Abstract
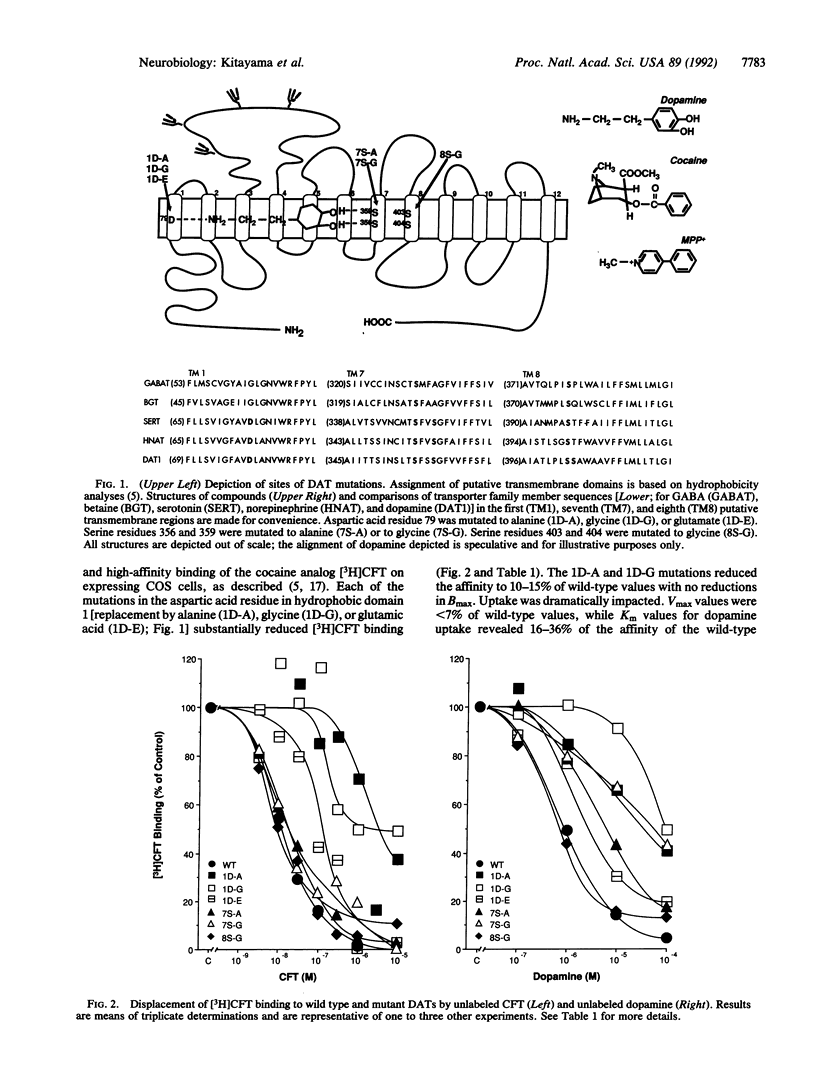
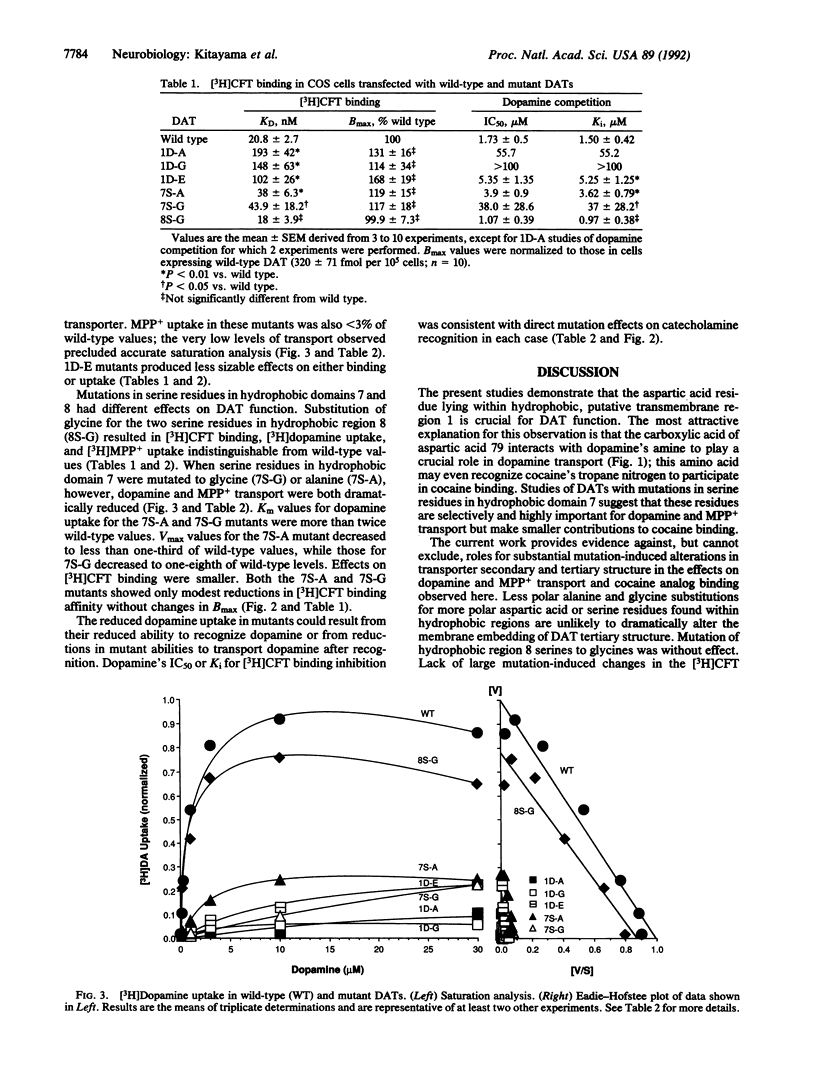
Polar amino acids lying within three hydrophobic regions of the dopamine transporter (DAT) are analogous to those important for ligand recognition by catecholamine receptors. Possible functional significance of these amino acids was examined by expressing DAT cDNAs mutated in these polar residues. Replacement of aspartate at position 79 with alanine, glycine, or glutamate dramatically reduced uptake of [3H]dopamine and the tritium-labeled Parkinsonism-inducing neurotoxin 1-methyl-4-phenylpyridinium (MPP+) and reduced the mutants' affinity for the tritium-labeled cocaine analog (-)-2 beta-carbomethoxy-3 beta-(4-fluorophenyl)tropane (CFT) without affecting Bmax. Replacement of the serine residues at positions 356 and 359 in the seventh hydrophobic region by alanine or glycine caused reductions in [3H]dopamine and [3H]MPP+ uptake, whereas [3H]CFT binding was less affected. Substitution of two serines in the eighth hydrophobic region yielded wild-type values for [3H]dopamine and [3H]MPP+ uptake and [3H]CFT binding. These results demonstrate that aspartate and serine residues lying within the first and seventh hydrophobic putative transmembrane regions are crucial for DAT function and provide identification of residues differentially important for cocaine binding and for dopamine uptake.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., Pacholczyk T. Sodium-dependent neurotransmitter reuptake systems. Curr Opin Neurobiol. 1991 Jun;1(1):84–90. doi: 10.1016/0959-4388(91)90014-x. [DOI] [PubMed] [Google Scholar]
- Blakely R. D., Berson H. E., Fremeau R. T., Jr, Caron M. G., Peek M. M., Prince H. K., Bradley C. C. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991 Nov 7;354(6348):66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Carroll F. I., Lewin A. H., Abraham P., Parham K., Boja J. W., Kuhar M. J. Synthesis and ligand binding of cocaine isomers at the cocaine receptor. J Med Chem. 1991 Mar;34(3):883–886. doi: 10.1021/jm00107a003. [DOI] [PubMed] [Google Scholar]
- Gray T. M., Matthews B. W. Intrahelical hydrogen bonding of serine, threonine and cysteine residues within alpha-helices and its relevance to membrane-bound proteins. J Mol Biol. 1984 May 5;175(1):75–81. doi: 10.1016/0022-2836(84)90446-7. [DOI] [PubMed] [Google Scholar]
- Guastella J., Nelson N., Nelson H., Czyzyk L., Keynan S., Miedel M. C., Davidson N., Lester H. A., Kanner B. I. Cloning and expression of a rat brain GABA transporter. Science. 1990 Sep 14;249(4974):1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Hoffman B. J., Mezey E., Brownstein M. J. Cloning of a serotonin transporter affected by antidepressants. Science. 1991 Oct 25;254(5031):579–580. doi: 10.1126/science.1948036. [DOI] [PubMed] [Google Scholar]
- Horn A. S. Dopamine uptake: a review of progress in the last decade. Prog Neurobiol. 1990;34(5):387–400. doi: 10.1016/0301-0082(90)90033-d. [DOI] [PubMed] [Google Scholar]
- Iversen L. L. Role of transmitter uptake mechanisms in synaptic neurotransmission. Br J Pharmacol. 1971 Apr;41(4):571–591. doi: 10.1111/j.1476-5381.1971.tb07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilty J. E., Lorang D., Amara S. G. Cloning and expression of a cocaine-sensitive rat dopamine transporter. Science. 1991 Oct 25;254(5031):578–579. doi: 10.1126/science.1948035. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Neve K. A., Cox B. A., Henningsen R. A., Spanoyannis A., Neve R. L. Pivotal role for aspartate-80 in the regulation of dopamine D2 receptor affinity for drugs and inhibition of adenylyl cyclase. Mol Pharmacol. 1991 Jun;39(6):733–739. [PubMed] [Google Scholar]
- Pacholczyk T., Blakely R. D., Amara S. G. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991 Mar 28;350(6316):350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Reith M. E., Meisler B. E., Sershen H., Lajtha A. Structural requirements for cocaine congeners to interact with dopamine and serotonin uptake sites in mouse brain and to induce stereotyped behavior. Biochem Pharmacol. 1986 Apr 1;35(7):1123–1129. doi: 10.1016/0006-2952(86)90148-6. [DOI] [PubMed] [Google Scholar]
- Ritz M. C., Cone E. J., Kuhar M. J. Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure-activity study. Life Sci. 1990;46(9):635–645. doi: 10.1016/0024-3205(90)90132-b. [DOI] [PubMed] [Google Scholar]
- Ritz M. C., Lamb R. J., Goldberg S. R., Kuhar M. J. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987 Sep 4;237(4819):1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Schaeffer J. C., Lin C. L., Kitayama S., Uhl G. R. Ligand autoradiographic receptor screening. II. Expression of receptor cDNA in transfected COS cells grown on polyester disks and its recovery. Brain Res Mol Brain Res. 1991 Feb;9(3):271–276. doi: 10.1016/0169-328x(91)90012-m. [DOI] [PubMed] [Google Scholar]
- Shimada S., Kitayama S., Lin C. L., Patel A., Nanthakumar E., Gregor P., Kuhar M., Uhl G. Cloning and expression of a cocaine-sensitive dopamine transporter complementary DNA. Science. 1991 Oct 25;254(5031):576–578. doi: 10.1126/science.1948034. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., D'Amato R. J. MPTP: a neurotoxin relevant to the pathophysiology of Parkinson's disease. The 1985 George C. Cotzias lecture. Neurology. 1986 Feb;36(2):250–258. doi: 10.1212/wnl.36.2.250. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Dixon R. A. Structural basis of beta-adrenergic receptor function. FASEB J. 1989 May;3(7):1825–1832. doi: 10.1096/fasebj.3.7.2541037. [DOI] [PubMed] [Google Scholar]
- Wang C. D., Buck M. A., Fraser C. M. Site-directed mutagenesis of alpha 2A-adrenergic receptors: identification of amino acids involved in ligand binding and receptor activation by agonists. Mol Pharmacol. 1991 Aug;40(2):168–179. [PubMed] [Google Scholar]
- Yamauchi A., Uchida S., Kwon H. M., Preston A. S., Robey R. B., Garcia-Perez A., Burg M. B., Handler J. S. Cloning of a Na(+)- and Cl(-)-dependent betaine transporter that is regulated by hypertonicity. J Biol Chem. 1992 Jan 5;267(1):649–652. [PubMed] [Google Scholar]



