Abstract
Background
Attentional bias (i.e., differences in reaction time between drug and neutral cues) has been associated with a variety of drug-use behaviors (e.g., craving, abstinence) and reduction of bias may ultimately reduce use.
Objective
The current study examined whether attentional bias modification therapy (ABMT) reduced the frequency of drug use behaviors in individuals with cocaine use disorder (CUD).
Method
Participants (n= 37) were randomly assigned to ABMT or control therapy, which systematically varied how frequently probes replaced neutral (ABMT = 100%; control = 50%) relative to drug stimuli. Each intervention included five training sessions comprising a total of 2640 trials over 4 weeks. Clinical assessments occurred at baseline, post-intervention, 2 weeks and 3 months post-treatment.
Results
There were no baseline differences between groups on drug-use behaviors or other clinical measures. Contrary to predictions, both groups exhibited slower rather than faster reaction times for cocaine stimuli (p = 0.005) at baseline, with no relationship between bias and baseline measures of drug-use behavior.
Conclusions
ABMT was not more effective than our control at reducing attentional bias, reducing craving or changing other drug use behaviors. Current results suggest additional replication studies are needed to assess ABMT’s efficacy in reducing drug-use behaviors in CUD.
Keywords: Cocaine, attentional bias modification, therapy, craving
Introduction
Cocaine use disorder (CUD) is associated with significant individual and societal costs with few known effective treatments (1). During the development of a substance use disorder (SUD), drug cues become progressively more emotionally salient with repeated drug use through classical conditioning (2). An increase in salience typically results in changes in how each individual selectively attends to drug cues relative to other stimuli. It has been hypothesized that this bias in selective attention directly increases drug craving and/or use through automatic processes (3–6). Although the degree of attentional bias has been associated with treatment outcome in both alcohol and cannabis use disorders (7, 8), current findings in CUD are mixed (7, 9).
In research settings, attentional bias is behaviorally measured through reaction time differences between drug and neutral stimuli, with the direction of the bias effect partially determined by the experimental paradigm. Attentional bias is therefore a non-specific term that has been used to describe both faster (i.e., facilitation) and slower (i.e., interference) reaction times for drug relative to neutral stimuli. For example, slower response times for drug relative to neutral cues (hereafter referred to as interference) during a Stroop task represents a common measure of attentional bias (3, 7, 10–13). Several studies demonstrate greater interference for cocaine cues in CUD relative to healthy controls during drug Stroop tasks (10, 14–16). Greater interference for cocaine cues has been associated with craving (14, 17), and is elevated when users are more tempted to use (13). However, other studies have reported greater interference in treatment-seeking relative to non-treatment-seeking CUD patients during a Stroop task, even though treatment seekers reported less experience using cocaine (12).
Attentional bias can also be measured during visual probe tasks, during which a probe appears in close spatial proximity to either drug or neutral stimuli (18–21). In the context of this task, response times are faster for probes near drug-related relative to neutral stimuli (hereafter referred to as facilitation), typically increasing as a function of the duration that cues are displayed. Facilitation has been observed in other SUD (21–25). However, the only study comparing CUD relative to healthy controls during a visual probe task reported interference for cocaine stimuli (26).
A variety of cognitive bias modification therapies have recently been proposed as potential treatments for SUD (27). For example, attentional bias modification training (ABMT) attempts to involuntarily shift bias away from drug cues as a mechanism for reducing use (18, 20, 21, 28). This is typically accomplished by having probes replace neutral rather than drug stimuli on all trials in the visual probe task, theoretically retraining individuals to disengage their attention away from drug stimuli. ‘Active’ training has been demonstrated to result in decreased attentional bias to alcohol cues relative to neutral cues (as measured by reduced facilitation during a visual probe task or reduced interference during a drug Stroop task) in both heavy drinkers (19, 20, 24) and individuals with alcohol use disorder (AUD; 21) dependent on the direction of training. In addition, ABMT resulted in significantly longer relapse times in individuals with AUD, but showed no significant effects on craving (21). Related trainings designed to increase control of distraction by alcohol cues (Alcohol Attention Control Training Program) also decreased alcohol use in hazardous and harmful drinkers for up to 3 months compared to a wait list control group (29). However, no differences between active and control training were recently observed when treatment was administered over the internet in AUD (30). To our knowledge, ABMT has not been investigated as a treatment for CUD.
The current study randomized 40 treatment-seeking CUD to either ABMT or a control therapy group while concurrently monitoring cocaine usage, craving and withdrawal symptoms. Based on previous work, we hypothesized that CUD would exhibit facilitation (reaction time for neutral > cocaine stimuli) at longer inter-stimulus intervals during a visual probe task at baseline. We also hypothesized that participants in the ABMT arm would show decreased attentional bias to cocaine cues (i.e. decreased facilitation during the visual probe task), decreased cocaine use and craving and longer time to relapse relative to the control group following intervention.
Methods
Participants
Forty adult participants (25 males; 37.8 ± 10.9 years of age) with a confirmed diagnosis of CUD participated in the study. Participants 1) between 18–55 years old, 2) self-reporting cocaine use in at least 4 of the prior 30 days, 3) seeking treatment and 4) not already in active treatment were included. Participants with self-reported history of major neurological disorder, medical condition, bipolar or schizophrenia spectrum disorder, contraindications for magnetic resonance imaging, pregnancy, or active legal problems with potential for incarceration were excluded. Preferred routes for cocaine administration included snorting (40.5%), smoking (51.4%) and intravenous use (5.4%). Drug screening (Integrated E-Z Split Key Cup II Drug Test) was performed on all visits. All participants completed a single therapeutic session of motivational interviewing to eliminate ethical concerns of non-treatment in the control group. Motivational interviewing has previously been shown to decrease cocaine use (31) and improve treatment retention (32).
Participants were then informed that they would be randomly assigned to an experimental therapy (ABMT) or control therapy. The initial assessment of AB occurred approximately 10 days post-consent/motivational interviewing session, followed by post-intervention assessments at the final treatment session (28 days after initial assessment), and follow-ups at approximately two weeks (short-term; 42 days) and at three months (long-term; 112 days). Neuroimaging data collected at baseline and post-intervention will be presented in a separate publication (see Table 1). Informed consent was obtained from all study participants, and the study was approved by the University of New Mexico Health Sciences Center.
Table 1.
Overall study schemata for proposed session dates (row 1), actual visit dates (row 2) and clinical measures (row 3).
| Session/ideal day | Clinical | Baseline/INT1 (0d) | INT2 (5d) | INT3 (10d) | INT4 (15d) | INT5 (20d) | Post-INT (28d) | Short-term (42d) | Long-term (112d) |
|---|---|---|---|---|---|---|---|---|---|
| Actual day (Mean±SD) | −11.4±6.1 | 0±0.0 | 5.9±2.4 | 11.2±2.9 | 17.4±4.4 | 23.0±5.7 | 31.4±10.1 | 44.2±11.0 | 120.2±16.1 |
| Measures | Consent, MI, SCID, all clinical measures | CSSA, CCQ-N, CCQ-G, TLFB, Urine Tox, Imaging | CSSA, CCQ-N, CCQ-G, TLFB, Urine Tox | CSSA, CCQ-N, CCQ-G, TLFBs, Urine Tox | CSSA, CCQ-N, CCQ-G, TLFBs, Urine Tox, TRQ | CSSA, CCQ-N, CCQ-G, TLFBs, Urine Tox | CSSA, CCQ-N, CCQ-G, TLFBs, Urine Tox, TRQ, STAI-S, BDI-II, SDS, Imaging | CSSA, CCQ-N, CCQ-G, TLFBs, Urine Tox, TRQ, STAI-S, BDI-II, SDS | CSSA, CCQ-N, CCQ-G, TLFBs, Urine Tox, TRQ, STAI-S, BDI-II, SDS |
BDI-II = Beck Depression Inventory; CCQ-G & CCQ-N = Cocaine Craving Questionnaire (Brief-General) & (Brief-Now); CSSA = Cocaine Selective Severity Assessment; INT = intervention; MI = Motivational Interviewing; SCID = Structured Clinical Interview for DSM Disorders; SD = standard deviation; SDS = Severity of Dependence Scale; STAI-S = State-Trait Anxiety Inventory (State); TLFB = Timeline Followback; TRQ = Treatment Review Questionnaire; Urine Tox = Integrated E-Z Split Key Cup II Drug Test.
One participant was eliminated due to poor behavioral performance on the AB task, resulting in 37 baseline assessments of AB (ABMT = 19; control = 18). Overall study retention was excellent, with only mild levels of attrition occurring at post-intervention (ABMT = 18; control = 16), short-term (ABMT = 18; control = 15) and long-term (ABMT = 17; control = 14) assessments. There were no significant differences (all p’s > 0.10) on primary outcome measures (cocaine craving, usage or withdrawal, or measures of attentional bias) at baseline for study completers (N = 31) versus non-completers (N = 6).
Clinical Assessment
Participants completed the Treatment Review Questionnaire, the Fagerstrom Test for Nicotine Dependence (33), the Alcohol Use Disorders Identification Test (34), the Cocaine Craving Questionnaire (Brief-NOW and Brief-GENERAL forms; 35), the Cocaine Selective Severity Assessment (36), the Severity of Dependence Scale (37) and the Structured Clinical Interview for DSM Disorders I Module E (38) for substance abuse and dependence. The Timeline Followback calendar (TLFB; 39) was used to determine self-reported cocaine, alcohol, and marijuana usage for all participants, as well as for any other self-reported drugs of use or as follow-up for positive urine screens. Participants were asked to refrain from smoking for one hour prior to each visit.
Self-reported percent days of cocaine use (primary outcome variable) was calculated for baseline (30 days prior to visit 2), post-intervention (between visits 2 and 7) short-term follow-up (between visits 7 and 8) and long-term follow-up (visit 8 to visit 9) phases, normalizing for the total number of days in each interval. The secondary outcome measure was time to relapse, defined as first reported usage of cocaine following the end of visit 7 (capped at 84 days for 12 treatment weeks). Finally, The Barratt Impulsiveness Scale (40), the Edinburgh Handedness Inventory (41), the Beck Depression Inventory (42), the State-Trait Anxiety Inventories (43, 44) and The Wechsler Test of Adult Reading (45) addressed potential confounding variables across groups (see Table 2).
Table 2.
Demographic characteristics and baseline clinical measures between ABMT and control therapy participants.
| ABMT | Control | P- value | Cohen’s d | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean | SD | Mean | SD | |||
| Demographic Characteristics | ||||||
| Sex | 5 F | 14 M | 8 F | 10 M | P > 0.10 | |
| Age | 37.4 | 11.2 | 38.9 | 11.1 | P > 0.10 | −0.24 |
| Education | 13.6 | 2.1 | 12.7 | 2.5 | P > 0.10 | 0.41 |
| HQ | 77.4 | 47.1 | 94.1 | 9.8 | P > 0.10 | −0.50 |
| WTAR | 48.9 | 8.7 | 48.4 | 9.0 | P > 0.10 | 0.06 |
| Baseline Clinical Measures | ||||||
| Cocaine Usea | 38.7 | 21.6 | 38.1 | 21.6 | P > 0.10 | 0.03 |
| CCQ-Ga | 34.6 | 16.9 | 41.7 | 17.9 | P > 0.10 | −0.42 |
| CSSAa | 25.2 | 17.6 | 36.0 | 21.2 | P > 0.10 | −0.57 |
| BIS | 71.6 | 13.9 | 79.7 | 13.8 | P = 0.09 | −0.60 |
| AUDIT | 12.5 | 9.6 | 10.5 | 8.9 | P > 0.10 | 0.23 |
| FTND | 2.0 | 2.5 | 1.9 | 2.3 | P > 0.10 | 0.05 |
| BDI-II | 57.4 | 10.2 | 60.5 | 12.6 | P > 0.10 | −0.27 |
| STAI-T | 62.7 | 14.2 | 67.4 | 16. 3 | P > 0.10 | −0.31 |
Notes:
indicates that data was derived from Visit 2. Percent days of cocaine use was normalized over baseline period.
ABMT = Attentional Bias Modification Therapy; AUDIT = Alcohol Use Disorders Identification Test; BDI-II = Beck Depression Inventory; BIS = Barrett Impulsiveness Scale; CCQ-G = Cocaine Craving Questionnaire (Brief-General); CSSA = Cocaine Selective Severity Assessment; FTND = Fagerstrom Test for Nicotine Dependence ; HQ = handedness quotient; STAI-T = State-Trait Anxiety Inventory (Trait); WTAR = Wechsler Test of Adult Reading.
ABMT Task
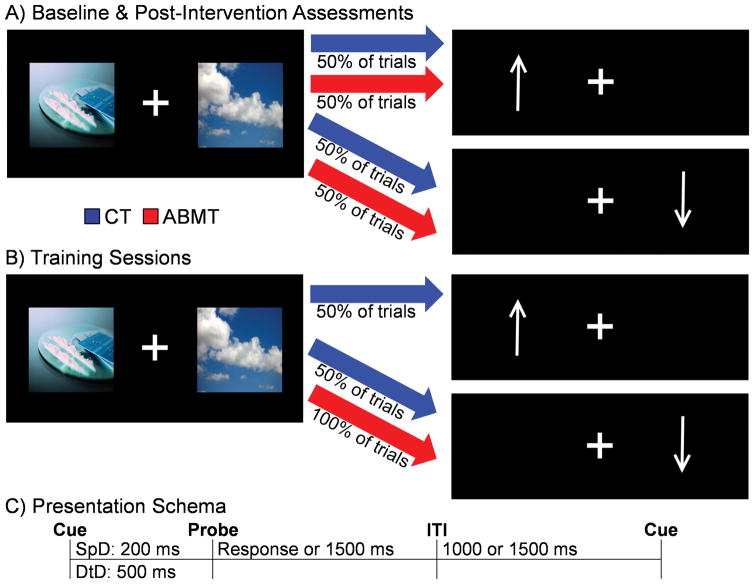
The current task was largely adapted from previous ABMT studies (21). During baseline, all participants were presented with picture pairs (Figure 1A) depicting cocaine/neutral stimulus (96 trials) or a neutral/neutral stimulus pairs (40 trials) using Presentation software. As depicted in Figure 1, cocaine and neutral stimuli were equivalent in size and visual angle, and generally matched for overall color and content. Stimuli were pseudo-randomly presented on either side of a fixation cross, with a total of 12 unique cocaine/neutral stimulus pairs (presented 8 times) and 16 unique neutral/neutral stimulus pairs. The stimulus pairs were displayed (Figure 1C) for either 200 (speed detection trials) or 500 (difficulty to disengage trials) ms at equal frequencies. The stimulus pairs were immediately replaced by a probe (arrow). The longer duration permitted the purposeful allocation of attentional resources to the cue’s spatial location (46). Participants classified the direction of the probe as quickly as possible with their right hand (index finger = up; middle finger = down). Attentional bias was measured for each stimulus duration by subtracting the median response time for cocaine trials (e.g., probe replaced cocaine stimulus) from neutral trials.
Fig. I. Attentional Bias Modification Trial Distribution and Presentation.
This figure illustrates the frequency with which a visual probe replaced either cocaine-related pictures or neutral pictures in the control (CT; blue) and attentional bias modification therapy (ABMT; red) conditions for both the pre- and post- intervention measures (Panel A) and during training sessions (Panel B). Cue pictures were presented (Panel C) for either 200 ms (speeded detection: SpD) or 500 ms (difficulty to disengage: DtD), with a response window of 1500 ms following cue presentation and a variable inter-trial interval (ITI).
There were a total of 5 intervention sessions (Figure 1B) during which participants were exposed to 36 novel cocaine/neutral pairs. Neutral/neutral pairs were eliminated. ABMT participants were instructed that probes would replace neutral stimuli on 100% of the trials (200 or 500 ms stimulus durations). Control therapy participants were instructed that the probe would replace either the cocaine or neutral pictures with equal likelihood. Although other control tasks have been utilized, the current task provided stringent control for stimulus exposure and manipulating attentional bias to cocaine stimuli. To increase task difficulty and participant engagement, some trial blocks presented three pictures simultaneously (two identical pictures). Each intervention training task consisted of 24 picture pairs repeated 22 times over seven blocks, totaling 2640 cumulative training trials. Post-task performance feedback was given, with goals set for the next session. The post-intervention session was identical to baseline with the exception of 12 novel neutral/cocaine stimulus pairs.
Behavioral Analyses
Median response time reduced positive skew in response time data. Anticipatory (within 100 ms of stimulus onset) and incorrect trials were excluded from all median calculations. On the AB task, there were no response time outliers (greater than 3 times the interquartile range) for either pre- or post-intervention measures, and accuracy was above chance levels for all post-intervention variables. One participant was excluded due to below-chance (below 70.8% based on binomial distribution) performance on more than one baseline variable. For all behavioral and clinical data, normality was first assessed with the Shapiro-Wilk test. Two different covariance matrices (compound symmetry and a first-order autoregressive model) were tested for best fit in linear mixed models using Akaike Information Criteria.
Results
Baseline Clinical and AB Data
There were no significant differences between ABMT and control therapy participants (p > 0.10) on major demographic variables at baseline (Table 2). There were no significant (p > 0.05) baseline differences between groups in terms of normalized cocaine usage, positive cocaine screens, cocaine craving, cocaine withdrawal symptoms, severe drinking, depression, impulsivity, or anxiety (Table 2; Figure 2). Effect sizes (as determined by ABMT - control) are presented in Table 2 to minimize power concerns. There were no group differences (p > 0.05) between control and ABMT participants on baseline measures of attentional bias for response time (Cohen’s d range 0.11–0.23) or accuracy.
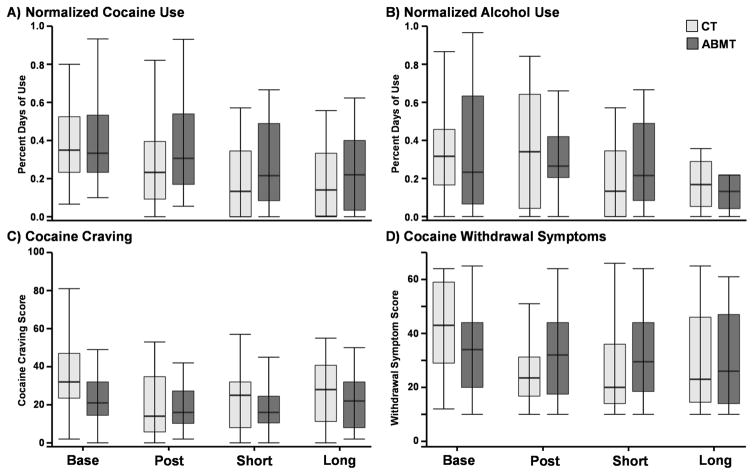
Fig. II. Substance Use Outcome Measures at Baseline, Post-Intervention, and Follow-ups.
This figure presents substance use data acquired at baseline and post-intervention, as well as at short (2 weeks) and long-term (3 months) follow-up. Box and whisker plots are used to present data from the control (CT; light grey) and attentional bias modification therapy (ABMT; dark grey) groups for normalized cocaine (Panel A) and alcohol (Panel B) usage over a 30 day period before intervention and in the interim between measures subsequently. Panel C presents data on a measure of general level of cocaine craving in the previous week. Panel D presents data on a measure of severity of cocaine withdrawal symptoms within the past 24-hour period
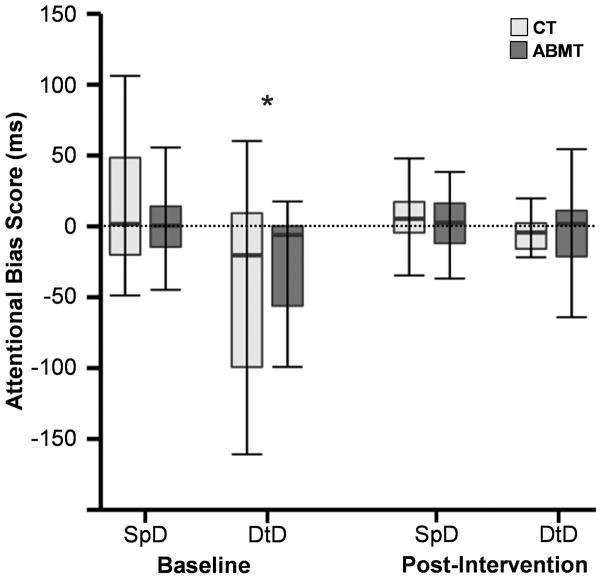
We next examined for bias at baseline (one-sample t-tests) across both groups, and potential relationships with cocaine usage, craving and withdrawal symptoms. Results indicated that response times were slower for cocaine relative to neutral trials (i.e., interference) at the 500 ms duration (t36 = −3.7; p = 0.001), opposite of the hypothesized direction (Figure 3). Multiple regression indicated that the degree of interference was not significantly (p > 0.10) associated with measures of craving, withdrawal or cocaine usage for the bias at the 500 ms stimulus duration.
Fig. III. Attentional Bias Differences Between Groups.
This figure presents attentional bias data for control (CT; light grey) and attentional bias modification therapy (ABMT; dark grey) patients at baseline and post-intervention. Attentional bias was measured by subtracting response times in trials where the probe replaced cocaine-related pictures from trials where a probe replaced neutral pictures. Box and whisker plots are used to present data for probes presented at 200 ms stimulus duration (SpD: speeded response) and 500 ms stimulus duration (DtD: difficulty to disengage). An asterisk denotes the presence of significant bias (here interference), measured as deviation from zero (dotted line)
Intervention
Participants in both the ABMT (50% correct) and control therapy (57.1% correct) groups performed at chance levels (p > 0.10) when guessing their group assignment during the post-intervention session. There were no differences between ABMT and control therapy participants in terms of overall accuracy (Mann-Whitney U tests) or response times ([Group (ABMT vs. control) × Novel (repeated vs. novel)] mixed-effect models ANOVAs) for either novel or repeated trials (p > 0.10; Cohen’s d range −0.18–0.48). All participants were significantly (p = 0.005) less accurate for novel (95.3%) relative to repeated (99.0%) cocaine trials at the 500 ms stimulus duration. A statistical trend for stimulus novelty (F1,32 = 3.1; p = 0.09) was observed for the 200 ms trials, with a different pattern of reaction times between neutral and cocaine stimuli for novel (mean = 17.4 ms) relative to repeated (mean = −10.0 ms) trials. As a result of the non-significant group effects (ABMT vs. control), results were collapsed across novelty for all subsequent analyses.
Two 2 × 2 [Group (ABMT vs. control) × Time (Pre- vs. Post-intervention)] linear mixed effect models examined whether ABMT affected attentional bias relative to baseline for the speeded detection or difficult to disengage trials. However, neither the main effects nor the interaction were significant (p > 0.10), with small post-intervention group effect sizes (Cohen’s d range −0.20–0.02). Data were therefore collapsed across groups, followed by one-sample t-tests to determine whether differences in reaction time were still present post-intervention. However, results indicated that there were no differences (p > 0.10) between cocaine and neutral stimuli for either trials post-intervention.
Chi-square analyses examined for group differences in positive urine screens for cocaine. There was a trend difference for more positive urine screens in the ABMT relative to control cohort at short-term follow-up (X2N=33 = 3.64, p=.056; ABMT=66.7%, control=33.3%), with no significant group differences immediately post-intervention or at the long-term follow-up visit (both p’s > 0.10). 2 × 4 [Group (ABMT vs. control) × Time (Baseline, Post-intervention, Short-Term Follow-up and Long-Term Follow-up)] linear mixed effect models examined whether intervention type (ABMT vs. control) affected cocaine use, cocaine craving, cocaine withdrawal symptoms, or alcohol consumption. A first order auto-regressive covariance structure provided the best fit for cocaine use, whereas the overall model fit for all other dependent variables was superior using compound symmetry.
The main effect of group was not significant (p > 0.10; Cohen’s d range for group differences at all visits −0.35–0.42) for all cocaine variables (Figure 2). The main effect of time was significant for normalized cocaine use (F3,90.8 = 4.7; p = 0.004), cocaine craving symptoms (F3,93.9 = 5.8; p = 0.001) and cocaine withdrawal symptoms (F3,93.6 = 4.5; p = 0.005). Pairwise comparisons indicated reductions in cocaine use, craving and withdrawal symptoms between baseline and all three follow-up assessments (all p’s < 0.05), with the exception of statistical trends for two of the intervals (cocaine use during intervention p = 0.08; long-term withdrawal symptoms p = 0.07). A significant Group × Time interaction was also present for cocaine craving (F3,93.9 = 3.2; p = 0.028), but simple effects tests comparing ABMT and control groups at each of the three follow-up visits were not significant (p > 0.10).
The linear mixed effect analysis of normalized alcohol consumption also resulted in a significant effect of time (F3,93.9 = 3.0; p = 0.034), with trends for decreased usage at short (p = 0.06) and long-term (p = 0.09) follow-up.
A multiple regression analysis examined whether the decrease in cocaine craving, withdrawal symptoms and use from the baseline to post-intervention was associated with changes in reaction time between cocaine and neutral trials. However, results from this analysis were also negative (p > 0.10).
Discussion
The current experiment examined the degree of attentional bias during a visual probe task in a cohort of treatment-seeking CUD at baseline and whether bias or drug-use behaviors were affected by ABMT. Contrary to a priori predictions, results indicated that interference (i.e., slower response time) rather than facilitation was present for visual probes following cocaine stimuli. However, the degree of interference was not related to other clinical measures. Following intervention, participants in both groups responded more accurately to repeated relative to novel stimuli. Participants in both groups (ABMT and control) reported decreased use and craving as a function of progression through treatment, but this was not driven by a specific therapeutic mechanism that could be directly linked to ABMT.
The randomization process was effective, with no observable baseline differences in clinical variables, demographic variables or degree of attentional bias for cocaine stimuli. Participants in both groups also performed at chance levels when guessing to which therapeutic arm they had been assigned. Similar to previous studies in CUD (26), reaction times were significantly slower for probes that replaced cocaine relative to neutral stimuli at the 500 ms stimulus duration (i.e., interference) during the baseline assessment. In contrast, studies involving other SUD (21–25) typically report faster reaction times for drug stimuli (i.e., facilitation) during visual probe tasks either at baseline or following training. Current and previous (26) findings of increased interference rather than facilitation for cocaine cues has multiple potential explanations. Foremost, participants may have intentionally shifted their attention away from cocaine stimuli due to their desire to reduce cocaine usage (i.e., treatment-seeking). In support of this, previous research (16) has reported greater interference using a cocaine Stroop task in treatment-seeking CUD relative to both controls and CUD not seeking treatment. Second, CUD use various forms of cocaine (snorted, smoked, etc.), and not all stimuli may have resulted in drug-related bias for each participant based on their preferred routes of administration. We controlled for this by including stimuli that depicted all routes of administration.
Collectively, findings of interference in two CUD studies imply that there may be something unique about either cocaine stimuli or CUD. Since alcohol is a legal (and therefore more socially acceptable) drug, it may not induce the same degree of aversion or approach-avoidance conflict as cocaine stimuli, even in individuals who use the drug. Similarly, ABMT may be more effective for drugs that are used on a more frequent basis (e.g., daily such as alcohol and cigarettes) rather than somewhat infrequently. Finally, previous studies in other SUD (22, 47) have compared bias between patient and control groups rather than between two patient groups. As previously noted (3), interactions between paradigm parameters (e.g., stimulus duration) and diagnostic status (CUD versus healthy control) exist during visual probe tasks, which would also influence outcomes.
Contrary to baseline findings, there was no significant evidence of interference from cocaine stimuli at the post-intervention stage. However, this decrease in interference was not linked to a specific intervention type (ABMT vs. control), as both groups showed similar evidence of reduced interference. Previous studies have also reported a decrease in attentional bias following ABMT, although these studies have typically been in other SUD (primarily alcohol) and also reported facilitation for drug stimuli at baseline (18, 20, 21, 28). An important consideration between current and previous studies (21) is the control task that was employed. Attentional bias is purportedly reduced during ABMT by consistently having the probe replace the neutral stimuli on 100% of trials. Our selected rate (50% of trials) for control therapy should therefore not have affected native bias while simultaneously controlling for secondary task-related effects such as stimulus exposure and habituation, providing the most rigorous test of the efficacy of ABMT. However, others have argued that this task design confounds ABMT by both affecting attentional control and contaminating the habitual bias for drug stimuli (21). Thus, further work is needed investigating the role of control therapies to rule-out non-specific effects of ABMT.
A strength of the current study was the high retention rate (83.8%), which permitted the assessment of more long-term (i.e., 3-month) changes in drug use behaviors. ABMT has been shown to result in longer relapse times relative to those in control training in AUD (21), and in at-risk drinkers, ABMT has been shown to reduced drinking quantities (48). Attentional bias has also been linked to outcome in other substance abusing populations (7, 8). In the current study, there was a general reduction in cocaine use, cocaine craving, and cocaine withdrawal symptoms following both interventions that persisted into short (2 weeks) and long-term (3 months) follow-up. However, this reduction was also not significantly different across the two treatment groups. These findings are similar to a recent study in AUD, which also indicated that a sham therapy was as efficacious as various cognitive bias modification therapies provided over the internet in reducing substance use (30).
Other studies have demonstrated an effect of ABMT on reducing bias but ultimately not significantly altering the degree of substance use relative to other control conditions (49, 50). General reductions in drug-related behaviors may be secondary to assessment reactivity, due to the repeated exposure to cocaine stimuli, or due to participants’ inherent desire to reduce their use (51–54). Results from previous studies are mixed on the relationship between increased interference from cocaine cues during Stroop tasks and craving (14, 17), as well as between interference and impulsivity (correlated: 11, uncorrelated: 16). Similarly in CUD, increased interference during a Stroop task has been associated with both worse treatment outcomes (7: more positive urine screens, shorter treatment program retention) and better outcomes (9: fewer long-term positive screens and longer program retention). In the current study, neither the degree of interference from cocaine stimuli at baseline nor the change in reaction times as a function of intervention were related to other drug-use behaviors such as craving, usage or severity of withdrawal symptoms.
Several limitations of the current experiment should be noted. Foremost, our sample sizes were relatively modest, which may have limited power. Effect sizes are therefore provided for all primary variables of interest, with all effects falling in the small to medium range (Cohen’s d between −0.35 and 0.48). Our sample sizes were also powered based on previous positive ABMT studies in other SUD, which used a similar number of participants (19, 21). Second, participants underwent a single session of motivational interviewing for ethical reasons (i.e., non-treatment), which may have affected the amount of attentional bias present in both groups. Although the initial assessment of bias occurred approximately 10 days following the motivational interviewing session (30 minutes) both past (55) and current (56) treatments have been found to be associated with attentional avoidance in AUD. Thus, although unlikely (57), it is possible that a single session of motivational interviewing influenced both baseline measures of bias and washed out the therapeutic effects of ABMT relative to control therapy. Importantly, future studies are needed to determine how different motivational therapies may interact with ABMT (58). Previous research has reported ABMT efficacy with five (21) or fewer (19) brief interventions. However, five sessions of ABMT may be insufficient in CUD, as efficacy generally increases with the number of treatment sessions (59). Finally, the current experiment did not assess motivation for change, which has been shown to be an important determinant for various outcome measures (60) and could also affect attentional bias.
In summary, there are multiple methods for assaying the degree of bias towards drug stimuli in CUD, including the visual probe task. Similar to other published studies in CUD (26), current results indicated evidence of interference rather than facilitation for cocaine probes at baseline. However, the degree of bias did not appear to be related to any other measured drug-use behaviors either at baseline or as a function of treatment in our sample. Moreover, ABMT did not appear to alter attentional bias relative to a robust control condition, which suggests that trainings targeting attentional bias would likely not succeed in large-scale studies of CUD. Recent studies highlight the role of symptom severity (27, 61), disease characteristics (59), presence of external stressors (59) and difficulty in disambiguating cognitive constructs (27) in determining the true efficacy of ABMT across various clinical samples. Thus, more work is needed to establish the ecological validity and inherent variability of attentional bias in treatment-seeking CUD specifically and how treatment generalizes to other drug-use behaviors (27), as has recently been done with other psychological constructs (62).
Acknowledgments
We would like to thank Cathy Smith and Diana South for their assistance with data collection, and Marion Cook for her help with the literature search.
This research was supported by grants NIH 01 R21 DA031380 from the National Institute of Drug Abuse to Andrew Mayer.
Footnotes
Declaration of Interest:
The authors report no conflicts of interest.
References
- 1.Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33(2):191–206. doi: 10.1080/00952990701199416. [DOI] [PubMed] [Google Scholar]
- 2.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 3.Field M, Cox WM. Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97(1–2):1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 4.Franken IH, van Strien JW, Franzek EJ, van de Wetering BJ. Error-processing deficits in patients with cocaine dependence. Biol Psychol. 2007;75(1):45–51. doi: 10.1016/j.biopsycho.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Wiers RW, Rinck M, Kordts R, Houben K, Strack F. Retraining automatic action-tendencies to approach alcohol in hazardous drinkers. Addiction. 2010;105(2):279–287. doi: 10.1111/j.1360-0443.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- 6.Wiers RW, Stacy AW. Implicit cognition and addiction. Curr Dir Psychol Sci. 2006;15(6):292–296. [Google Scholar]
- 7.Carpenter KM, Schreiber E, Church S, McDowell D. Drug Stroop performance: Relationships with primary substance of use and treatment outcome in a drug-dependent outpatient sample. Addict Behav. 2006;31(1):174–181. doi: 10.1016/j.addbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Cox WM, Hogan LM, Kristian MR, Race JH. Alcohol attentional bias as a predictor of alcohol abusers’ treatment outcome. Drug Alcohol Depend. 2002;68(3):237–243. doi: 10.1016/s0376-8716(02)00219-3. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter KM, Martinez D, Vadhan NP, Barnes-Holmes D, Nunes EV. Measures of attentional bias and relational responding are associated with behavioral treatment outcome for cocaine dependence. Am J Drug Alcohol Abuse. 2012;38(2):146–154. doi: 10.3109/00952990.2011.643986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox WM, Fadardi JS, Pothos EM. The addiction-stroop test: Theoretical considerations and procedural recommendations. Psychol Bull. 2006;132(3):443–476. doi: 10.1037/0033-2909.132.3.443. [DOI] [PubMed] [Google Scholar]
- 11.Field M, Christiansen P, Cole J, Goudie A. Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction. 2007;102(4):579–586. doi: 10.1111/j.1360-0443.2007.01743.x. [DOI] [PubMed] [Google Scholar]
- 12.Vadhan NP, Carpenter KM, Copersino ML, Hart CL, Foltin RW, Nunes EV. Attentional bias towards cocaine-related stimuli: Relationship to treatment-seeking for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33(5):727–736. doi: 10.1080/00952990701523722. [DOI] [PubMed] [Google Scholar]
- 13.Waters AJ, Marhe R, Franken IH. Attentional bias to drug cues is elevated before and during temptations to use heroin and cocaine. Psychopharmacology (Berl) 2012;219(3):909–921. doi: 10.1007/s00213-011-2424-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Copersino ML, Serper MR, Vadhan N, Goldberg BR, Richarme D, Chou JC, Stitzer M, et al. Cocaine craving and attentional bias in cocaine-dependent schizophrenic patients. Psychiatry Res. 2004;128(3):209–218. doi: 10.1016/j.psychres.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Hester R, Dixon V, Garavan H. A consistent attentional bias for drug-related material in active cocaine users across word and picture versions of the emotional Stroop task. Drug Alcohol Depend. 2006;81(3):251–257. doi: 10.1016/j.drugalcdep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Lane SD, Schmitz JM, Waters AJ, Cunningham KA, Moeller FG. Relationship between attentional bias to cocaine-related stimuli and impulsivity in cocaine-dependent subjects. Am J Drug Alcohol Abuse. 2011;37(2):117–122. doi: 10.3109/00952990.2010.543204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franken IH, Kroon LY, Hendriks VM. Influence of individual differences in craving and obsessive cocaine thoughts on attentional processes in cocaine abuse patients. Addict Behav. 2000;25(1):99–102. doi: 10.1016/s0306-4603(98)00112-9. [DOI] [PubMed] [Google Scholar]
- 18.Field M. Cannabis ‘dependence’ and attentional bias for cannabis-related words. Behav Pharmacol. 2005;16(5–6):473–476. doi: 10.1097/00008877-200509000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Field M, Duka T, Eastwood B, Child R, Santarcangelo M, Gayton M. Experimental manipulation of attentional biases in heavy drinkers: do the effects generalise? Psychopharmacology (Berl) 2007;192(4):593–608. doi: 10.1007/s00213-007-0760-9. [DOI] [PubMed] [Google Scholar]
- 20.Schoenmakers T, Wiers RW, Jones BT, Bruce G, Jansen AT. Attentional re-training decreases attentional bias in heavy drinkers without generalization. Addiction. 2007;102(3):399–405. doi: 10.1111/j.1360-0443.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- 21.Schoenmakers TM, de Bruin M, Lux IF, Goertz AG, Van Kerkhof DH, Wiers RW. Clinical effectiveness of attentional bias modification training in abstinent alcoholic patients. Drug Alcohol Depend. 2010;109(1–3):30–36. doi: 10.1016/j.drugalcdep.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Bradley B, Field M, Mogg K, De HJ. Attentional and evaluative biases for smoking cues in nicotine dependence: Component processes of biases in visual orienting. Behav Pharmacol. 2004;15(1):29–36. doi: 10.1097/00008877-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Ehrman RN, Robbins SJ, Bromwell MA, Lankford ME, Monterosso JR, O’Brien CP. Comparing attentional bias to smoking cues in current smokers, former smokers, and non-smokers using a dot-probe task. Drug Alcohol Depend. 2002;67(2):185–191. doi: 10.1016/s0376-8716(02)00065-0. [DOI] [PubMed] [Google Scholar]
- 24.Field M, Mogg K, Zetteler J, Bradley BP. Attentional biases for alcohol cues in heavy and light social drinkers: the roles of initial orienting and maintained attention. Psychopharmacology (Berl) 2004;176(1):88–93. doi: 10.1007/s00213-004-1855-1. [DOI] [PubMed] [Google Scholar]
- 25.Waters AJ, Shiffman S, Bradley BP, Mogg K. Attentional shifts to smoking cues in smokers. Addiction. 2003;98(10):1409–1417. doi: 10.1046/j.1360-0443.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery C, Field M, Atkinson AM, Cole JC, Goudie AJ, Sumnall HR. Effects of alcohol preload on attentional bias towards cocaine-related cues. Psychopharmacology (Berl) 2010;210(3):365–375. doi: 10.1007/s00213-010-1830-y. [DOI] [PubMed] [Google Scholar]
- 27.Wiers RW, Gladwin TE, Hofmann W, Salemink E, Ridderinkhof KR. Cognitive bias modification and cognitive control training in addiction and related psychopathology mechanisms, clinical perspectives, and ways forward. Clin Psychol Science. 2013;1(2):192–212. [Google Scholar]
- 28.Field M, Munafo MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull. 2009;135(4):589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fadardi JS, Cox WM. Reversing the sequence: reducing alcohol consumption by overcoming alcohol attentional bias. Drug Alcohol Depend. 2009;101(3):137–145. doi: 10.1016/j.drugalcdep.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Wiers RW, Houben K, Fadardi JS, van BP, Rhemtulla M, Cox WM. Alcohol cognitive bias modification training for problem drinkers over the web. Addict Behav. 2015;40:21–26. doi: 10.1016/j.addbeh.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Stein MD, Herman DS, Anderson BJ. A motivational intervention trial to reduce cocaine use. J Subst Abuse Treat. 2009;36(1):118–125. doi: 10.1016/j.jsat.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Carroll KM, Ball SA, Nich C, Martino S, Frankforter TL, Farentinos C, Kunkel LE, et al. Motivational interviewing to improve treatment engagement and outcome in individuals seeking treatment for substance abuse: a multisite effectiveness study. Drug Alcohol Depend. 2006;81(3):301–312. doi: 10.1016/j.drugalcdep.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 34.Reinert DF, Allen JP. The alcohol use disorders identification test: an update of research findings. Alcohol Clin Exp Res. 2007;31(2):185–199. doi: 10.1111/j.1530-0277.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- 35.Heinz AJ, Epstein DH, Schroeder JR, Singleton EG, Heishman SJ, Preston KL. Heroin and cocaine craving and use during treatment: measurement validation and potential relationships. J Subst Abuse Treat. 2006;31(4):355–364. doi: 10.1016/j.jsat.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Kampman KM, Volpicelli JR, Mulvaney F, Rukstalis M, Alterman AI, Pettinati H, Weinrieb RM, et al. Cocaine withdrawal severity and urine toxicology results from treatment entry predict outcome in medication trials for cocaine dependence. Addict Behav. 2002;27(2):251–260. doi: 10.1016/s0306-4603(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 37.Kaye S, Darke S. Determining a diagnostic cut-off on the Severity of Dependence Scale (SDS) for cocaine dependence. Addiction. 2002;97(6):727–731. doi: 10.1046/j.1360-0443.2002.00121.x. [DOI] [PubMed] [Google Scholar]
- 38.First MB, Spitzer RL, Gibbon M, Williams JB. Administration Booklet. American Psychiatric Pub; 2012. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version. [Google Scholar]
- 39.Sobell LC, Sobell MB. Timeline followback user’s guide: A calendar method for assessing alcohol and drug use. Toronto: Addiction Research Foundation; 1996. [Google Scholar]
- 40.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 41.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Cognitive Neuropsychology. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 42.Richter P, Werner J, Heerlein A, Kraus A, Sauer H. On the validity of the Beck Depression Inventory. A review. Psychopathology. 1998;31(3):160–168. doi: 10.1159/000066239. [DOI] [PubMed] [Google Scholar]
- 43.Spielberger CD, Ritterband LM, Sydeman SJ, Reheiser EC, Unger KK. Assessment of emotional states and personality traits: Measuring psychological vital signs. Clinical personality assessment: Practical approaches. 1995;42:59. [Google Scholar]
- 44.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory. 1970. [Google Scholar]
- 45.Wechsler D. Wechsler Test of Adult Reading: WTAR. Psychological Corporation; 2001. [Google Scholar]
- 46.Mayer AR, Dorflinger JM, Rao SM, Seidenberg M. Neural networks underlying endogenous and exogenous visual-spatial orienting. Neuroimage. 2004;23(2):534–541. doi: 10.1016/j.neuroimage.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 47.Bradley BP, Mogg K, Wright T, Field M. Attentional bias in drug dependence: Vigilance for cigarette-related cues in smokers. Psychol Addict Behav. 2003;17(1):66–72. doi: 10.1037/0893-164x.17.1.66. [DOI] [PubMed] [Google Scholar]
- 48.McGeary JE, Meadows SP, Amir N, Gibb BE. Computer-delivered, home-based, attentional retraining reduces drinking behavior in heavy drinkers. Psychol Addict Behav. 2014;28(2):559–562. doi: 10.1037/a0036086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kerst WF, Waters AJ. Attentional retraining administered in the field reduces smokers’ attentional bias and craving. Health Psychol. 2014 doi: 10.1037/a0035708. [DOI] [PubMed] [Google Scholar]
- 50.Lopes FM, Pires AV, Bizarro L. Attentional bias modification in smokers trying to quit: a longitudinal study about the effects of number of sessions. J Subst Abuse Treat. 2014;47(1):50–57. doi: 10.1016/j.jsat.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Clifford PR, Maisto SA. Subject reactivity effects and alcohol treatment outcome research. J Stud Alcohol. 2000;61(6):787–793. doi: 10.15288/jsa.2000.61.787. [DOI] [PubMed] [Google Scholar]
- 52.Clifford PR, Maisto SA, Davis CM. Alcohol treatment research assessment exposure subject reactivity effects: part I. Alcohol use and related consequences. J Stud Alcohol Drugs. 2007;68(4):519–528. doi: 10.15288/jsad.2007.68.519. [DOI] [PubMed] [Google Scholar]
- 53.Donovan DM, Bogenschutz MP, Perl H, Forcehimes A, Adinoff B, Mandler R, Oden N, et al. Study design to examine the potential role of assessment reactivity in the Screening, Motivational Assessment, Referral, and Treatment in Emergency Departments (SMART-ED) protocol. Addict Sci Clin Pract. 2012;7(1):16. doi: 10.1186/1940-0640-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maisto SA, Clifford PR, Davis CM. Alcohol treatment research assessment exposure subject reactivity effects: part II. Treatment engagement and involvement. J Stud Alcohol Drugs. 2007;68(4):529–533. doi: 10.15288/jsad.2007.68.529. [DOI] [PubMed] [Google Scholar]
- 55.Noel X, Colmant M, Van Der Linden M, Bechara A, Bullens Q, Hanak C, Verbanck P. Time course of attention for alcohol cues in abstinent alcoholic patients: the role of initial orienting. Alcohol Clin Exp Res. 2006;30(11):1871–1877. doi: 10.1111/j.1530-0277.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 56.Townshend JM, Duka T. Avoidance of alcohol-related stimuli in alcohol-dependent inpatients. Alcohol Clin Exp Res. 2007;31(8):1349–1357. doi: 10.1111/j.1530-0277.2007.00429.x. [DOI] [PubMed] [Google Scholar]
- 57.Saitz R, Palfai TP, Cheng DM, Alford DP, Bernstein JA, Lloyd-Travaglini CA, Meli SM, et al. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA. 2014;312(5):502–513. doi: 10.1001/jama.2014.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boffo M, Pronk T, Wiers RW, Mannarini S. Combining cognitive bias modification training with motivational support in alcohol dependent outpatients: study protocol for a randomised controlled trial. Trials. 2015;16:63. doi: 10.1186/s13063-015-0576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychol Bull. 2011;137(6):940–958. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- 60.DiClemente CC, Bellino LE, Neavins TM. Motivation for change and alcoholism treatment. Alcohol Res Health. 1999;23(2):86–92. [PMC free article] [PubMed] [Google Scholar]
- 61.Emmelkamp PM. Attention bias modification: the Emperor’s new suit? BMC Med. 2012;10:63. doi: 10.1186/1741-7015-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Open Science Collaboration. Estimating the reproducibility of psychological science. Psychobiology. 2015;349(6251):aac4716. doi: 10.1126/science.aac4716. [DOI] [PubMed] [Google Scholar]