts, Chinese hamster ovary cells, human fibroblasts23–25 and resting human T cells.26 The pan-nuclear γH2AX response can be caused by replication-mediated DSBs in cancer cells treated with camptothecin,27 indenoisoquinolines28 or gemcitabine,29 which are not related to apoptotic DNA fragmentation. Moreover, small DNA molecules, which mimic DNA DSBs (called D bait) and disorganize the DNA repair system in cancer cells,30,31 and adeno-associated virus replication in infected host cells, which could be explained as a reaction to the single-stranded DNA part of the virus that compromises DNA repair, also can induce the pan-nuclear γH2AX response.32,33 The changes in chromatin structure after hypotonic treatment are sufficient to elicit extensive pan-nuclear γH2AX formation in the absence of DNA strand breaks, which are not related to apoptosis.34
Human peripheral blood lymphocytes (HPBLs) are extremely sensitive to IR and represent the most common target cells for biological dosimetric,35 radiosensitive36 and immune function studies.37,38 γH2AX foci have been shown to be the most sensitive dosimeter and radiosensitive indicator.39 HPBLs are non-dividing cells in the G0 phase of the cell cycle, and can proliferate to generate the immune response if stimulated. Therefore, HPBLs are able to efficiently repair DSBs to prevent apoptosis and mutations. Low LET γ-ray irradiation induces the activation of DDR proteins through phosphorylation of ATM at Ser 1981, DNA-PKcs at tyrosine 2609 and H2AX at Ser 139 to form DNA repair foci in HPBLs.19,40,41 If the DNA damage is not repaired after IR, HPBLs undergo p53-dependent apoptosis through the caspase cascade,42–47 and subsequently, functional inhibition of HPBLs may result in immune suppression in individuals exposed to acute or chronic IR at high doses and in radio/chemotherapy patients.48,49 Therefore, it is of interest to understand the relationships among pan-nuclear γH2AX signals, apoptosis, DSB repair, chromosomal aberration and proliferative function in resting HPBLs exposed to low LET X-irradiation, and to explore the pharmacological approaches to protect HPBLs against IR-induced pan-nuclear γH2AX response/apoptosis. In this study, we demonstrated that X-ray-induced pan-nuclear γH2AX response represents an apoptotic signal in resting HPBLs, and is mediated by transient pan-nuclear ATM and DNA-PKcs activation and dependent on p53 and caspase activation. Our studies suggest that caspase inhibitors are better than ATM/DNA-PKcs inhibitors and p53 inhibitors to block pan-nuclear γH2AX response/apoptosis and protect HPBLs from IR.
Results
The kinetic patterns of the pan-nuclear responses are different from that of the DDR foci in resting HPBLs after X-irradiation
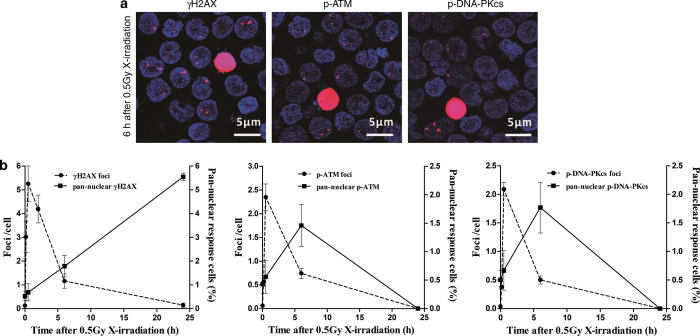
As shown in Figure 1a, we detected the nuclear-wide γH2AX, p-ATM and p-DNA-PKcs responses and the γH2AX, p-ATM and p-DNA-PKcs foci in resting HPBLs after X-irradiation. Importantly, the kinetic patterns of the pan-nuclear responses are different from that of the DDR foci (Figure 1b). For the γH2AX signals, although the percentages of pan-nuclear γH2AX-positive cells increased steadily after X-irradiation, the frequencies of the γH2AX foci peaked at 30 min after X-irradiation, decreased rapidly during 6 h post irradiation and was reduced to approximately 8% of the peak value at 24 h post irradiation (Figure 1b, left panel). For the p-ATM and p-DNA-PKcs signals, the kinetic patterns of the p-ATM and p-DNA-PKcs foci were similar to that of the γH2AX foci, but the percentages of pan-nuclear p-ATM- and p-DNA-PKcs-positive cells peaked at 6 h post irradiation and decreased to zero at 24 h post irradiation (Figure 1b, middle and right panel). These data suggest that the nuclear-wide γH2AX, p-ATM and p-DNA-PKcs responses are not related to DSB repair in resting HPBLs after X-irradiation.
Figure 1.
Distinct kinetic patterns between the pan-nuclear responses and the repair foci of γH2AX, p-ATM and p-DNA-PKcs. (a) Representative images of X-irradiation-induced pan-nuclear signals and foci of γH2AX, p-ATM and p-DNA-PKcs in resting HPBLs at 6 h after 0.5 Gy X-ray exposure. ×60 magnification, ×1.0 zoom. (b) HPBLs were irradiated with 0.5 Gy X-ray, and the pan-nuclear signals and foci of γH2AX (left panel), p-ATM (middle panel) and p-DNA-PKcs (right panel) were examined at the indicated time points after irradiation and quantified by counting 1000–5000 HPBLs from each sample. γH2AX, p-ATM and p-DNA-PKcs were labeled in red, and the nuclei were stained in blue with DAPI. Data are presented as the means±S.D. of four to six donors.
X-irradiation-induced pan-nuclear γH2AX, p-ATM and p-DNA-PKcs signals are colocalized with TUNEL apoptotic staining in resting HPBLs
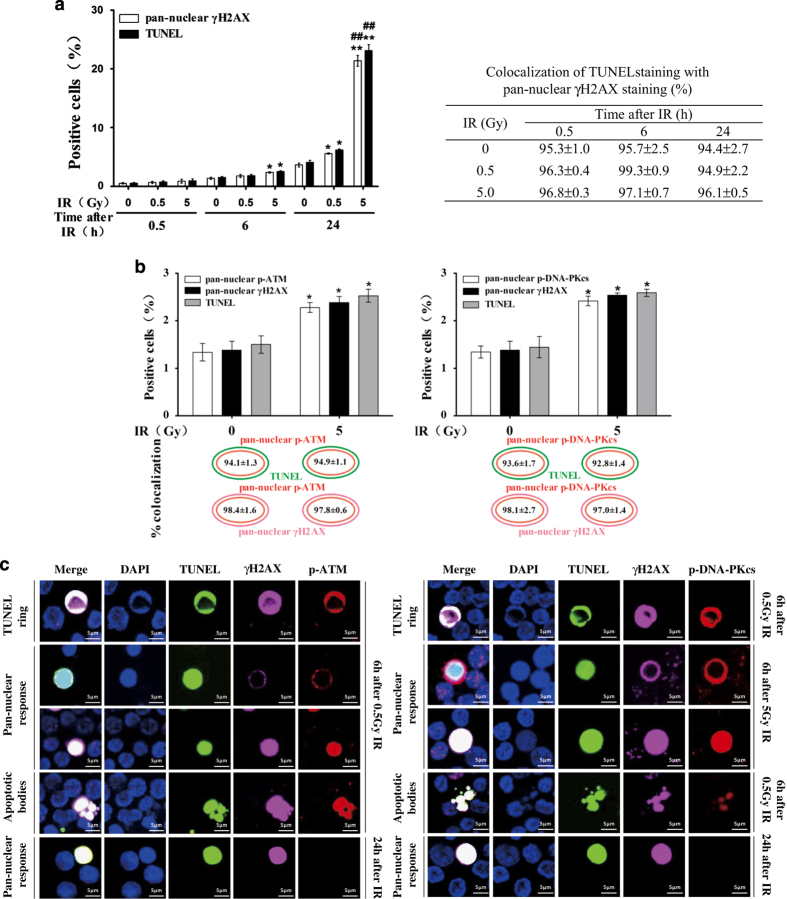
It was reported that pan-nuclear formation of γH2AX in undamaged chromatin induced by high-LET localized ion irradiation was not related to apoptosis in human fibroblasts, tumor cells and hamster ovary cells.20 However, we found that the kinetics of the increase of the pan-nuclear γH2AX response was similar to that of apoptotic induction at 0.5, 6 and 24 h post irradiation (0.5 or 5.0 Gy) in HPBLs. Moreover, the percentage of TUNEL-positive cells was similar to the percentage of pan-nuclear γH2AX-positive cells at each time point after the same dose radiation (Figure 2a, left panel). More importantly, 94–99% of the TUNEL-positive cells also displayed pan-nuclear γH2AX staining in a dose- and time-independent manner (Figure 2a, right panel; Figure 2c), indicating that pan-nuclear γH2AX staining was associated with X-irradiation-induced apoptosis in resting HPBLs.
Figure 2.
Relationship between pan-nuclear γH2AX, p-ATM and p-DNA-PKcs responses and apoptosis in resting HPBLs exposed to X-ray irradiation. (a) HPBLs were irradiated with 0.5 and 5 Gy X-ray. The pan-nuclear γH2AX, TUNEL-positive cells (left panel) and their colocalization (right panel) were examined at the indicated time points after irradiation. (b) HPBLs were irradiated with 5 Gy X-ray. The pan-nuclear p-ATM with the pan-nuclear γH2AX and TUNEL-positive cells, pan-nuclear p-DNA-PKcs with the pan-nuclear γH2AX and TUNEL-positive cells and their colocalization were examined at 6h after irradiation. (c) Representative images of X-irradiation-induced pan-nuclear γH2AX, p-ATM, p-DNA-PKcs and TUNEL staining and their colocalization in resting HPBLs at the indicated time points after irradiation. γH2AX was labeled in infrared, p-ATM and p-DNA-PKcs in red and TUNEL in green, and nuclei were stained blue with DAPI. A total of 5000 HPBLs from each sample were examined to calculate the percentage of pan-nuclear γH2AX, p-ATM, p-DNA-PKcs and TUNEL-positive cells. ×60 magnification, ×1.0 zoom. Data shown in panel (a) and panel (b) are presented as the means±S.D. of six donors. *P<0.05 and ** P<0.01 compared with corresponding non-irradiated HPBLs at 6 or 24 h post irradiation; ## P<0.01 compared with corresponding HPBLs exposed to 0.5 Gy X-ray at 24 h post irradiation.
Furthermore, pan-nuclear p-ATM and p-DNA-PKcs signals were also colocalized with pan-nuclear γH2AX or TUNEL staining at 6 h post irradiation in resting HPBLs. It was found that 93–95% of the TUNEL-positive cells also exhibited pan-nuclear p-ATM or p-DNA-PKcs staining at 6 h post irradiation (5 Gy) (Figures 2b and c). We further found that 97–98% of cells with pan-nuclear γH2AX staining showed pan-nuclear p-ATM and p-DNA-PKcs staining (Figures 2b and c). Notably, HPBLs with pan-nuclear γH2AX staining did not exhibit pan-nuclear p-ATM and p-DNA-PKcs staining at 24 h post irradiation (Figure 2c), suggesting that ATM and DNA-PKcs were only activated at early stages after irradiation.
According to the morphological signs of apoptosis progression such as apoptotic rings, pan-nuclear staining and apoptotic bodies, we observed three types of morphological characterization of the apoptotic γH2AX response: γH2AX ring versus TUNEL ring and very weak TUNEL pan-staining of the nucleus; pan-nuclear γH2AX versus strong TUNEL pan-staining of the nucleus; and the γH2AX pan-staining within apoptotic bodies. Notably, no DDR foci were observed in the pan-nuclear responses.
X-irradiation-induced pan-nuclear γH2AX responses and apoptosis in resting HPBLs are mediated by ATM and DNA-PKcs
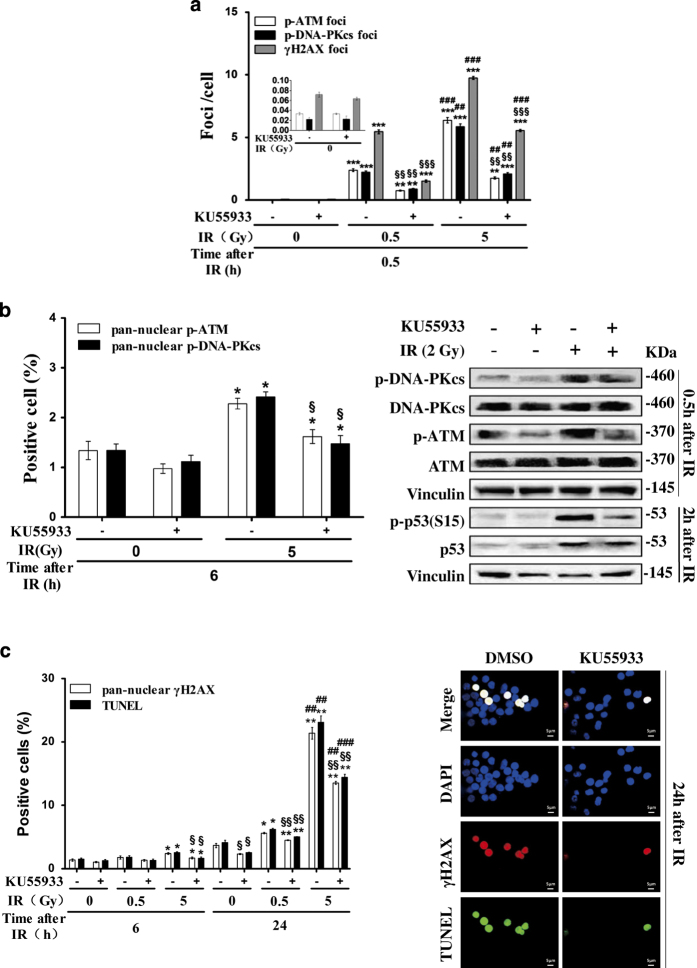
We next investigated the role of ATM and DNA-PKcs in nuclear-wide H2AX phosphorylation and apoptosis. It was found that pretreatment with KU55933, a potent inhibitor of ATM/DNA-PKcs, resulted in significant inhibition of X-irradiation-induced γH2AX, p-ATM and p-DNA-PKcs focus formation at 30 min post irradiation (0.5 or 5 Gy) in resting HPBLs (Figure 3a), suggesting that KU55933 inhibited X-irradiation-induced DSB repair. In addition, KU55933 significantly inhibited the pan-nuclear p-ATM and p-DNA-PKcs signals at 6 h post irradiation (5 Gy) (Figure 3b, left panel) in HPBLs. The inhibitory effects of KU55933 on p-ATM and p-DNA-PKcs expression were also demonstrated by western blotting analysis (Figure 3b, right panel). We found that KU55933 also inhibited X-irradiation-induced p53 phosphorylation (Figure 3b, right panel). Correspondingly, KU55933 inhibited the pan-nuclear γH2AX signals and TUNEL apoptosis at 6 and 24 h post irradiation (0.5 or 5 Gy) in resting HPBLs (Figure 3c). Together, our results suggest that KU55933 decreases X-irradiation-induced pan-nuclear γH2AX responses and apoptosis by inhibiting transient pan-nuclear p-ATM and p-DNA-PKcs responses and p53 phosphorylation in resting HPBLs.
Figure 3.
Inhibition of X-irradiation-induced pan-nuclear γH2AX response and apoptosis in resting HPBLs by KU55933. (a) HPBLs were treated with 10 μM KU55933 for 2 h before X-irradiation (0.5 or 5 Gy). γH2AX, p-ATM and p-DNA-PKcs foci were measured in 500–1000 HPBLs from each sample. (b) HPBLs were treated with 10 μM KU55933 for 2 h before X-irradiation (5 Gy). Pan-nuclear p-ATM and p-DNA-PKcs cells (left panel) were counted, and western blotting assays (right panel) were performed to examine the expression of p-ATM/ATM, p-DNA-PKcs/DNA-PKcs and p-p53/p53 proteins at the indicated time points after irradiation. (c) HPBLs were treated with 10 μM KU55933 for 2 h before X-irradiation (0.5 or 5 Gy). Pan-nuclear γH2AX and TUNEL-positive cells were measured, and their representative images were captured at 24 h after X-irradiation; γH2AX was labeled in red and TUNEL in green, and nuclei were stained in blue with DAPI. A total of 2000–5000 HPBLs from each sample were examined to determine the percentage of the pan-nuclear γH2AX, p-ATM, p-DNA-PKcs and TUNEL-positive cells. ×100 magnification, ×1.0 zoom. The values shown in panel (a), panel (b) and panel (c) represent the means±S.D. obtained from three to four donors. *P<0.05, **P<0.01 and ***P<0.001 compared with corresponding non-irradiated HPBLs; ## P<0.01 and ### P<0.001 compared with corresponding HPBLs exposed to 0.5 Gy X-ray irradiation with/without inhibitor treatment; § P<0.05, §§ P<0.01 and §§§ P<0.01 compared with corresponding HPBLs without inhibitor treatment.
X-irradiation-induced pan-nuclear γH2AX response and apoptosis in resting HPBLs were also mediated by p53 and pan-caspases
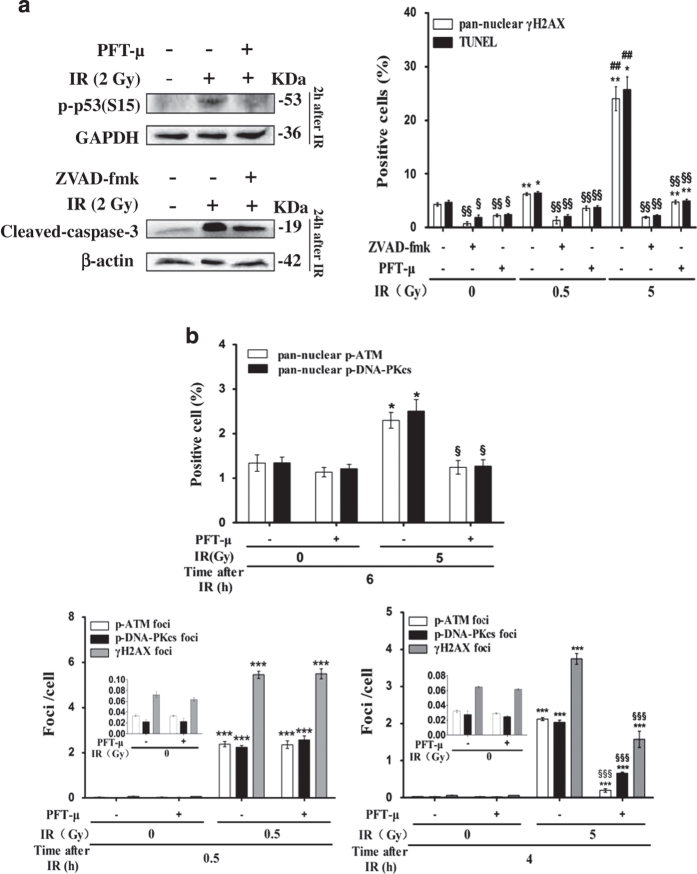
It has been shown that apoptosis of HPBLs is p53- and caspase-dependent.26,43–47 We next investigated the roles of p53 and caspases in nuclear-wide H2AX phosphorylation and apoptosis. It was found that pretreatment with the p53 inhibitor PFT-μ and pan-caspase inhibitor ZVAD-fmk significantly reduced X-irradiation-induced p53 phosphorylation and cleaved-caspase-3 expression in resting HPBLs (Figure 4a, left panel). Similar to the inhibitory effects of KU55933 on X-irradiation-induced pan-nuclear γH2AX signals and TUNEL staining, pretreatment with PFT-μ or ZVAD-fmk also resulted in the significant suppression of X-irradiation-induced pan-nuclear γH2AX signals and apoptosis at 24 h post irradiation (0.5 or 5 Gy) in resting HPBLs (Figure 4a, right panel), suggesting that p53 and caspases are involved in the regulation of the nuclear-wide γH2AX response. Moreover, PFT-μ inhibited X-irradiation-induced pan-nuclear p-ATM and p-DNA-PKcs responses at 6 h post irradiation (5 Gy) and X-irradiation-induced p-ATM and p-DNA-PKcs focus formation at 4 h post irradiation, although it did not decrease X-irradiation-induced p-ATM and p-DNA-PKcs focus formation at 30 min post irradiation (Figure 4b), suggesting that p53 is involved in mediating the pan-nuclear p-ATM and p-DNA-PKcs responses, and has an important role in enhancing DSB repair in HPBLs after X-irradiation.
Figure 4.
Inhibition of X-irradiation-induced pan-nuclear γH2AX response and apoptosis in resting HPBLs by PFT-μ and ZVAD-fmk. (a) HPBLs were treated with 20 μM PFT-μ for 5 h or 100 μM ZVAD-fmk for 1 h before X-irradiation (2 Gy). The levels of p-p53 expression (left upper panel) and cleaved caspase-3 expression (left bottom panel) in HPBLs at indicated time points post irradiation were examined by western blotting. Furthermore, pan-nuclear γH2AX and TUNEL-positive cells were counted at 24 h post irradiation. (b) HPBLs was treated with 20 μM PFT-μ for 5 h before X-irradiation. Pan-nuclear p-ATM- and p-DNA-PKcs-positive cells were counted at 6 h post irradiation (upper panel). γH2AX, p-ATM and p-DNA-PKcs foci were measured in HPBLs at 0.5 h (left bottom panel) and 4 h (right bottom panel) post irradiation. A total of 1000 HPBLs from each sample were examined for quantitation of the p-ATM, p-DNA-PKcs and γH2AX foci, and 2000–5000 HPBLs from each sample were examined to calculate the percentage of pan-nuclear γH2AX, p-ATM, p-DNA-PKcs and TUNEL-positive cells. The values shown in panel (a) and panel (b) represent the means±S.D. obtained from three to four donors. *P<0.05, **P<0.01 and ***P<0.001 compared with corresponding non-irradiated HPBLs with/without inhibitor treatment; ## P<0.01 compared with corresponding HPBLs exposed to 0.5 Gy X-ray irradiation with/without inhibitor treatment; § P<0.05, §§ P<0.01 and §§§ P<0.01 compared with corresponding HPBLs without inhibitor treatment.
Effects of KU55933, PFT-μ and ZVAD-fmk on X-irradiation-induced chromosomal aberrations and proliferative response in resting HPBLs
Although KU55933, PFT-μ and ZVAD-fmk protect HPBLs from X-irradiation-induced apoptosis, it is not clear whether they affect X-irradiation-induced genomic instability and protect HPBLs function. Table 1 shows that X-irradiation significantly induced chromosomal aberrations including breaks, dicentrics plus rings in resting HPBLs and increased the percentage of aberrant cells. KU55933 and PFT-μ, but not ZVAD-fmk, further significantly enhanced X-irradiation-induced chromosomal aberrations.
Table 1. Effects of KU55933, PFT-μ and ZVAD-fmk on frequencies of X-irradiation-induced chromosomal aberrations at 48 h post irradiation in resting HPBLs.
| Treatment |
Chromosomal aberrations/100 cells
|
% Aberrant cells | |
|---|---|---|---|
| Breaks | Dicentrics+rings | ||
| Ctrl | 0.7±0.3 | 0.5±0.5 | 1.2±0.8 |
| KU55933 | 0.7±0.6 | 0 | 0.7±0.6 |
| PFT-μ | 0 | 0 | 0 |
| ZVAD-fmk | 0.3±0.6 | 0 | 0.3±0.6 |
| IR | 13.8±2.5** | 14.1 ±2.2** | 21.3±3.3** |
| KU55933+IR | 24.2±2.3**§§ | 23.7±3.7***§ | 40.7±5.0**§§ |
| PFT-μ+IR | 22.3±2.4***§ | 20.7±2.8**§ | 34.7±2.4**§§ |
| ZVAD-fmk+IR | 17.9±5.8* | 13.5±4.9* | 25.6±4.8** |
Resting HPBLs were pretreated with 10 μM KU55933 for 2 h, 20 μM PFT-μ for 5 h or 100 μM ZVAD-fmk for 1 h followed by irradiation with 2 Gy X-rays, and incubated with PHA and colcemid for 48 h. The metaphase cells were then harvested, and a total of 100 metaphase cells were analyzed. The values represent the means±S.D. of HPBLs from three individual donors. *P<0.05, **P<0.01 and ***P<0.001, compared with non-irradiated control and inhibitor treatment alone; § P<0.05 and §§ P<0.01, compared with IR alone.
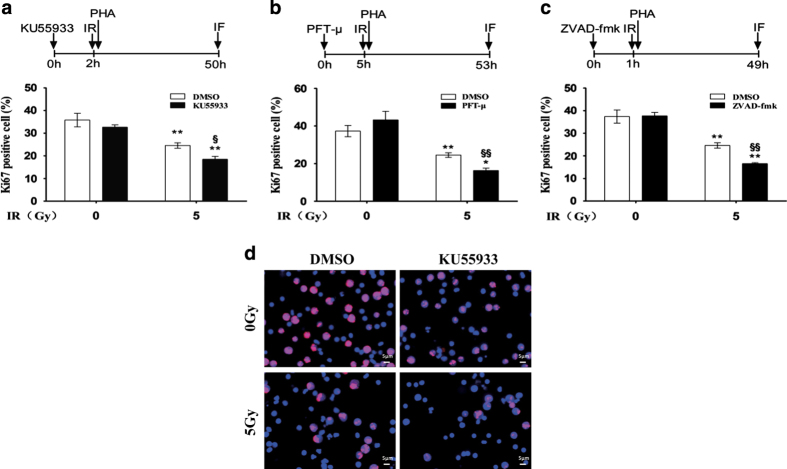
We investigated the impact of KU55933, PFT-μ and ZVAD-fmk on the proliferation of X-irradiated HPBLs. We found that the inhibitor (KU55933, PFT-μ or ZVAD-fmk) combined with X-irradiation resulted in a more significant decrease of the percentage of Ki67-positive cells than the inhibitor or X-irradiation alone in resting HPBLs, suggesting that KU55933, PFT-μ and ZVAD-fmk aggravated the inhibitory effect of X-irradiation on the proliferative response in resting HPBLs (Figures 5a–d).
Figure 5.
KU55933, PFT-μ and ZVAD-fmk inhibited the proliferative response to mitogen in resting HPBLs exposed to X-ray irradiation. HPBLs were treated with 10 μM KU55933 for 2 h (a), 20 μM PFT-μ for 5 h (b) or 100 μM ZVAD-fmk for 1 h (c) followed by irradiation with 5 Gy X-ray, and were cultured with PHA and colcemid for 48 h. HPBLs were then collected and transformed HPBLs were detected using the Ki67 antibody. A total of 1000 HPBLs from each sample were examined to determine the proliferation ratio. Data are presented as the means±S.D. obtained from three donors. *P<0.05 and **P<0.01 compared with corresponding non-irradiated HPBLs with/without inhibitor treatment; § P<0.05 and §§ P<0.01 compared with corresponding HPBLs without inhibitor treatment. (d) Representative images of HPBLs labeled with Ki67. The cells were treated with KU55933 and irradiated with X-ray as described above. Ki67 was labeled in red and nuclei were stained in blue with DAPI. ×40 magnification, ×1.0 zoom.
Discussion
Unlike the kinetics of localized ion-induced transient pan-nuclear γH2AX response with a peak at ~1 h post irradiation, we demonstrated herein that X-irradiation-induced pan-nuclear γH2AX response and apoptosis were increased in a dose- and time-dependent manner in resting HPBLs exposed to X-irradiation. This phenomenon is similar to the kinetics of the anticancer agent etoposide-induced pan-nuclear γH2AX response and apoptosis in resting human T cells.26 More importantly, we provide direct evidence that the X-irradiation-induced pan-nuclear γH2AX response is presented in the apoptotic HPBLs. The apoptotic γH2AX pan-nuclear response is a novel feature of the low LET IR-induced apoptosis in HPBLs, and could serve as a biomarker to distinguish apoptotic cells from the DNA-damaged cells with a focal pattern and to monitor the extent of radiation injury and side effects of radiotherapy.
In contrast, X-irradiation-induced pan-nuclear p-ATM and p-DNA-PKcs responses in resting HPBLs were transient with a maximum response at 6 h post irradiation and complete disappearance at 24 h post irradiation. The transient X-irradiation-induced pan-nuclear p-ATM and p-DNA-PKcs responses are also presented in the apoptotic HPBLs. Moreover, ATM/DNA-PKcs inhibitor KU55933 not only inhibited X-irradiation-induced p-ATM and p-DNA-PKcs foci and the pan-nuclear p-ATM and p-DNA-PKcs responses but also suppressed the pan-nuclear γH2AX response and apoptosis in resting HPBLs. DNA-PK can be strongly activated during apoptotic DNA fragmentation24 and ATM also can be activated by small double-stranded DNA fragments.50 Our results suggest that the pan-nuclear γH2AX response is triggered by transient pan-nuclear p-ATM and p-DNA-PKcs signals in resting HPBLs, and that the pan-nuclear γH2AX response has a key role in X-ray-induced apoptosis in resting HPBLs.
γH2AX was recently shown to be a crucial component in modulating apoptosis.18,51 Lu et al.51 showed that H2AX S139 phosphorylation by ultraviolet A-activated c-Jun N-terminal kinase (JNK) is required for DNA ladder formation mediated by caspase-3/caspase-activated DNase (CAD) in apoptotic cells. Cook et al.18 reported that phosphorylation of tyrosine 142 of H2AX promoted the apoptotic response and inhibited the DDR to DNA damage. When H2AX tyrosine 142 was mutated to alanine, only γH2AX foci, but not pan-nuclear γH2AX and TUNEL staining, occurred after irradiation in H2AX−/− mouse embryonic fibroblast cells transfected with mutant H2AX (Y142F).18 These observations support our results that X-irradiation-induced pan-nuclear γH2AX is involved in X-irradiation-induced apoptosis in resting HPBLs. Our results also suggest that there are no H2AX mutations at S139 and Y142 sites in HPBLs, and that pan-nuclear γH2AX staining can be used to detect the apoptosis of HPBLs exposed to low LET IR. In addition, no γH2AX, p-ATM and p-DNA-PKcs foci were observed in the X-ray-induced pan-nuclear response and TUNEL-positive HPBLs. One possible explanation is that the condensed chromatin in the late stages of apoptosis blocks the expression of DNA repair proteins.19 All together, we proposed that γH2AX regulates apoptosis in a pan-nuclear response pattern and is involved in the DSB repair response in a focal pattern in resting HPBLs.
It has been reported that IR-induced HPBLs apoptosis is mediated by caspase cascade and is p53-dependent,42–47 which raises a question of whether X-irradiation-induced pan-nuclear γH2AX response is also dependent on p53 and caspases. In the present study, we demonstrated that p53 inhibitor PFT-μ and caspase inhibitor ZVAD-fmk inhibited the X-irradiation-induced nuclear-wide γH2AX response and apoptosis. A possible explanation is that the pan-nuclear γH2AX response is involved in the initiation and execution of apoptosis in HPBLs, and that p53 and caspases mediate the pan-nuclear γH2AX response by priming and executing apoptosis, respectively. Coincident with the inhibition of X-irradiation-induced pan-nuclear γH2AX response, we found that p53 inhibitor PFT-μ decreased the X-irradiation-induced pan-nuclear p-ATM and p-DNA-PKcs responses, suggesting that p53 may mediate the pan-nuclear γH2AX response through activating the pan-nuclear p-ATM and p-DNA-PKcs responses. It has been found that wild-type p53 protein is able to rejoin DNA with DSBs to facilitate precise ligation through direct physical interaction of p53 with DNA-PK of NHEJ,52–54 and that p53 phosphorylation by ATM is involved in the initial steps of DNA damage signaling.55 In the present study, we also demonstrated that p53 inhibition by PFT-μ results in a decrease of DSB repair and an increase of chromosome breaks by inhibiting the X-irradiation-induced p-ATM and p-DNA-PKcs focus formation. Similar to ATM/DNA-PKcs inhibitor KU55933, PFT-μ can inhibit the pan-nuclear γH2AX response, the formation of p-ATM and p-DNA-PKcs foci, and pan-nuclear p-ATM and p-DNA-PKcs responses. However, we also found that caspase inhibitor ZVAD-fmk decreased the nuclear-wide γH2AX response and apoptosis but did not affect the X-ray-induced chromosome breaks, suggesting that the nuclear-wide γH2AX response is involved in caspase-mediated apoptotic execution, but caspases are not involved in DSB repair, as caspases are activated at the execution phase of apoptosis when DSB repair process is finished.
It is well established that the IR-induced massive cell death in radiosensitive tissues is not due to irreversible damage of cells but rather to activation of apoptosis, therefore blockage of apoptosis has become a pharmacological approach to radioprotection of normal tissues.56 Similarly, inhibition of DSB repair has been well known as an efficient approach to facilitate the apoptosis and sensitize tumor cells to IR and DSB-inducing chemotherapeutic agents such as etoposide, doxorubicin and camptothecin.57 Surprisingly, we found that ATM/DNA-PKcs inhibitor KU55933 protected resting HPBLs from the X-ray-induced pan-nuclear γH2AX response and apoptosis, although it inhibited the X-ray-induced DSBs repair and enhanced chromosomal breaks. Our results are consistent with the literature reports that inhibition of ATM and DNA-PKcs phosphorylation by KU55933 can result in the suppression of H2AX phosphorylation and etoposide-induced apoptosis in resting HPBLs.26,58 It is believed that HPBLs in a resting status tolerate a limited un-repair and inaccurate repair, and that there are opposite actions of KU55933 on normal resting cells and proliferating cancer cells. Owing to dual functions of p53 in mediating the DSB repair and apoptosis in resting HPBLs, p53 inhibitor PFT-μ, like ATM/DNA-PKcs inhibitor KU55933, protected resting HPBLs from the X-ray-induced pan-nuclear γH2AX response and apoptosis, and concurrently induced a decrease of DSB repair and an increase of chromosome breaks and frequencies of chromosomal aberrations. Our observations are consistent with the findings that PFT-α decreases the repair efficiency of X-ray-induced DNA lesions, leading to increased frequencies of chromosomal aberrations and reduced apoptosis in HPBLs.46 Unlike the dual role of KU55933 and PFT-μ, caspase inhibitor ZVAD-fmk protected HPBLs from X-ray-induced nuclear-wide γH2AX response and apoptosis, and did not worsen the X-ray-induced chromosomal instability. More importantly, we found that the inhibition of X-ray-induced nuclear-wide γH2AX response and apoptosis by KU55933, PFT-μ and ZVAD-fmk aggravated the inhibitory effects of X-irradiation on the proliferative response of HPBLs to mitogens, suggesting that the inhibition of the nuclear-wide γH2AX response and apoptosis does not protect the function of HPBLs. It is possible that the inhibition of DSB repair by KU55933 and PFT-μ does not result in correction of chromosome aberrations in HPBLs, and that the increased genomic instability leads to further suppression of the proliferative response of HPBLs exposed to X-irradiation, which limits the pharmacological use of DSB repair inhibitors and p53 inhibitors in radioprotection of normal tissues. Nevertheless, the aggravated effect of ZVAD-fmk on IR-induced proliferative inhibition may be related to suppression of the expression of lymphocyte-stimulated factor receptors, such as IL-2.59 Lawrence et al.59 found that ZVAD-fmk inhibits the proliferative response of human T lymphocytes induced by mitogens and IL-2 via inhibiting the expression of the IL-2 receptor, and that suppression of human T-cell proliferation by ZVAD-fmk is independent of its caspase inhibition properties, as ZVAD-fmk inhibits caspase processing during apoptosis but not during T-cell activation. Because caspase inhibitors are capable of providing reversible inhibitory effects on proliferative response in HPBLs by temporarily blocking the expression of proliferative response receptors, we proposed that inhibition of caspases would be a promising pharmacological approach for radioprotection of HPBLs.
In summary, we showed herein that X-irradiation induced not only the pan-nuclear γH2AX response but also the transient pan-nuclear p-ATM and p-DNA-PKcs responses in addition to the formation of nuclear DDR foci containing activated ATM, DNA-PKcs and γH2AX in resting HPBLs. The pan-nuclear γH2AX response and the transient pan-nuclear p-ATM and p-DNA-PKcs responses were directly related to X-ray-induced apoptosis. Moreover, the pan-nuclear γH2AX response and apoptosis were mediated not only by transient pan-nuclear p-ATM and p-DNA-PKcs signals but also by p53 and pan-caspase activities in resting HPBLs exposed to X-irradiation. ATM/DNA-PKcs inhibitor and p53 inhibitor attenuate the X-ray-induced pan-nuclear γH2AX response and apoptosis, but worsen the X-ray-induced functional impairment and genomic instability, suggesting that these inhibitors cannot be used as radioprotective agents for HPBLs. The pan-caspase inhibitor attenuates X-ray-induced pan-nuclear γH2AX response and apoptosis with no effect on X-ray-induced genomic instability and holds developmental promise.
Materials and Methods
Peripheral blood sample collection and lymphocyte isolation and culture
Human peripheral blood samples were obtained from informed healthy male and female volunteers (22–25 years old), and the research was approved by Fudan University School of Public Health Institutional Review Board. For each experiment, blood was collected in sterile heparinized Vacutainers from three to six different donors. Lymphocytes were isolated from blood using density gradient centrifugation in Ficoll-Paque (Cedarlane Co., Burlington, ON, Canada) according to the manufacturer’s instructions. Isolated HPBLs at a density of 1×106 cell/ml were cultured in RPMI 1640 medium (Gibco, Invitrogen Technologies, Carlsbad, CA, USA) supplemented with 15% fetal calf serum (Invitrogen Technologies), 5% (v/v) plasma, 100 U/ml penicillin and 100 μg/ml streptomycin at 5% CO2 and 37°C in a humidified incubator. The viability of the isolated HPBLs was above 95% as measured with the trypan blue exclusion assay.
Irradiation and treatment
An X-Rad 320 biological irradiator (Precision X-Ray, Inc., North Branford, CT, USA) was used for the X-ray irradiation, and the dose rate was 1.83 Gy/min. The HPBLs were pretreated with 10 μM KU55933 (Selleckchem, Houston, TX, USA) for 2 h, 20 μM PFT-μ (Selleckchem) for 5 h or 100 μM ZVAD-fmk (Selleckchem) for 1 h, and then irradiated in a flask at room temperature at doses of 0.5, 2 or 5 Gy. After irradiation, the cells were incubated in a CO2 incubator at 37 °C and harvested after various repair periods for immunofluorescence staining, apoptosis assay and western blotting analysis. For the lymphocyte proliferative assay, phytohemagglutinin (Shanghai Jikong Biotechnology, Shanghai, China) was added to the culture medium after IR, and the cells were further incubated for 48 h.
Immunofluorescence analysis
The suspension of irradiated and sham-irradiated HPBLs was immediately placed on ice for 10 min and washed in ice-cold PBS, and the cells were spun onto the triple chambered slides precoated with 50 mg/l poly-L-lysine (Sigma, St. Louis, MO, USA) using a cytospin centrifuge (Iris sample processing, Beckman Coulter Inc., Miami, FL, USA), fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.5% Triton X-100 for 15 min and followed by blocking with 10% (v/v) fetal calf serum in PBS at 37 °C for 1 h. The cells were then incubated overnight at 4 °C with anti-γH2AX (S139) antibody (Cell Signaling Technology, Danvers, MA, USA), anti-phospho-ATM (S1981) antibody (Abcam, Hong Kong, China) and anti-phospho-DNA-PKcs (T2609) antibody (Abcam) or anti-Ki67 antibody (Cell Signaling Technology), followed by secondary antibody labeled with Alexa Fluor 488-conjugated donkey anti-rabbit (Molecular probe, Life Technologies, Grand Island, NY, USA), Alexa Fluor 555-conjugated donkey anti-mouse or Alexa Fluor 647-conjugated donkey anti-rabbit (Molecular probe) at room temperature for 1 h. Fluorescence images were acquired by an Olympus BX51 fluorescent microscope (Olympus Optical Co., Tokyo, Japan) equipped with a COHU CCD camera (Audio Video Supply, San Diego, CA, USA) and VideoTesT-FISH software (VideoTesT, Saint Petersburg, Russia). Images used for comparisons between different treatments were acquired and processed with the same instrument settings. The normalized levels of pan-nuclear staining or foci of γ-H2AX, p-ATM (S1981) and p-DNA-PKcs (T2609) were the ratios of the number of pan-nuclear staining or foci to the number of cells scored. In addition, colocalization of TUNEL staining with pan-nuclear γ-H2AX, p-ATM or p-DNA-PKcs staining, and colocalization of pan-nuclear γ-H2AX staining with pan-nuclear p-ATM or p-DNA-PKcs staining were examined. The colocalization ratios were calculated as follows: number of colocalized pan-nuclear staining/number of TUNEL staining or pan-nuclear γ-H2AX staining.
Apoptosis detection
After pretreatment with inhibitors of ATM/DNA-PKcs, p53 and pan-caspases or/and X-irradiation, the cell monolayers were spun down on slides, and subjected to terminal deoxynucleotidyl transferase-mediated dUTP (2′-deoxyuridine 5′-triphosphate) biotin nick end labeling (TUNEL) staining using a TUNEL Apoptosis Detection Kit (Vazyme Biotech, Nanjing, Jiangsu Province, China) for in situ detection of apoptosis according to the manufacturer’s instructions. For combination with immunofluorescence analysis, fluorescein isothiocyanate-12-dUTP labeling mix was added and incubated for 1 h after incubation with the primary antibody, followed by incubation with the secondary antibody. The TUNEL-stained cells were counter-stained with mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Santa Cruz Biotechnology, Santa Cruz, CA, USA); the fluorescein isothiocyanate-labeled TUNEL-positive cells were imaged using an Olympus BX51 fluorescent microscope, and the TUNEL-positive cells were counted to calculate the TUNEL indices.
Chromosomal aberration analysis
After pretreatment with inhibitors of ATM/DNA-PKcs, p53 and pan-caspases, HPBLs were irradiated by X-rays. After a 2-h repair period, the cells were incubated with colcemid and phytohemagglutinin, and the levels of X-ray-induced chromosomal aberrations in HPBLs were determined at 48 h after irradiation. Chromosome preparations were made using standard techniques after the incubation of the cells in hypotonic solution (0.075 M KCl) and fixation with methanol/acetic acid (3 : 1). For each sample, 100 metaphases per donor were scored. The chromosomal aberrations were classified according to Savage.60
Western blotting
Cell extracts were prepared in radioimmunoprecipitation assay buffer containing 1% (v/v) proteinase inhibitor, 1% (v/v) phosphatase inhibitor cocktail, 1 mM phenylmethylsulfonyl fluoride and 1 mM sodium orthovanadate. Aliquots (60 μg protein) from each sample were loaded into each lane of sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and subjected to gel electrophoresis, and followed by transfer to polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). The membranes were incubated overnight at 4 °C with the primary anti-γ-H2AX (S139) antibody (1 : 1000) (Cell Signaling Technology), anti-phospho-ATM (S1981) antibody (1 : 1000) (Abcam), anti-ATM antibody (1 : 500) (Santa Cruz), anti-phospho-DNA-PKcs (T2609) antibody (1 : 1000) (Abcam), anti-DNA-PCcs antibody (1 : 500) (Santa Cruz), anti-phospho-p53 (S15) antibody (1 : 1000) (Cell Signaling Technology), anti-p53 antibody (1 : 500) (Santa Cruz) or anti-cleaved-caspase-3 antibody (1 : 1000) (Cell Signaling Technology). The membranes were then incubated for 1 h at room temperature with the secondary antibody: IgG-HPR antibody (1 : 2500) (Immunology Consultants Laboratory, Inc., Portland, OR, USA). Enhanced chemiluminescence was then performed (ECL Kit, Millipore) according to the manufacturer's protocol. The densitometry analysis was performed with the ChemiDoc detection system (Bio-Rad Laboratories, Hercules, CA, USA) and Quantity One software (Bio-Rad). The membranes were also probed with anti-GAPDH antibody (1 : 1000) (Huaan Biotechnology, Hangzhou, Zhejiang Province, China), anti-β-actin antibody (1 : 5000) (Huaan Biotechnology) or anti-vinculin antibody (1 : 10000) (Abcam) as the internal control.
Statistical analysis
Data are presented as the means±standard deviation. Statistical analysis of the data was performed using SPSS 17.0 software. Independent-samples T test was applied to analyze the differences. A difference with P<0.05 was considered to be statistically significant.
Acknowledgments
We thank Meijun Zhou for chromosome aberration analysis; Advanced Research Scientist Yonghe Li of Department of Biochemistry and Molecular Biology, Southern Research Institute in Birmingham for manuscript editing. This work was supported by the National Nature Science Foundation of China (81273000, 31370839).
Glossary
- HPBLs
human peripheral blood lymphocytes
- IR
ionizing radiation
- γH2AX
Phosphorylated histone variant H2AX
- dUTP
2′-deoxyuridine 5′-triphosphate
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP biotin nick end labeling
- LET
linear energy transfer
- p-ATM
phosphorylated Ataxia telangiectasia mutated
- p-DNA-PKcs
phosphorylated DNA-dependent protein kinase catalytic subunit
- ATM
Ataxia telangiectasia mutated
- DNA-PKcs
DNA-dependent protein kinase catalytic subunit
- PFT-μ
Pifithrin-μ
- PFT-α
Pifithrin-α
- DSBs
double strand breaks
- DDR
DNA damage response
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- JNK
c-Jun N-terminal kinase
- CAD
caspase-3/caspase-activated DNase
- NHEJ
non-homologous end-joining
- IL-2
Interleukin-2
- RPMI
Roswell Park Memorial Institute
- PHA
phytohemagglutinin
- PBS
phosphate-buffered saline
- DAPI
4′,6-diamidino-2-phenylindole
- S.D.
standard deviation.
Footnotes
Edited by I Harris
The authors declare no conflict of interest.
References
- Jackson SP , Bartek J . The DNA-damage response in human biology and disease. Nature 2009; 461: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou EP , Boon C , Redon C , Bonner WM . Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol 1999; 146: 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou EP , Pilch DR , Orr AH , Ivanova VS , Bonner WM . DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 1998; 273: 5858–5868. [DOI] [PubMed] [Google Scholar]
- Burma S , Chen BP , Murphy M , Kurimasa A , Chen DJ . ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 2001; 276: 42462–42467. [DOI] [PubMed] [Google Scholar]
- Stiff T , O'Driscoll M , Rief N , Iwabuchi K , Lobrich M , Jeggo PA . ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res 2004; 64: 2390–2396. [DOI] [PubMed] [Google Scholar]
- Wang H , Wang M , Wang H , Bocker W , Iliakis G . Complex H2AX phosphorylation patterns by multiple kinases including ATM and DNA-PK in human cells exposed to ionizing radiation and treated with kinase inhibitors. J Cell Physiol 2005; 202: 492–502. [DOI] [PubMed] [Google Scholar]
- Lane DP . Cancer - P53, guardian of the genome. Nature 1992; 358: 15–16. [DOI] [PubMed] [Google Scholar]
- Canman CE , Lim DS , Cimprich KA , Taya Y , Tamai K , Sakaguchi K et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 1998; 281: 1677–1679. [DOI] [PubMed] [Google Scholar]
- Rathmell WK , Kaufmann WK , Hurt JC , Byrd LL , Chu G . DNA-dependent protein kinase is not required for accumulation of p53 or cell cycle arrest after DNA damage. Cancer Res 1997; 57: 68–74. [PubMed] [Google Scholar]
- Jimenez GS , Bryntesson F , Torres-Arzayus MI , Priestley A , Beeche M , Saito Si et al. DNA-dependent protein kinase is not required for the p53-dependent response to DNA damage. Nature 1999; 400: 81–83. [DOI] [PubMed] [Google Scholar]
- Jhappan C , Yusufzai TM , Anderson S , Anver MR , Merlino G . The p53 response to DNA damage in vivo is independent of DNA-dependent protein kinase. Mol Cell Biol 2000; 20: 4075–4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S , Guo M , Ouyang H , Li XL , Cordon-Cardo C , Kurimasa A et al. The catalytic subunit of DNA-dependent protein kinase selectively regulates p53-dependent apoptosis but not cell-cycle arrest. Proc Natl Acad Sci USA 2000; 97: 1584–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callen E , Jankovic M , Wong N , Zha S , Chen HT , Difilippantonio S et al. Essential role for DNA-PKcs in DNA double-strand break repair and apoptosis in ATM-deficient lymphocytes. Mol Cell 2009; 34: 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S , Harris CC . p53: traffic cop at the crossroads of DNA repair and recombination. Nat Rev Mol Cell Biol 2005; 6: 44–55. [DOI] [PubMed] [Google Scholar]
- Ding HF , Lin YL , McGill G , Juo P , Zhu H , Blenis J et al. Essential role for caspase-8 in transcription-independent apoptosis triggered by p53. J Biol Chem 2000; 275: 38905–38911. [DOI] [PubMed] [Google Scholar]
- Mihara M , Erster S , Zaika A , Petrenko O , Chittenden T , Pancoska P et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell 2003; 11: 577–590. [DOI] [PubMed] [Google Scholar]
- Leist M , Jaattela M . Four deaths and a funeral: From caspases to alternative mechanisms. Nat Rev Mol Cell Bio 2001; 2: 589–598. [DOI] [PubMed] [Google Scholar]
- Cook PJ , Ju BG , Telese F , Wang X , Glass CK , Rosenfeld MG . Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature 2009; 458: 591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markova E , Torudd J , Belyaev I . Long time persistence of residual 53BP1/gamma-H2AX foci in human lymphocytes in relationship to apoptosis, chromatin condensation and biological dosimetry. Int J Radiat Biol 2011; 87: 736–745. [DOI] [PubMed] [Google Scholar]
- Meyer B , Voss KO , Tobias F , Jakob B , Durante M , Taucher-Scholz G . Clustered DNA damage induces pan-nuclear H2AX phosphorylation mediated by ATM and DNA-PK. Nucleic Acids Res 2013; 41: 6109–6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psonka-Antonczyk K , Elsasser T , Gudowska-Nowak E , Taucher-Scholz G . Distribution of double-strand breaks induced by ionizing radiation at the level of single DNA molecules examined by atomic force microscopy. Radiat Res 2009; 172: 288–295. [DOI] [PubMed] [Google Scholar]
- Solier S , Sordet O , Kohn KW , Pommier Y . Death receptor-induced activation of the Chk2- and histone H2AX-associated DNA damage response pathways. Mol Cell Biol 2009; 29: 68–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou EP , Nieves-Neira W , Boon C , Pommier Y , Bonner WM . Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem 2000; 275: 9390–9395. [DOI] [PubMed] [Google Scholar]
- Mukherjee B , Kessinger C , Kobayashi J , Chen BP , Chen DJ , Chatterjee A et al. DNA-PK phosphorylates histone H2AX during apoptotic DNA fragmentation in mammalian cells. DNA Repair (Amst) 2006; 5: 575–590. [DOI] [PubMed] [Google Scholar]
- de Feraudy S , Revet I , Bezrookove V , Feeney L , Cleaver JE . A minority of foci or pan-nuclear apoptotic staining of gammaH2AX in the S phase after UV damage contain DNA double-strand breaks. Proc Natl Acad Sci USA 2010; 107: 6870–6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korwek Z , Sewastianik T , Bielak-Zmijewska A , Mosieniak G , Alster O , Moreno-Villanueva M et al. Inhibition of ATM blocks the etoposide-induced DNA damage response and apoptosis of resting human T cells. DNA Repair (Amst) 2012; 11: 864–873. [DOI] [PubMed] [Google Scholar]
- Furuta T , Hayward R , Meng L , Takemura H , Aune G , Bonner W et al. p21CDKN1A allows the repair of replication-mediated DNA double-strand breaks induced by topoisomerase I and is inactivated by the checkpoint kinase inhibitor 7-hydroxystaurosporine. Oncogene 2006; 25: 2839–2849. [DOI] [PubMed] [Google Scholar]
- Antony S , Agama KK , Miao ZH , Takagi K , Wright MH , Robles AI et al. Novel indenoisoquinolines NSC 725776 and NSC 724998 produce persistent topoisomerase I cleavage complexes and overcome multidrug resistance. Cancer Res 2007; 67: 10397–10405. [DOI] [PubMed] [Google Scholar]
- Ewald B , Sampath D , Plunkett W . H2AX phosphorylation marks gemcitabine-induced stalled replication forks and their collapse upon S-phase checkpoint abrogation. Mol Cancer Ther 2007; 6: 1239–1248. [DOI] [PubMed] [Google Scholar]
- Quanz M , Berthault N , Roulin C , Roy M , Herbette A , Agrario C et al. Small-molecule drugs mimicking DNA damage: a new strategy for sensitizing tumors to radiotherapy. Clin Cancer Res 2009; 15: 1308–1316. [DOI] [PubMed] [Google Scholar]
- Quanz M , Chassoux D , Berthault N , Agrario C , Sun JS , Dutreix M . Hyperactivation of DNA-PK by double-strand break mimicking molecules disorganizes DNA damage response. Plos One 2009; 4: e6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragkos M , Breuleux M , Clement N , Beard P . Recombinant adeno-associated viral vectors are deficient in provoking a DNA damage response. J Virol 2008; 82: 7379–7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RA , Carson CT , Schuberth C , Weitzman MD . Adeno-associated virus replication induces a DNA damage response coordinated by DNA-dependent protein kinase. J Virol 2009; 83: 6269–6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baure J , Izadi A , Suarez V , Giedzinski E , Cleaver JE , Fike JR et al. Histone H2AX phosphorylation in response to changes in chromatin structure induced by altered osmolarity. Mutagenesis 2009; 24: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IAEA. Cytogenetic Analysis for Radiation Dose Assessment: A Manual, IAEA (Interational Atomic Energy Agency) Technical Reports Series. No.405.: Vienna, 2001. [Google Scholar]
- Dikomey E , Borgmann K , Peacock J , Jung H . Why recent studies relating normal tissue response to individual radiosensitivity might have failed and how new studies should be performed. Int J Radiat Oncol 2003; 56: 1194–1200. [DOI] [PubMed] [Google Scholar]
- Cogoli-Greuter M . The lymphocyte story - an overview of selected highlights on the in vitro activation of human lymphocytes in space. Microgravity Sci Tec 2014; 25: 343–352. [Google Scholar]
- Bufi N , Saitakis M , Dogniaux S , Buschinger O , Bohineust A , Richert A et al. Human primary immune cells exhibit distinct mechanical properties that are modified by inflammation. Biophys J 2015; 108: 2181–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkamm K , Lobrich M . Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci U S A 2003; 100: 5057–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourton EC , Plowman PN , Smith D , Arlett CF , Parris CN . Prolonged expression of the gamma-H2AX DNA repair biomarker correlates with excess acute and chronic toxicity from radiotherapy treatment. Int J Cancer 2011; 129: 2928–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabacik S , Ortega-Molina A , Efeyan A , Finnon P , Bouffler S , Serrano M et al. A minimally invasive assay for individual assessment of the ATM/CHEK2/p53 pathway activity. Cell Cycle 2011; 10: 1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y , White E . p53-dependent apoptosis pathways. Adv Cancer Res 2001; 82: 55–84. [DOI] [PubMed] [Google Scholar]
- Bassi L , Carloni M , Meschini R , Fonti E , Palitti F . X-irradiated human lymphocytes with unstable aberrations and their preferential elimination by p53/survivin-dependent apoptosis. Int J Radiat Biol 2003; 79: 943–954. [DOI] [PubMed] [Google Scholar]
- Belloni P , Meschini R , Czene S , Harms-Ringdahl M , Palitti F . Studies on radiation-induced apoptosis in G0 human lymphocytes. Int J Radiat Biol 2005; 81: 587–599. [DOI] [PubMed] [Google Scholar]
- Vilasova Z , Rezacova M , Vavrova J , Tichy A , Vokurkova D , Zoelzer F et al. Changes in phosphorylation of histone H2A.X and p53 in response of peripheral blood lymphocytes to gamma irradiation. Acta Biochim Pol 2008; 55: 381–390. [PubMed] [Google Scholar]
- Meschini R , Berni A , Ortenzi V , Mancinelli P , Palitti F . Relation between DNA repair, apoptosis and chromosomal aberrations in presence of pifithrin-α, an inhibitor of p53. Mutat Res 2010; 701: 92–97. [DOI] [PubMed] [Google Scholar]
- Yu YJ , Little JB . p53 is involved in but not required for ionizing radiation-induced caspase-3 activation and apoptosis in human lymphoblast cell lines. Cancer Res 1998; 58: 4277–4281. [PubMed] [Google Scholar]
- UNSCEAR. Effects of Ionizing Radiation: Report to the General Assembly, with Scientific Annexes, vol. 1: UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation): New York: United Nations, 2008. [Google Scholar]
- ICRP. ICRP statement on tissue reactions/early and late effects of radiation in normal tissues and organs--threshold doses for tissue reactions in a radiation protection context. ICRP (International Commissin On Radiologcal Protection) Publication 118. Ann ICRP vol. 41, 2012. [DOI] [PubMed] [Google Scholar]
- Shiotani B , Zou L . Single-stranded DNA orchestrates an ATM-to-ATR Switch at DNA Breaks. Mol Cell 2009; 33: 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C , Zhu F , Cho Y-Y , Tang F , Zykova T , Ma WY et al. Cell apoptosis: requirement of H2AX in DNA ladder formation, but not for the activation of caspase-3. Mol Cell 2006; 23: 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T , Namba H , Hara T , Takmura N , Nagayama Y , Fukata S et al. p53 induced by ionizing radiation mediates DNA end-jointing activity, but not apoptosis of thyroid cells. Oncogene 1997; 14: 1511–1519. [DOI] [PubMed] [Google Scholar]
- Tang W , Willers H , Powell SN . p53 directly enhances rejoining of DNA double-strand breaks with cohesive ends in gamma-irradiated mouse fibroblasts. Cancer Res 1999; 59: 2562–2565. [PubMed] [Google Scholar]
- Lin Y , Waldman BC , Waldman AS . Suppression of high-fidelity double-strand break repair in mammalian chromosomes by pifithrin-alpha, a chemical inhibitor of p53. DNA Repair (Amst) 2003; 2: 1–11. [DOI] [PubMed] [Google Scholar]
- Al Rashid ST , Dellaire G , Cuddihy A , Jalali F , Vaid M , Coackley C et al. Evidence for the direct binding of phosphorylated p53 to sites of DNA breaks in vivo. Cancer Res 2005; 65: 10810–10821. [DOI] [PubMed] [Google Scholar]
- Gudkov AV , Komarova EA . Radioprotection: smart games with death. J Clin Invest 2010; 120: 2270–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson I , Zhao Y , Richardson CJ , Green SJ , Martin NM , Orr AI et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res 2004; 64: 9152–9159. [DOI] [PubMed] [Google Scholar]
- Sordet O , Redon CE , Guirouilh-Barbat J , Smith S , Solier S , Douarre C et al. Ataxia telangiectasia mutated activation by transcription- and topoisomerase I-induced DNA double-strand breaks. Embo Rep 2009; 10: 887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence CP , Chow SC . Suppression of human T cell proliferation by the caspase inhibitors, z-VAD-FMK and z-IETD-FMK is independent of their caspase inhibition properties. Toxicol Appl Pharmacol 2012; 265: 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage J . Classification and relationships of induced chromosomal structual changes. J Med Genet 1976; 13: 103–122. [DOI] [PMC free article] [PubMed] [Google Scholar]