Abstract
Extramammary Paget disease (EMPD) is a rare perineal neoplasia associated with a high rate of local recurrence. Surgical excision is the standard treatment; however, this has high rates of post-operative morbidity in combination with potentially mutilating results. Previous literature has demonstrated good response with imiquimod 5% cream in patients with vulval EMPD, yet its effectiveness in primary perianal disease is unknown.
We describe the case of a 40-year-old woman presenting with EMPD of the perianal region, providing detailed histological and pictoral evidence of its response to topical imiquimod 5% cream over a 16-week period, which initially resulted in remission prior to metastatic lymph node recurrence. This case demonstrates the potential for topical imiquimod cream to avoid major surgery and its associated complications in patients presenting with EMPD of the perianal region. We discuss the current evidence for treating this rare condition with medical therapy, how this case adds to current literature and possible future directions.
INTRODUCTION
Extramammary Paget disease (EMPD) is similar histologically to Paget disease of the breast and predominantly affects external genitalia. It is a rare malignancy and primary cutaneous disease usually involves the vulva [1]. Secondary EMPD disease is defined as a neoplasia of a non-cutaneous tissue [2], the associated malignancy is frequently anal and/or rectal adenocarcinoma with involvement of the anogenital skin [3]. Histologically, primary or cutaneous EMPD is defined as a non-invasive intra-epidermal adenocarcinoma that can spread into neighbouring epithelial glandular appendices [4].
There are several treatment modalities described in the management of EMPD including surgery, chemotherapy and irradiation with variable curative rates. However, relapse can occur in >30% of patients [5]. Surgery is the first-line treatment; however, surgical margins are frequently positive, resulting in high recurrence rates after surgery [6].
Imiquimod (Aldara®, 3 M Health Care Limited) is an immune stimulator that is highly effective in the treatment of vulval intra-epithelial neoplasia [7]. Growing evidence is beginning to suggest a role for imiquimod 5% cream in EMPD, particularly EMPD of vulval origin [5], in achieving disease-control or complete response. However, limited evidence exists for the treatment of primary EMPD of the perianal region with imiquimod.
In this case report, we present a female patient of 40 years old with perianal EMPD, treated with imiquimod 5% cream.
CASE REPORT
A 40-year-old woman presented to the Dermatology outpatient clinic with a 2-year history of a painful rash in the perianal region associated with pruritus and erythema. The patient denied any bowel or gynaecological symptoms. Her medical history included erythema nodosum, polycystic ovary disease and a body mass index (BMI) >30.
On examination, an erythematous perianal lesion was present with associated superficial excoriation (Fig. 1). An initial punch biopsy was taken (Fig. 2A), which demonstrated a unilateral well-demarcated eroded lesion. Immunohistochemical staining (Fig. 2B) showed malignant cells in the epidermis, staining positively for CK7 and EMA. The tissue stained negative for CK20 and oestrogen receptors. A diagnosis of EMPD was given.
Figure 1:
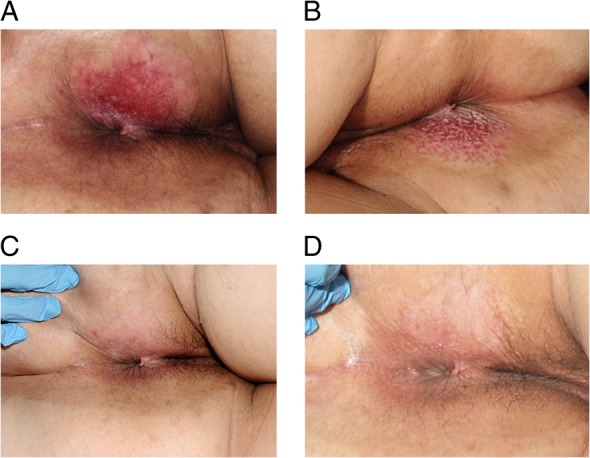
(A) Initial presentation with an erythematous perianal lesion with associated superficial excoriation. (B) Appearance of rash following 5 weeks and (C) 16 weeks of treatment with 5% imiquimod cream, demonstrating stepwise improvement. (D) Appearance of perineum at 6-month follow-up following completion of treatment.
Figure 2:
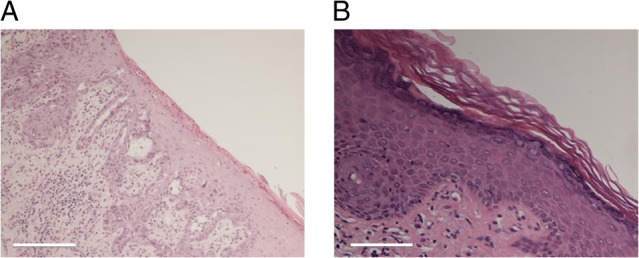
(A) Initial punch biopsy of the perianal lesion demonstrated a well-demarcated erosive lesion with associated fibrin deposition and acute inflammation. (B) Following the 16-week course of imiquimod cream, repeat biopsies demonstrated chronic inflammation with epidermal reaction and prominent vascular proliferation, but no evidence of EMPD. Both produced with haematoxylin & eosin staining. Scale bar 500 μm (A) and 200 μm (B).
Breast and gynaecological examinations of the patient were normal, while a colonoscopy did not identify any evidence of extramammary bowel disease. A computed tomography (CT) chest, abdomen and pelvis demonstrated subcutaneous inflammation of the right buttock but no evidence of disease spread beyond the perianal skin.
Due to her elevated BMI and the perianal location of her EMPD, it was felt that the standard option of surgical resection by performing Mohs micrographic surgery or wide local excision would be associated with a high risk of perioperative morbidity. The potential for local control in a disease with a well-described pattern for local recurrence, while avoiding potentially disfiguring surgery, was felt to be a viable alternative option.
A trial of imiquimod cream (5%) was commenced with a regimen of one application each night for 5 days together with 2 rest days per week. The rest period was used to allow time for any skin inflammation caused by imiquimod to settle. Analgesia (Paracetamol, Diclofenac and Tramadol) was prescribed to provide pain relief for potential local skin reactions. On review at the first 2 weeks of treatment, the patient had tolerated the imiquimod 5% cream, with only two applications missed due to vomiting secondary to a viral infection.
By Week 5, the area of erythema had visibly reduced, with a 1-cm rim of granulation tissue around its edge (Fig. 1B). The patient's progress was reviewed at 12 and 16 weeks, with a stepwise regression of the EMPD identified. At 16-week examination demonstrated almost complete resolution of the perianal erythema (Fig. 1C). Further multiple, random punch biopsies of the perianal skin were taken 1 month later, which demonstrated variable evidence of chronic inflammation with epidermal reaction (Fig. 2C), but no residual EMPD.
A follow-up MRI of the pelvis 16 weeks after the discontinuation of treatment showed residual thickening consistent with inflammation at the site of previous subcutaneous nodules. A CT chest, abdomen and pelvis did not identify any distal EMPD deposits.
The patient remained in remission for 18 months, however inguinal lymphadenopathy was noted at this time and a biopsy demonstrated adenocarcinoma suggesting malignant transformation of her EMPD.
DISCUSSION
We describe the primary local treatment of perianal EMPD with imiquimod 5% cream over a 16-week period initially resulting in remission prior to lymph node spread at 18 months. This provides further evidence, both in disease response and optimal treatment time, which imiquimod might potentially be considered as a first-line treatment for perineal EMPD.
Imiquimod induces the immune response by activating macrophages and other inflammatory cells by increasing synthesis of pro-inflammatory cytokines while also producing distinctive apoptosis in epithelial cells [7]. A retrospective case-series of 23 patients demonstrated up to 80% clinical response with imiquimod for EMPD of the vulva [2]. The clinical response appears to be related to duration of imiquimod therapy in weeks [2]; however, the optimal length of treatment time remains to be defined.
Surgical resection of perianal EMPD is an invasive procedure associated with a high perioperative morbidity. Furthermore, the potential of disfiguring surgery and permanent stoma formation may have a profound psychological impact on the patient. Recurrence rates could approach up to 50% despite adequate surgical resection margins [8].
Close monitoring of patients during the treatment period is particularly important, both to monitor response of EMPD to treatment and the side-effect profile experienced by patients secondary to topical imiquimod. Following the completion of imiquimod treatment, biopsies are mandatory in order to assess residual disease, as recurrence is common. Longer term data regarding recurrence following topical therapy, and adequacy of subsequent surgical resection is awaited [6]. In this case recurrence was noted by palpable inguinal lymph nodes, with no skin or colonic lesion found. This highlights the importance of regular follow-up in perineal EMPD, with a high clinical index of suspicion for relapse or malignant change.
Developing an evidence-base for the use of imiquimod, as either first-line therapy or for disease recurrence, will be limited by the potential number of new EMPD cases. An international register [9] to collate data on each patient case should be considered to determine the optimal length of imiquimod treatment, together with the long-term follow-up of patients following treatment. In addition, it may also aid the identification of patient cohorts most likely to respond to imiquimod therapy.
CONFLICT OF INTEREST STATEMENT
None declared.
PATIENT CONSENT
Written consent was gained from the patient prior to article submission.
REFERENCES
- 1.Novak MA, Guerriere-Kovach P, Pathan A, Campbell TE, Deppisch LM. Perianal Paget's disease: distinguishing primary and secondary lesions using immunohistological studies including gross cystic disease fluid protein-15 and cytokeratin 20 expression. Arch Pathol Lab Med 1998;122:1077–81. [PubMed] [Google Scholar]
- 2.Luyten A, Sörgel P, Clad A, Gieseking F, Maass-Poppenhusen K, Lellé RJ, et al. Treatment of extramammary Paget disease of the vulva with imiquimod: a retrospective, multicentre study by the German Colposcopy Network. J Am Acad Dermatol 2014;70:644–50. [DOI] [PubMed] [Google Scholar]
- 3.Goldblum JR, Hart WR. Perianal Paget's disease: a histologic and immunohistochemical study of 19 cases. Am J Surg Pathol 1997;21:1178–87. [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson EJ, Brown HM. Vulvar Paget disease of urothelial origin: a report of three cases and a proposed classification of vulvar Paget disease. Hum Pathol 2002;33:549–54. [DOI] [PubMed] [Google Scholar]
- 5.Luyten A, Brummer O, Kühnle H, Reinecke-Lüthge A, Petry KU. New options for the treatment of Pagets disease of the vulva. Geburtshilfe Frauenheilkd 2006;66:1081–6. [Google Scholar]
- 6.Shaco-Levy R, Bean SM, Vollmer RT, Jewell E, Jones EL, Valdes CL, et al. Paget disease of the vulva: a study of 56 cases. Eur J Obstet Gynecol Reprod Biol 2010;149:86–91. [DOI] [PubMed] [Google Scholar]
- 7.Schon MP, Wienrich BG, Drewnlok C, Bong AB, Eberle J, Geilen CC, et al. Death receptor-independent apoptosis in malignant melanoma induced by the small-molecule immune response modifier imiquimod. J Invest Dermatol 2004;122:1266–76. [DOI] [PubMed] [Google Scholar]
- 8.Zollo JD, Zeitouni NC.. The Roswell Park Cancer Institute experience with extramammary Paget's disease. Br J Dermatol 2000;142:59–65. [DOI] [PubMed] [Google Scholar]
- 9.Genetic and Rare Diseases Information Centre http://rarediseases.info.nih.gov/gard (22 April 2015, date last accessed).


