Abstract
Vascularization of large bone grafts is one of the main challenges of bone tissue engineering (BTE), and has held back the clinical translation of engineered bone constructs for two decades so far. The ultimate goal of vascularized BTE constructs is to provide a bone environment rich in functional vascular networks to achieve efficient osseointegration and accelerate restoration of function after implantation. To attain both structural and vascular integration of the grafts, a large number of biomaterials, cells, and biological cues have been evaluated. This review will present biological considerations for bone function restoration, contemporary approaches for clinical salvage of large bone defects and their limitations, state-of-the-art research on the development of vascularized bone constructs, and perspectives on evaluating and implementing novel BTE grafts in clinical practice. Success will depend on achieving full graft integration at multiple hierarchical levels, both between the individual graft components as well as between the implanted constructs and their surrounding host tissues. The paradigm of vascularized tissue constructs could not only revolutionize the progress of bone tissue engineering, but could also be readily applied to other fields in regenerative medicine for the development of new innovative vascularized tissue designs.
Keywords: vascularized bone scaffolds, vascular grafts, osseointegration, angiogenesis, anastomosis
Introduction
Current medical practice still faces significant challenges in treating large bone defects caused by trauma or disease. Developing bone grafts that can restore vascular function to the regenerating bone tissue has arguably been the most difficult aspect to address.104 The main causes of large graft failure are inner graft necrosis and lack of integration with the host tissue. Host tissue remodeling capabilities for severely damaged vascular beds are limited,115 and integrating a fully functional vasculature deep inside bone grafts is technically and biologically challenging. Incomplete and inhomogeneous graft viability is therefore a problem, and generally results in premature failure of the implanted constructs.
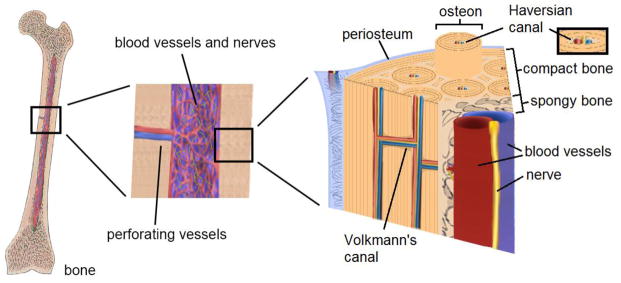
Bone is a very complex tissue with multiple intricate hierarchical architectures (Figure 1). The innermost region consists of cancellous bone tissue, which is comprised of a highly porous layer of rigid struts (trabeculae), red marrow tissue (site of blood cell formation) and blood vessels. Surrounding the cancellous bone is dense cortical bone, which has mechanical properties that can be up to two orders of magnitude greater than those of the inner trabecular region.5 Enveloping the outside of the bone structure is the periosteum, a thin highly vascularized membrane that contains the osteoblast precursor cells responsible for forming new bone tissue. The basic units of cortical bone are layered cylindrical structures called osteons, which are joined together by interstitial lamellae. Vascular networks spread throughout the cortical bone structure via transverse (Volkmann) and longitudinal (Haversian) canals.16, 77 Several specialized cell types such as osteoblasts and osteoclasts are interspersed within the tissue in order to carry out the biological processes necessary for bone remodeling and homeostasis. At the microscale, cells and vascular networks are separated by distances ranging from 100 to 300 μm,25, 33, 97, 125 which guarantees effective nutrient supply and waste removal to individual cells.
Figure 1.
Schematic of the bone structure. Bone is highly vascularized, from the intramedullary cavity to the periosteal mineral matrix.
All bones share common constituents: an extracellular matrix (ECM), signaling factors, and bone cells.12 Bone ECM is an organic and inorganic composite framework that gives the tissue its mechanical strength and toughness and provides a surface for new bone deposition. Molecular signaling factors included in the ECM actively promote the differentiation of precursor or progenitor cells into functional bone cells. These bone cells (osteoblasts, osteoclasts, and osteocytes) in turn regulate the formation and remodeling of the other components of bone. Thus, natural bone is considered to be osteoconductive, osteoinductive, and osteogenic.3
Any graft intended to repair or regenerate bone should also express one or more of these properties.8 As a result, bone tissue engineering (BTE) has traditionally focused on combining osteoconductive scaffolds, osteoinductive growth factors, and osteogenic precursor cells or osteoblasts to repair and regenerate bone. It is worth noting that current BTE efforts are not developed in parallel with the innovations in vascular tissue engineering, while state-of-the-art vascular substitutes are not designed for bone applications. This severe disjunction between bone and vascular tissue engineering overlooks the inherent importance of well-developed vascular beds in the skeletal system. As a fact, nutrient and waste exchange between individual cells and capillary vessels in bone is limited to distances of 100 to 300 μm.25, 33, 97, 125 Because designing around this diffusion limit is vital for graft success, vascularization is arguably the greatest challenge in tissue engineering and regenerative medicine, including BTE. In addition, the difference between BTE and other tissue engineering fields is that the former requires integration between components having multiple order-of-magnitude differences in mechanical properties. To date, limited successes have been seen in clinical implementation of BTE approaches because of the challenge of reproducibly achieving hierarchical integration of the graft components, from cell-cell interactions to macroscale incorporation into the host tissue. In particular, vasculature repair and integration must be achieved at multiple levels: larger perfusable blood vessel grafts are needed for restoration of blood flow into the site of injury, while smaller microvascular beds are needed to provide thorough distribution of blood across the entire scaffold volume, sustaining osteogenesis and osseointegration until bone function is restored (Figure 2). In fact, the consensus through all tissue engineering fields is that improved vascularization is at the crux of all future scaffold designs.36, 47, 61
Figure 2.
Hierarchical levels in bone vasculature. Large vessels branch out internally into smaller capillary units to perfuse blood throughout the scaffold.
We83, 91 and others58, 90 have already addressed the necessity to target the limitations in current approaches for BTE, but here we emphasize integrative strategies for complete restoration of bone function. We will examine current surgical treatments for large segmental bone defects, BTE approaches for the design of synthetic vascularized bone grafts, strategies for translational implementation of these constructs into clinical practice, and future perspectives on vascular BTE.
Current surgical approaches to vascularized bone repair
Annually, about 2.2 million bone graft procedures are performed around the world, with more than half a million in the United States alone, at a cost surpassing $2 billion.76 Currently, in clinical settings, there are three types of bone grafts used to repair defects in long bones: synthetic bone void fillers, allografts, and autografts.124 Synthetic fillers can provide an osteoconductive scaffold into which bone depositing cells migrate and deposit new bone tissue.53 Allografts are made of decellularized bone tissue, harvested from other human donors, that still possess ECM and their associated growth factors that are able to recruit host bone cells and stimulate bone deposition.30 As such, allografts demonstrate osteoinductivity in addition to the osteoconductivity displayed by synthetic fillers. But the gold standard of bone repair is autografting,106 which involves removing a section of the patient’s own bone tissue from elsewhere in the body in order to transplant it at the defect site, providing a graft with all three formative bone properties and a pre-existing vascular network. Autologous grafts constitute the most common surgical approach to large bone defect repair. Harvesting autografts from highly vascularized regions of bone tissue can improve revascularization of the bone defect. For example, vascularized fibular grafting (Figure 3A) takes advantage of the small vessels from harvested fibula grafts to repair bone defects by microvascular anastomosis.111 Free vascularized flap techniques, including those from the iliac crest, scapula, and radial forearm, can help restore the vascularity of the damaged bone and its surrounding tissue.17 However, autograft supply is limited and can cause pain, damage, or morbidity at the donor site.11 Graft usage is also restricted by outcome unpredictability, which increases when bone defect size exceeds 4 cm.74
Figure 3.
Current techniques used for the repair of vascularized bones. (A) In vascularized fibular grafting, a highly vascularized section of the fibula, with its associated muscle and skin flaps, are removed for implantation in other affected areas. (B) In the induced membrane technique, an autograft is placed inside a highly vascularized tissue envelope formed around a temporary bone cement spacer. (C) A general schematic of an Ilizarov apparatus around a tibial defect. Distraction osteogenesis utilizes several mechanical devices such as this one to repair long bone defects.
Some clinical approaches that have been adopted to fix larger segmental bone defects are induced membranes,112 distraction osteogenesis,93 insertion of vascular bundles,48 and cement casting.13 The induced membrane, for example (Figure 3B), is one of the methods used to increase the density of the vasculature surrounding the defect site and has been reported to successfully repair large segmental defects up to 20 cm.73 For this technique, a polymethyl methacrylate cement block is placed on the bone defect and is surrounded with the adjacent soft tissues. The host slowly creates a membrane rich in vasculature around the block, which is then removed and replaced with an autograft. Bone repair is then prompted by host remodeling in this vascularized environment. The main disadvantages of this method are the required burdensome second operative procedure, potential for infection, and the possible need of repeated debridement.46 Another applied technique for restoring bone function is distraction osteogenesis. In this procedure the spacing of the defect is slowly augmented by mechanical means to control the formation of new bone and new vascular beds (Figure 3C).35 The main limitations involve the slow recovery period, the long duration of treatment time with a cumbersome mechanical apparatus in place around the affected limb, associated pain from the surgical method, possibility of infection at the site of injury, and relatively high rates of failure.14 In addition, the limited availability of vascular bundles for massive extremity trauma, and lack of remodeling capability of non-degradable cements are the drawbacks for these approaches.
The methods discussed above have shown the ability to treat bone with a certain degree of success. The future of clinical treatments will depend on decreasing surgical complexity, accelerating the time of regeneration, and reducing the potential for treatment failure. To this end, BTE constructs have been the focus of recent research.
Tissue engineering approaches for vascularized bone repair
Traditional approaches
Initially, the field of BTE focused on synthesizing biomaterials to use as bone scaffolds or fillers; however, the focus has been shifting gradually toward understanding the role that cells and biological cues play in the reestablishment of bone functionality and structure. The current methodologies involve studying the components of the “golden triad” of tissue engineering: scaffolds, cells, and signals.
Rigid porous scaffolds form the basic support frames of vascularized BTE constructs. The balance between porosity and mechanical strength is the key factor in the fabrication of scaffolds.102 Several methods have been used for porous scaffold fabrication, including molding,57 foaming,21 leaching,64 template-casting,45 machining,27 layer-by-layer assembly (LBL),71 lithographic techniques,67 and additive manufacturing.107 Metals, such as titanium,18 tantalum,118 and magnesium,109 ceramics such as calcium phosphate,113 and polymers ranging from polyesters,79 polyurethanes,15 and polycarbonates,65 to more specialized chemistries, such as polyanhydrides,99 polyphosphazenes,86 and polypropylene fumarates,23 have provided a basic array of materials that can be used for scaffold fabrication. By themselves, however, rigid scaffold frameworks cannot promote the full biofunctionality that a vascularized BTE construct requires. We will discuss our perspectives on improving these traditional approaches in upcoming sections.
Similar to the rigid bone scaffolds, synthetic tissue engineered vascular grafts have also been made from a variety of tough biocompatible and hemocompatible materials, though it is worth noting that traditional BTE design has not included tissue engineered vessel grafts as a component. Elastomers have shown excellent properties for bone scaffold and vascular graft fabrication due to their biocompatibility, resorbability, and multiaxial load-bearing elastic properties.80 For example, Yadong Wang and coworkers developed a heparin-coated porous polyglycerol sebacate (PGS) vascular graft with an outer polycaprolactone (PCL) shell for added mechanical strength.117 More recently, we developed and characterized elastomeric hollow fiber membranes as small diameter vascular grafts,81 with intended use in BTE constructs to initiate and establish vascular beds.
Hydrogels and other soft materials have been proposed as matrix candidates for osteogenesis and angiogenesis due to their capacity to be loaded with cells and signals at relatively high density and their ability to sustain cell viability for relatively long periods of time.22 Biologically derived gels, such as collagen,26 elastin,6 hyaluronic acid,95 chitosan,51 and alginate87 provide excellent biocompatibility, but low availabilities and high costs are usually associated limitations of these materials. Synthetic materials22 and synthetic biologically derived composites49 have been able to relieve these issues. However, since a hydrogel structure is easy to disrupt, integration with the host tissue and interface stabilization becomes a critical problem.22
Cells constitute the biologically functional units of vascularized BTE constructs. Osteoblasts and stem cells are the major causative agents of bone tissue formation, and they have consistently been shown to promote bone formation inside scaffolds. MSCs,122 endothelial progenitor cells (EPCs),70 human umbilical vein endothelial cells (HUVECs),43 embryonic stem cells,72 adult human circulating CD34+,75 and even adipose-derived stem cells121 have been successfully tested for assisting vascularization and mineral deposition inside scaffolds. The angiogenic potential of endothelial cells (ECs) has been observed on a number of scaffolds.105, 116 Smooth muscle cells (SMCs)89 or pericytes9 are required for proper function, strength, and structural stability of endothelialized neovessels in scaffolds.84, 123 Numerous studies have been performed on cell mono- or co-cultures in scaffolds for improved vascular formation.1, 20, 44, 70 Even though cells in scaffolds can promote the formation of microvascular networks, it usually takes a relatively long period, from days to weeks, to develop functional vasculature in vitro and in vivo,7, 43, 44 so ischemia could set in before this process is sufficiently developed to restore blood perfusion.55 As a result, cellularized constructs have met with limited implementation in clinical practice due to long-term loss of viability and adverse host responses.52 More research is needed to understand and optimize the underlying mechanisms of vascular tissue maturation and organization, both in vivo and in vitro, from cells inside the scaffold.60, 103, 104
Cellular responses have been shown to be highly regulated by biochemical, biomechanical and biophysical signals.100 Growth factors (GFs) are biological macromolecules that direct cell growth, differentiation, and migration by binding to receptors located on the cell membrane to conduct signals between cells. A number of powerful GFs have been used to induce differentiation in cells. For example, bone morphogenetic proteins (BMPs), including bone morphogenetic protein-2 (BMP-2)40, 42 BMP-4,24 BMP-6,85 and BMP-710 have been used to promote osteogenic and chondrogenic differentiation of cells on scaffolds. Fibroblast growth factor-2 (FGF-2)101 and vascular endothelial growth factor (VEGF)119 have been used to enhanced tissue and vascular growth in ceramic scaffolds. Other derived molecules, such as transforming growth factor beta-1 (TGF-β1),96 insulin growth factor-1 (IGF-1),108 and growth factor derived peptides82 have also shown to induce osteogenic and vasculogenic responses from progenitor cells. GF combinations have also been studied due to synergistic effects.94 Other stimuli, such as physicochemical features,100 electrical impulses,37 temperature,32 and mechanical stresses,100 can also direct cell proliferation and differentiation. Collectively, spatiotemporal control of cell and signal presentation has been investigated as a strategy to facilitate tissue formation and scaffold remodeling.88 One promising scheme is to mimic biological cascades by sequential delivery of growth factors50, 68 or cells39, 44, 78, 92 to enhance tissue regeneration as compared to approaches involving simultaneous GF release68 or uniform cell loading.39
The next step: Integration
Although promising results have been obtained, it has become increasingly evident that new research should be aimed at hierarchical integration of bone and vascular devices to yield fully functional BTE constructs with enhanced biological properties that can promote concurrent osteogenic and angiogenic growth and seamlessly assimilate with the host bone matrix and vasculature. To achieve tissue regeneration and integration, cells and signals need to be incorporated into the constructs in a fashion that can establish stable graft-host tissue interfaces, and facilitate instant or rapid blood perfusion across the constructs to sustain cellular viability under the mechanical loading of daily activity.38 It is worth noting that the final integration is probably only achievable by the remodeling of the regenerating bone during functional loading. A promising strategy for engineering vascularized bone tissue constructs would be the incorporation of cell-laden hydrogels and synthetic tissue engineered vascular grafts into a porous osteoconductive rigid frame in a hierarchical manner. More specifically, the hydrogel can be used to deliver signals and encapsulate and support the cells necessary for osteogenesis and microvascularization, the large scale tissue engineered vascular grafts can accommodate immediate blood supply as well as initiate and establish microvascular beds in hydrogel microenvironment, and the rigid frame offers improved structural guidance and mechanical integrity (Figure 4). The question, however, is how to reconcile the properties of different components efficiently to achieve maximum integration between each other and with the host.
Figure 4.
Integration of BTE strategies into a vascularized construct.
In general, a mechanically robust frame can improve integration to the host by stabilizing interfaces between engineered and host tissues under weight-bearing conditions. The scaffold should provide structure and mechanical properties resembling those of bone to promote ideal integration and facilitate load transfer. For this, a graded structure would be favored over a homogeneously designed scaffold. Although uniform porosity is the dominant trend in rigid scaffold fabrication, we and others have demonstrated that a scaffold with a pore size gradient could match the architecture of bones by combining a highly porous component that promotes host tissue ingrowth with a less porous component that provides load-bearing support to make an ideal construct for restoring the functionality of damaged bone.69, 120 A porous design also allows for addition of cell-laden soft materials, which can facilitate and guide remodeling to produce new bone tissue with properties similar, or ideally, identical, to that of the host. In order to better mimic the properties of bone, a top-down approach could also be adopted, in which microstructures could be actually incorporated into the bone scaffold frame to facilitate osseous tissue remodeling and inclusion, ingrowth, or development of new vascular beds.41, 56, 83, 91 However, it remains to be determined if mimicking the complex geometry of bone as closely as possible would be necessary or if it would represent an improvement on graded scaffolds.
Soft hydrogel fillers can deliver and control the 3D spatial distribution of encapsulated or migrating cells.110 These cells, in turn, can grow to form mineralized matrix and vascular structures inside scaffolds. However, it is very challenging to preform a complex hydrogel-based vasculature within a mechanically-sound macroporous scaffold for load-bearing applications.43, 114, 123 Moreover, hydrogel-based microvascular beds are not suitable for surgical anastomosis to major vessels due to low mechanical strength. Notwithstanding these limitations, hydrogels are so far the most promising alternative to creating preformed synthetic vascular networks on a relatively small scale. By customizing and conditioning these soft matrices for a particular vascular response in cells, networks allowing microcirculation through the scaffolds are possible. Though tissue engineered vessel grafts are traditionally not incorporated into and designated for BTE, instant or rapid blood perfusion across the constructs and the interface of graft-host tissue is highly desirable to overcome the long-lasting problems of necrosis at the center of large grafts. As such, the incorporation of connectable, perfusable vessel graft beds into the newly designed vascularized bone constructs would be a promising approach and present a future direction for vascularized BTE. The new generation vascular substitutes within the scaffold require sufficient hemocompatibility to maintain patency and compliance to withstand blood pressure and body loads.28 More importantly, they should possess remodeling capabilities to initiate and form functional vessel networks and to integrate with the host vessels, leading to rapid efficient blood perfusion through the construct.62 As indicated before, several materials have been used for development of synthetic grafts, which can be designed to exhibit the aforementioned characteristics. However, fabrication of functional perfusable vessels with improved vessel compatibility and bioremodeling properties are necessary for the development of future vascularized BTE constructs. For example, L’Heureux59 and Niklason63 developed tissue engineered blood vessels from deposited vascular ECM which was left behind as autogenous cells were washed away after growth in a polymer mesh. This deposited ECM presents a biocompatible environment with sufficient bioactivity to support neovessel stabilization and vasculogenic development.19 In another recent study, Gurtner and coworkers were also able to sustain the viability of explanted vascular beds ex vivo, and seed them with progenitor cells to create neo-organs for implantation.29 Suturable explanted beds such as these ones can offer a fast way to connect to the host vasculature, provide a stable blood supply, and support viability inside BTE constructs. The feasibility of this is noted in macrochanneled tissue engineered constructs, like those fabricated by Akita2 and Haholu,31 in which relatively large host vessels were directly inserted into the channels to promote vascular formation and infiltration. Moreover, Kneser showed high vascular development on scaffolds, consisting of arteriovenous loops integrated to processed bovine cancellous bone, implanted on rat femoral defects.54, 55 These approaches could bridge gaps between tissue engineering and modern surgery.
Overall, mechanical, chemical, and biological gradients will constitute the base for seamless integration inside the constructs. The structural integrity of vascular networks included in the scaffold design will depend on the level of integration between the rigid scaffold frame and the developing vascularized matrix. A successful BTE construct would contain the graded structure of bone tissue and the branching structure of vessel networks, from major vessel conduits to small capillary beds inside the scaffold, working synergistically to promote both osteogenesis and angiogenesis. A number of BTE construct designs are possible by going through this approach. We have proposed integrating connectable vascular graft beds into a rigid channeled macroporous composite scaffold with infiltrated cell-laden hydrogels, aiming at a surgical implant that can both promote angiogenic growth and supply blood immediately throughout the construct. Other possible designs could be the combination of rigid scaffolds and computer-designed or explanted decellularized vascular beds that could provide the thorough coverage needed for fully vascularized constructs.
Translation and implementation
Regulations and standards
The testing of medical devices must observe the needs of the medical community, based on generally approved standards and regulations. There have been a number of agencies, local and international, that have regulated the design of new bone scaffolds. However, there are variations between the standards set by different regulatory agencies.4, 34 Currently, concerted efforts have been taken to produce more standardized protocols and increase the efficiency of evaluation. For example, there has been a call to simplify and expedite the evaluation of specialized medical devices (requiring special control and marketing assessment) without compromising their strict risk evaluation.66 Moreover, American regulators have moved towards adding vascularized composite allografts in the list of “organs”, which will lead to more generalized definitions and enhance the evaluation process.98 It is to be expected, thus, that vascularized construct regulation and eventual implementation will closely depend on the coordinated work of regulatory agencies and governments.
Clinical evaluation of implanted constructs
Following the in vivo pre-clinical implementation of a novel vascularized BTE construct, it is important to evaluate whether the development constitutes a significant improvement over current methods of treatment. Methods of construct evaluation focus on three levels of graft integration with surrounding tissues (Figure 5). The first level corresponds to osseointegration. This stage considers the degree of bone tissue repair and regeneration, which is heavily dependent on interface stability and cell activity. The second level corresponds to vascular integration. It is necessary that blood flow be seamlessly restored throughout the construct to prevent complications related to insufficient circulation and promote cell and tissue viability. The last level involves gradual substitution of BTE constructs by bone remodeling under functional loading to achieve complete integration. Osteogenic and vascular regeneration need to be complementary and should proceed at satisfactory rates for effective repair and functional restoration. Though the majority of current practices implement vascular repair and scaffold implantation as separate procedures, the translational future of vascularized bone scaffolds will greatly depend on our capability to combine both processes into a single system. To achieve this, BTE approaches should be developed together with advances of vascular graft beds for a more efficient and integrated design in bone repair.
Figure 5.
Levels of graft integration into the host body: (A) osseointegration, (B) vascular anastomosis, (C) vascular scaffold integration.
The ultimate measure of the success of the vascularized scaffold will be accelerated function restoration, and the most immediate indicative measure will be the extent of integration and anastomosis to host tissues, both osseous and vascular. For example, the rigid porous frame should match the structural and mechanical properties of bone for improved interface stabilization and integration. Large tissue engineered vascular grafts should be able to surgically connect to major vessels, while the inner microvasculature should be capable of spontaneous anastomosis with both larger vessel grafts and surrounding host vascular networks. Ultimately, the most effective vascularized scaffold would be one that allows the greatest extent of angiogenesis and anastomosis following implantation.
Future outlook and conclusions
Although the search for an ideal BTE construct is ongoing, and significant limitations remain for osseointegration, degree of internal revascularization, anastomotic potential, reproducibility, and long-term viability, definitive progress has been achieved. These include the fabrication of novel bioactive materials, controlled distribution of cells and signaling cues, and clinical modifications aimed at improving the effectiveness of current surgical methods. Multifunctional approaches are needed for concurrent angiogenesis and osteogenesis, which will lead to effective vascular graft beds integration within synthetic scaffolds. This has been possible due to coordinated efforts in integrated, multi-interdisciplinary fields to provide increasingly streamlined translational processes. Future BTE constructs are expected to have an architecture that can be easily incorporated into the injured area and the surrounding vascular beds for complete restoration of tissue function. Success of multifunctional BTE constructs will depend on integration at different levels, from cell-scaffold interactions, to combinations of surgical methods and materials, to the hierarchical arrangements of the synthetic vessel grafts within the rigid scaffolds. Clinical translation will strongly depend on combining multiple strategies into a single platform that can provide both accelerated osteogenesis and an increased incorporation rate into the host vasculature at the implant site.
Acknowledgments
We would like to acknowledge the financial support of the following agencies: NIH R01AR057837 (NIAMS), NIH R01DE021468 (NIDCR), and DOD W81XWH-10-1-0966 (PRORP).
Footnotes
Conflict of interest
The authors have no conflicts of interest with respect to this review.
References
- 1.Aguirre A, Planell JA, Engel E. Dynamics of bone marrow-derived endothelial progenitor cell/mesenchymal stem cell interaction in co-culture and its implications in angiogenesis. Biochem Biophys Res Commun. 2010;400:284–291. doi: 10.1016/j.bbrc.2010.08.073. [DOI] [PubMed] [Google Scholar]
- 2.Akita S, Tamai N, Myoui A, Nishikawa M, Kaito T, Takaoka K, Yoshikawa H. Capillary vessel network integration by inserting a vascular pedicle enhances bone formation in tissue-engineered bone using interconnected porous hydroxyapatite ceramics. Tissue Eng. 2004;10:789–795. doi: 10.1089/1076327041348338. [DOI] [PubMed] [Google Scholar]
- 3.Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001;10:S96–S101. doi: 10.1007/s005860100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Society for Testing and Materials. ASTM F756-00: standard practice for assessment of hemolytic properties of materials. Philadelphia, PA: ASTM International; 2000. [Google Scholar]
- 5.An YH. Mechanical properties of bone. In: An YH, Draughn RA, editors. Mechanical Testing of Bone and the Bone – Implant Interface. Boca Raton, FL: CRC Press; 2000. pp. 41–63. [Google Scholar]
- 6.Annabi N, Mithieux SM, Weiss AS, Dehghani F. Cross-linked open-pore elastic hydrogels based on tropoelastin, elastin and high pressure CO2. Biomaterials. 2010;31:1655–1665. doi: 10.1016/j.biomaterials.2009.11.051. [DOI] [PubMed] [Google Scholar]
- 7.Baranski JD, Chaturvedi RR, Stevens KR, Eyckmans J, Carvalho B, Solorzano RD, Yang MT, Miller JS, Bhatia SN, Chen CS. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc Natl Acad Sci USA. 2013;110:7586–7591. doi: 10.1073/pnas.1217796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer T, Muschler G. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000;371:10–27. [PubMed] [Google Scholar]
- 9.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berner A, Boerckel J, Saifzadeh S, Steck R, Ren J, Vaquette C, Zhang JQ, Nerlich M, Guldberg RE, Hutmacher D. Biomimetic tubular nanofiber mesh and platelet rich plasma-mediated delivery of BMP-7 for large bone defect regeneration. Cell Tissue Res. 2012;347:603–612. doi: 10.1007/s00441-011-1298-z. [DOI] [PubMed] [Google Scholar]
- 11.Betz R. Limitations of autograft and allograft: new synthetic solutions. Orthopedics. 2002;25:s561–s570. doi: 10.3928/0147-7447-20020502-04. [DOI] [PubMed] [Google Scholar]
- 12.Bi Y, Stuelten CH, Kilts T, Wadhwa S, Iozzo RV, Robey PG, Chen X-D, Young MF. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem. 2005;280:30481–30489. doi: 10.1074/jbc.M500573200. [DOI] [PubMed] [Google Scholar]
- 13.Black L, Purnell P, Hill J. Current themes in cement research. Adv Appl Ceram. 2010;109:253–259. [Google Scholar]
- 14.Blum ALL, Bongiovanni JC, Morgan SJ, Flierl MA, dos Reis FB. Complications associated with distraction osteogenesis for infected nonunion of the femoral shaft in the presence of a bone defect: a retrospective series. J Bone Joint Surg Br. 2010;92-B:565–570. doi: 10.1302/0301-620X.92B4.23475. [DOI] [PubMed] [Google Scholar]
- 15.Bonzani IC, Adhikari R, Houshyar S, Mayadunne R, Gunatillake P, Stevens MM. Synthesis of two-component injectable polyurethanes for bone tissue engineering. Biomaterials. 2007;28:423–433. doi: 10.1016/j.biomaterials.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Buckwalter J, Glimcher M, Cooper R, Recker R. Bone biology. J Bone Joint Surg Am. 1995;77:1256–1275. [Google Scholar]
- 17.Chim H, Salgado CJ, Mardini S, Chen H-C. Reconstruction of mandibular defects. Semin Plast Surg. 2010;24:188–197. doi: 10.1055/s-0030-1255336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dabrowski B, Swieszkowski W, Godlinski D, Kurzydlowski KJ. Highly porous titanium scaffolds for orthopaedic applications. J Biomed Mater Res B Appl Biomater. 2010;95B:53–61. doi: 10.1002/jbm.b.31682. [DOI] [PubMed] [Google Scholar]
- 19.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 20.Deb S, Mandegaran R, Di Silvio L. A porous scaffold for bone tissue engineering/45S5 Bioglass® derived porous scaffolds for co-culturing osteoblasts and endothelial cells. J Mater Sci Mater Med. 2010;21:893–905. doi: 10.1007/s10856-009-3936-5. [DOI] [PubMed] [Google Scholar]
- 21.Dehghani F, Annabi N. Engineering porous scaffolds using gas-based techniques. Curr Opin Biotechnol. 2011;22:661–666. doi: 10.1016/j.copbio.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 23.Farshid B, Lalwani G, Sitharaman B. Cytotoxicity of polypropylene fumarate nanocomposites used in bone tissue engineering. 39th Annual Northeast Bioengineering Conference (NEBEC); Syracuse, NY. 2013. pp. 119–120. [Google Scholar]
- 24.Ferreira CL, Abreu FAMd, Silva GAB, Silveira FF, Barreto LBA, Paulino TdP, Miziara MN, Alves JB. TGF-β1 and BMP-4 carried by liposomes enhance the healing process in alveolar bone. Arch Oral Biol. 2013;58:646–656. doi: 10.1016/j.archoralbio.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Folkman J, Hochberg M. Self-regulation of growth in three dimensions. J Exp Med. 1973;138:745–753. doi: 10.1084/jem.138.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glowacki J, Mizuno S. Collagen scaffolds for tissue engineering. Biopolymers. 2008;89:338–344. doi: 10.1002/bip.20871. [DOI] [PubMed] [Google Scholar]
- 27.Grayson WL, Fröhlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C, Wan LQ, Liu XS, Guo XE, Vunjak-Novakovic G. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci USA. 2010;107:3299–3304. doi: 10.1073/pnas.0905439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenwald SE, Berry CL. Improving vascular grafts: the importance of mechanical and haemodynamic properties. J Pathol. 2000;190:292–299. doi: 10.1002/(SICI)1096-9896(200002)190:3<292::AID-PATH528>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 29.Gurtner GC, Bhatt KA, Wong VW. Composite tissue engineering and organ regeneration using explanted microvascular beds (EMBs) Plast Reconstr Surg. 2009;124:106–107. [Google Scholar]
- 30.Habibovic P, de Groot K. Osteoinductive biomaterials-properties and relevance in bone repair. J Tissue Eng Regen Med. 2007;1:25–32. doi: 10.1002/term.5. [DOI] [PubMed] [Google Scholar]
- 31.Haholu A, Sever C, Uygur F, Kose G, Urhan M, Sinan O, Omer O, Cihan S, Kulahci Y. Prefabrication of vascularized bone graft using an interconnected porous calcium hydroxyapatite ceramic in presence of vascular endothelial growth factor and bone marrow mesenchymal stem cells: experimental study in rats. Indian J Plast Surg. 2012;45:444–452. doi: 10.4103/0970-0358.105939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hastings CL, Kelly HM, Murphy MJ, Barry FP, O’Brien FJ, Duffy GP. Development of a thermoresponsive chitosan gel combined with human mesenchymal stem cells and desferrioxamine as a multimodal pro-angiogenic therapeutic for the treatment of critical limb ischaemia. J Control Release. 2012;161:73–80. doi: 10.1016/j.jconrel.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 33.Heslop BF, Zeiss IM, Nisbet NW. Studies on Transference of Bone: I. A Comparison of Autologous and Homologous Bone Implants with Reference to Osteocyte Survival, Osteogenesis and Host Reaction. Br J Exp Pathol. 1960;41:269–287. [PMC free article] [PubMed] [Google Scholar]
- 34.Hess JR, Sparrow RL, Van Der Meer PF, Acker JP, Cardigan RA, Devine DV. Blood components: red blood cell hemolysis during blood bank storage: using national quality management data to answer basic scientific questions. Transfusion. 2009;49:2599–2603. doi: 10.1111/j.1537-2995.2009.02275.x. [DOI] [PubMed] [Google Scholar]
- 35.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989;239:263–285. [PubMed] [Google Scholar]
- 36.Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat Biotechnol. 2005;23:821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 37.Jin G, Kim G. The effect of sinusoidal AC electric stimulation of 3D PCL/CNT and PCL/β-TCP based bio-composites on cellular activities for bone tissue regeneration. J Mater Chem B. 2013;1:1439–1452. doi: 10.1039/c2tb00338d. [DOI] [PubMed] [Google Scholar]
- 38.Jung S, Kleinheinz J. Angiogenesis — the key to regeneration. In: Andrades JA, editor. Regenerative Medicine and Tissue Engineering. Rijeka, Croatia: InTech; 2013. pp. 453–473. [Google Scholar]
- 39.Kang JK, Lee MH, Kwon BJ, Kim HH, Shim IK, Jung MR, Lee SJ, Park J-C. Effective layer by layer cell seeding into non-woven 3D electrospun scaffolds of poly-L-lactic acid microfibers for uniform tissue formation. Macromol Res. 2012;20:795–799. [Google Scholar]
- 40.Kang S-W, Kim J-S, Park K-S, Cha B-H, Shim J-H, Kim JY, Cho D-W, Rhie J-W, Lee S-H. Surface modification with fibrin/hyaluronic acid hydrogel on solid-free form-based scaffolds followed by BMP-2 loading to enhance bone regeneration. Bone. 2011;48:298–306. doi: 10.1016/j.bone.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 41.Kang Y, Jabbari E, Yang Y. Integrating top-down and bottom-up scaffolding tissue engineering approach for bone regeneration. In: Ramalingam M, Jabbari E, Ramakrishna S, Khademhosseini A, editors. Micro and Nanotechnologies in Engineering Stem Cells and Tissues. Hoboken, NJ: John Wiley & Sons, Inc; 2013. pp. 142–159. [Google Scholar]
- 42.Kang Y, Kim S, Khademhosseini A, Yang Y. Creation of bony microenvironment with CaP and cell-derived ECM to enhance human bone-marrow MSC behavior and delivery of BMP-2. Biomaterials. 2011;32:6119–6130. doi: 10.1016/j.biomaterials.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang Y, Mochizuki N, Khademhosseini A, Fukuda J, Yang Y. Engineering a vascularized collagen-β-tricalcium phosphate graft using an electrochemical approach. Acta Biomater. 2015;11:449–458. doi: 10.1016/j.actbio.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang Y, Ren L, Yang Y. Engineering vascularized bone grafts by integrating a biomimetic periosteum and β-TCP scaffold. ACS Appl Mater Interfaces. 2014;6:9622–9633. doi: 10.1021/am502056q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang Y, Scully A, Young DA, Kim S, Tsao H, Sen M, Yang Y. Enhanced mechanical performance and biological evaluation of a PLGA coated ε-TCP composite scaffold for load-bearing applications. Eur Polym J. 2011;47:1569–1577. doi: 10.1016/j.eurpolymj.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karger C, Kishi T, Schneider L, Fitoussi F, Masquelet AC. Treatment of posttraumatic bone defects by the induced membrane technique. Orthop Traumatol Surg Res. 2012;98:97–102. doi: 10.1016/j.otsr.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Kaully T, Kaufman-Francis K, Lesman A, Levenberg S. Vascularization — the conduit to viable engineered tissues. Tissue Eng Part B Rev. 2009;15:159–169. doi: 10.1089/ten.teb.2008.0193. [DOI] [PubMed] [Google Scholar]
- 48.Khira YM, Badawy HA. Pedicled vascularized fibular graft with Ilizarov external fixator for reconstructing a large bone defect of the tibia after tumor resection. J Orthop Traumatol. 2013;14:91–100. doi: 10.1007/s10195-013-0225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim S, Bedigrew K, Guda T, Maloney WJ, Park S, Wenke JC, Yang YP. Novel osteoinductive photo-cross-linkable chitosan-lactide-fibrinogen hydrogels enhance bone regeneration in critical size segmental bone defects. Acta Biomater. 2014;10:5021–5033. doi: 10.1016/j.actbio.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim S, Kang Y, Krueger CA, Sen M, Holcomb JB, Chen D, Wenke JC, Yang Y. Sequential delivery of BMP-2 and IGF-1 using a chitosan gel with gelatin microspheres enhances early osteoblastic differentiation. Acta Biomater. 2012;8:1768–1777. doi: 10.1016/j.actbio.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S, Kang Y, Mercado-Pagán ÁE, Maloney WJ, Yang Y. In vitro evaluation of photo-crosslinkable chitosan-lactide hydrogels for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2014;102:1393–1406. doi: 10.1002/jbm.b.33118. [DOI] [PubMed] [Google Scholar]
- 52.Kim S, Recum Hv. Endothelial stem cells and precursors for tissue engineering: cell source, differentiation, selection, and application. Tissue Eng Part B Rev. 2008;14:133–147. doi: 10.1089/teb.2007.0304. [DOI] [PubMed] [Google Scholar]
- 53.Kirkpatrick JS, Cornell CN, Hoang BH, Hsu W, Watson JT, Watters WC, Turkelson CM, Wies JL, Anderson S. Bone void fillers. J Am Acad Orthop Surg. 2010;18:576–579. doi: 10.5435/00124635-201009000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Kneser U, Polykandriotis E, Ohnolz J, Heidner K, Grabinger L, Euler S, Amann KU, Hess A, Brune K, Greil P. Engineering of vascularized transplantable bone tissues: induction of axial vascularization in an osteoconductive matrix using an arteriovenous loop. Tissue Eng. 2006;12:1721–1731. doi: 10.1089/ten.2006.12.1721. [DOI] [PubMed] [Google Scholar]
- 55.Kneser U, Schaefer D, Polykandriotis E, Horch R. Tissue engineering of bone: the reconstructive surgeon’s point of view. J Cell Mol Med. 2006;10:7–19. doi: 10.1111/j.1582-4934.2006.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koffler J, Kaufman-Francis K, Shandalov Y, Egozi D, Amiad Pavlov D, Landesberg A, Levenberg S. Improved vascular organization enhances functional integration of engineered skeletal muscle grafts. Proc Natl Acad Sci USA. 2011;108:14789–14794. doi: 10.1073/pnas.1017825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kramschuster A, Turng L-S. An injection molding process for manufacturing highly porous and interconnected biodegradable polymer matrices for use as tissue engineering scaffolds. J Biomed Mater Res B Appl Biomater. 2010;92B:366–376. doi: 10.1002/jbm.b.31523. [DOI] [PubMed] [Google Scholar]
- 58.Krishnan L, Willett N, Guldberg R. Vascularization strategies for bone regeneration. Ann Biomed Eng. 2014;42:432–444. doi: 10.1007/s10439-014-0969-9. [DOI] [PubMed] [Google Scholar]
- 59.L’Heureux N, Pâquet S, Labbé R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 60.Langer R. Tissue engineering: perspectives, challenges, and future directions. Tissue Eng. 2007;13:1–2. doi: 10.1089/ten.2006.0219. [DOI] [PubMed] [Google Scholar]
- 61.Laschke MW, Harder Y, Amon M, Martin I, Farhadi J, Ring A, Torio-Padron N, Schramm R, Rücker M, Junker D. Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissue Eng. 2006;12:2093–2104. doi: 10.1089/ten.2006.12.2093. [DOI] [PubMed] [Google Scholar]
- 62.Laschke MW, Mussawy H, Schuler S, Kazakov A, Rücker M, Eglin D, Alini M, Menger MD. Short-term cultivation of in situ prevascularized tissue constructs accelerates inosculation of their preformed microvascular networks after implantation into the host tissue. Tissue Eng Part A. 2010;17:841–853. doi: 10.1089/ten.TEA.2010.0329. [DOI] [PubMed] [Google Scholar]
- 63.Lawson J, Dahl S, Prichard H, Manson R, Gage S, Kypson A, Blum J, Pilgrim A, Tente W, Niklason L. VS5 human tissue-engineered grafts for hemodialysis: development, preclinical data, and early investigational human implant experience. J Vasc Surg. 2014;59:32S–33S. [Google Scholar]
- 64.Lee J-H, Kim S-H, Oh S-H, Kim S-J, Hah Y-S, Park B-W, Kim DR, Rho G-J, Maeng G-H, Jeon R-H, Lee H-C, Kim J-R, Kim G-C, Kim U-K, Byun J-H. Tissue-engineered bone formation using periosteal-derived cells and polydioxanone/pluronic F127 scaffold with pre-seeded adipose tissue-derived CD146 positive endothelial-like cells. Biomaterials. 2011;32:5033–5045. doi: 10.1016/j.biomaterials.2011.03.081. [DOI] [PubMed] [Google Scholar]
- 65.Liao J, Zhang L, Zuo Y, Wang H, Li J, Zou Q, Li Y. Development of nanohydroxyapatite/polycarbonate composite for bone repair. J Biomater Appl. 2009;24:31–45. doi: 10.1177/0885328209102756. [DOI] [PubMed] [Google Scholar]
- 66.Liberti L, Breckenridge A, Eichler HG, Peterson R, McAuslane N, Walker S. Expediting patients’ access to medicines by improving the predictability of drug development and the regulatory approval process. Clin Pharmacol Ther. 2009;87:27–31. doi: 10.1038/clpt.2009.179. [DOI] [PubMed] [Google Scholar]
- 67.Liska R, Schuster M, Inführ R, Turecek C, Fritscher C, Seidl B, Schmidt V, Kuna L, Haase A, Varga F, Lichtenegger H, Stampfl J. Photopolymers for rapid prototyping. J Coatings Tech Res. 2007;4:505–510. [Google Scholar]
- 68.Liu G, Fan W, Miao X, Xiao Y, Good D, Wei MQ. Sequential release of BMP-7 and VEGF from the PLGA/AK-gelatin composite scaffolds. J Biomim Biomater Tissue Eng. 2011;11:81–91. [Google Scholar]
- 69.Liu Y, Kim JH, Young D, Nishimoto SK, Heck R, Yang Y. Biomimetic macroporous scaffolds with high mechanical strength and biological evaluation. 38th Annual Meeting of the American Association for Dental Research; Miami, FL. 2009. p. 120808. [Google Scholar]
- 70.Liu Y, Teoh S-H, Chong MSK, Yeow C-H, Kamm RD, Choolani M, Chan JKY. Contrasting effects of vasculogenic induction upon biaxial bioreactor stimulation of mesenchymal stem cells and endothelial progenitor cells cocultures in three-dimensional scaffolds under in vitro and in vivo paradigms for vascularized bone tissue engineering. Tissue Eng Part A. 2012;19:893–904. doi: 10.1089/ten.TEA.2012.0187. [DOI] [PubMed] [Google Scholar]
- 71.Macdonald ML, Samuel RE, Shah NJ, Padera RF, Beben YM, Hammond PT. Tissue integration of growth factor-eluting layer-by-layer polyelectrolyte multilayer coated implants. Biomaterials. 2011;32:1446–1453. doi: 10.1016/j.biomaterials.2010.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marolt D, Campos IM, Bhumiratana S, Koren A, Petridis P, Zhang G, Spitalnik PF, Grayson WL, Vunjak-Novakovic G. Engineering bone tissue from human embryonic stem cells. Proc Natl Acad Sci USA. 2012;109:8705–8709. doi: 10.1073/pnas.1201830109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Masquelet A, Fitoussi F, Begue T, Muller G. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann Chir Plast Esthet. 2000;45:346–353. [PubMed] [Google Scholar]
- 74.Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin N Am. 2010;41:27–37. doi: 10.1016/j.ocl.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 75.Matsumoto T, Kawamoto A, Kuroda R, Ishikawa M, Mifune Y, Iwasaki H, Miwa M, Horii M, Hayashi S, Oyamada A, Nishimura H, Murasawa S, Doita M, Kurosaka M, Asahara T. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am J Pathol. 2006;169:1440–1457. doi: 10.2353/ajpath.2006.060064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mauffrey C, Madsen M, Bowles RJ, Seligson D. Bone graft harvest site options in orthopaedic trauma: a prospective in vivo quantification study. Injury. 2012;43:323–326. doi: 10.1016/j.injury.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 77.McCarthy I. The physiology of bone blood flow: a review. J Bone Joint Surg Am. 2006;88:4–9. doi: 10.2106/JBJS.F.00890. [DOI] [PubMed] [Google Scholar]
- 78.McFadden TM, Duffy GP, Allen AB, Stevens HY, Schwarzmaier SM, Plesnila N, Murphy JM, Barry FP, Guldberg RE, O’Brien FJ. The delayed addition of human mesenchymal stem cells to pre-formed endothelial cell networks results in functional vascularization of a collagen–glycosaminoglycan scaffold in vivo. Acta Biomater. 2013;9:9303–9316. doi: 10.1016/j.actbio.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 79.Meng ZX, Li HF, Sun ZZ, Zheng W, Zheng YF. Fabrication of mineralized electrospun PLGA and PLGA/gelatin nanofibers and their potential in bone tissue engineering. Mater Sci Eng C. 2013;33:699–706. doi: 10.1016/j.msec.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 80.Mercado-Pagán ÁE, Kang Y, Ker DFE, Park S, Yao J, Bishop J, Yang YP. Synthesis and characterization of novel elastomeric poly(D,L-lactide urethane) maleate composites for bone tissue engineering. Eur Polym J. 2013;49:3337–3349. doi: 10.1016/j.eurpolymj.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mercado-Pagán ÁE, Ker DFE, Yang Y. Hemocompatibility evaluation of small elastomeric hollow fiber membranes as vascular substitutes. J Biomater Appl. 2014;29:557–565. doi: 10.1177/0885328214537541. [DOI] [PubMed] [Google Scholar]
- 82.Mercado AE, Yang X, He X, Jabbari E. Effect of grafting BMP2-derived peptide to nanoparticles on osteogenic and vasculogenic expression of stromal cells. J Tissue Eng Regen Med. 2014;8:15–28. doi: 10.1002/term.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mercado ÁE, Yang Y. Strategies towards engineering vascularized bone graft substitutes. In: Laurencin C, Jiang T, editors. Bone Graft Substitutes and Bone Regenerative Engineering. West Conshohocken, PA: ASTM International; 2014. pp. 299–334. [Google Scholar]
- 84.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen D-HT, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, Chaturvedi R, Bhatia SN, Chen CS. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mizrahi O, Sheyn D, Tawackoli W, Kallai I, Oh A, Su S, Da X, Zarrini P, Cook-Wiens G, Gazit D, Gazit Z. BMP-6 is more efficient in bone formation than BMP-2 when overexpressed in mesenchymal stem cells. Gene Ther. 2013;20:370–377. doi: 10.1038/gt.2012.45. [DOI] [PubMed] [Google Scholar]
- 86.Morozowich NL, Nichol JL, Allcock HR. Investigation of apatite mineralization on antioxidant polyphosphazenes for bone tissue engineering. Chem Mater. 2012;24:3500–3509. [Google Scholar]
- 87.Moshaverinia A, Ansari S, Chen C, Xu X, Akiyama K, Snead ML, Zadeh HH, Shi S. Co-encapsulation of anti-BMP2 monoclonal antibody and mesenchymal stem cells in alginate microspheres for bone tissue engineering. Biomaterials. 2013;34:6572–6579. doi: 10.1016/j.biomaterials.2013.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nauth A, Giannoudis PV, Einhorn TA, Hankenson KD, Friedlaender GE, Li R, Schemitsch EH. Growth factors: beyond bone morphogenetic proteins. J Orthop Trauma. 2010;24:543–546. doi: 10.1097/BOT.0b013e3181ec4833. [DOI] [PubMed] [Google Scholar]
- 89.Neff LP, Tillman BW, Yazdani SK, Machingal MA, Yoo JJ, Soker S, Bernish BW, Geary RL, Christ GJ. Vascular smooth muscle enhances functionality of tissue-engineered blood vessels in vivo. J Vasc Surg. 2011;53:426–434. doi: 10.1016/j.jvs.2010.07.054. [DOI] [PubMed] [Google Scholar]
- 90.Nguyen B-NB, Fisher JP. In vivo techniques and strategies for enhanced vascularization of engineered bone. In: Brey EM, editor. Vascularization - Regenerative Medicine and Tissue Engineering. Boca Raton, FL: CRC Press; 2014. pp. 263–282. [Google Scholar]
- 91.Nguyen LH, Annabi N, Nikkhah M, Bae H, Binan L, Park S, Kang Y, Yang Y, Khademhosseini A. Vascularized bone tissue engineering: approaches for potential improvement. Tissue Eng Part B Rev. 2012;18:363–382. doi: 10.1089/ten.teb.2012.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Papadimitropoulos A, Scherberich A, Güven S, Theilgaard N, Crooijmans HJA, Santini F, Scheffler K, Zallone A, Martin I. A 3D in vitro bone organ model using human progenitor cells. Eur Cells Mater. 2011;21:445–458. doi: 10.22203/ecm.v021a33. [DOI] [PubMed] [Google Scholar]
- 93.Papakostidis C, Bhandari M, Giannoudis P. Distraction osteogenesis in the treatment of long bone defects of the lower limbs: effectiveness, complications and clinical results; a systematic review and meta-analysis. Bone Joint J. 2013;95:1673–1680. doi: 10.1302/0301-620X.95B12.32385. [DOI] [PubMed] [Google Scholar]
- 94.Patel ZS, Young S, Tabata Y, Jansen JA, Wong MEK, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Patterson J, Siew R, Herring SW, Lin AS, Guldberg R, Stayton PS. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials. 2010;31:6772–6781. doi: 10.1016/j.biomaterials.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pepper MS. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997;8:21–43. doi: 10.1016/s1359-6101(96)00048-2. [DOI] [PubMed] [Google Scholar]
- 97.Phemister DB. Changes in bones and joints resulting from interruption of circulation: I. General considerations and changes resulting from injuries. Arch Surg. 1940;41:436–472. [Google Scholar]
- 98.Pondrom S. The AJT report: news and issues that affect organ and tissue transplantation. Am J Transplant. 2010;10:1953–1954. doi: 10.1111/j.1600-6143.2009.03122.x. [DOI] [PubMed] [Google Scholar]
- 99.Poshusta AK, Burdick JA, Mortisen DJ, Padera RF, Ruehlman D, Yaszemski MJ, Anseth KS. Histocompatibility of photocrosslinked polyanhydrides: a novel in situ forming orthopaedic biomaterial. J Biomed Mater Res A. 2003;64:62–69. doi: 10.1002/jbm.a.10274. [DOI] [PubMed] [Google Scholar]
- 100.Prodanov L, Semeins CM, van Loon JJWA, te Riet J, Jansen JA, Klein-Nulend J, Walboomers XF. Influence of nanostructural environment and fluid flow on osteoblast-like cell behavior: a model for cell-mechanics studies. Acta Biomater. 2013;9:6653–6662. doi: 10.1016/j.actbio.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 101.Qu D, Li J, Li Y, Gao Y, Zuo Y, Hsu Y, Hu J. Angiogenesis and osteogenesis enhanced by bFGF ex vivo gene therapy for bone tissue engineering in reconstruction of calvarial defects. J Biomed Mater Res A. 2011;96A:543–551. doi: 10.1002/jbm.a.33009. [DOI] [PubMed] [Google Scholar]
- 102.Ritchie RO. The conflicts between strength and toughness. Nat Mater. 2011;10:817–822. doi: 10.1038/nmat3115. [DOI] [PubMed] [Google Scholar]
- 103.Rivron N, Liu J, Rouwkema J, Boer Jd, Blitterswijk Cv. Engineering vascularised tissues in vitro. Eur Cells Mater. 2008;15:27–40. doi: 10.22203/ecm.v015a03. [DOI] [PubMed] [Google Scholar]
- 104.Rouwkema J, Westerweel PE, de Boer J, Verhaar MC, van Blitterswijk CA. The use of endothelial progenitor cells for prevascularized bone tissue engineering. Tissue Eng Part A. 2009;15:2015–2027. doi: 10.1089/ten.tea.2008.0318. [DOI] [PubMed] [Google Scholar]
- 105.Santos MI, Tuzlakoglu K, Fuchs S, Gomes ME, Peters K, Unger RE, Piskin E, Reis RL, Kirkpatrick CJ. Endothelial cell colonization and angiogenic potential of combined nano- and micro-fibrous scaffolds for bone tissue engineering. Biomaterials. 2008;29:4306–4313. doi: 10.1016/j.biomaterials.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 106.Sen M, Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury. 2007;38:S75–S80. doi: 10.1016/j.injury.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 107.Shanjani Y, Hu Y, Toyserkani E, Grynpas M, Kandel RA, Pilliar RM. Solid freeform fabrication of porous calcium polyphosphate structures for bone substitute applications: in vivo studies. J Biomed Mater Res B Appl Biomater. 2013;101B:972–980. doi: 10.1002/jbm.b.32905. [DOI] [PubMed] [Google Scholar]
- 108.Sheng MHC, Lau KHW, Baylink DJ. Role of osteocyte-derived insulin-like growth factor I in developmental growth, modeling, remodeling, and regeneration of the bone. J Bone Metab. 2014;21:41–54. doi: 10.11005/jbm.2014.21.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Staiger MP, Pietak AM, Huadmai J, Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006;27:1728–1734. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 110.Stevens MM. Biomaterials for bone tissue engineering. Mater Today. 2008;11:18–25. [Google Scholar]
- 111.Sun Y, Zhang C, Jin D, Sheng J, Cheng X, Liu X, Chen S, Zeng B. Free vascularised fibular grafting in the treatment of large skeletal defects due to osteomyelitis. Int Orthop. 2010;34:425–430. doi: 10.1007/s00264-009-0761-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taylor BC, French BG, Fowler TT, Russell J, Poka A. Induced membrane technique for reconstruction to manage bone loss. J Am Acad Orthop Surg. 2012;20:142–150. doi: 10.5435/JAAOS-20-03-142. [DOI] [PubMed] [Google Scholar]
- 113.Teixeira S, Fernandes H, Leusink A, van Blitterswijk C, Ferraz MP, Monteiro FJ, de Boer J. In vivo evaluation of highly macroporous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res A. 2010;93A:567–575. doi: 10.1002/jbm.a.32532. [DOI] [PubMed] [Google Scholar]
- 114.Therriault D, White SR, Lewis JA. Chaotic mixing in three-dimensional microvascular networks fabricated by direct-write assembly. Nat Mater. 2003;2:265–271. doi: 10.1038/nmat863. [DOI] [PubMed] [Google Scholar]
- 115.Tremblay P-L, Hudon V, Berthod F, Germain L, Auger FA. Inosculation of tissue-engineered capillaries with the host’s vasculature in a reconstructed skin transplanted on mice. Am J Transplant. 2005;5:1002–1010. doi: 10.1111/j.1600-6143.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 116.Unger RE, Peters K, Wolf M, Motta A, Migliaresi C, Kirkpatrick CJ. Endothelialization of a non-woven silk fibroin net for use in tissue engineering: Growth and gene regulation of human endothelial cells. Biomaterials. 2004;25:5137–5146. doi: 10.1016/j.biomaterials.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 117.Wu W, Allen RA, Wang Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat Med. 2012;18:1148–1153. doi: 10.1038/nm.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang H, Li J, Zhou Z, Ruan J. Structural preparation and biocompatibility evaluation of highly porous tantalum scaffolds. Mater Lett. 2013;100:152–155. [Google Scholar]
- 119.Yang P, Wang C, Shi Z, Huang X, Dang X, Xu S, Wang K. Prefabrication of vascularized porous three-dimensional scaffold induced from rhVEGF165: a preliminary study in rats. Cells Tissues Organs. 2009;189:327–337. doi: 10.1159/000142162. [DOI] [PubMed] [Google Scholar]
- 120.Yang Y, Kang Y, Sen M, Park S. Bioceramics in tissue engineering. In: Burdick J, Mauck R, editors. Biomaterials for Tissue Engineering: A Review of the Past and Future Trends. New York, NY: Springer Wien; 2010. pp. 179–208. [Google Scholar]
- 121.Zanetti AS, Sabliov C, Gimble JM, Hayes DJ. Human adipose-derived stem cells and three-dimensional scaffold constructs: A review of the biomaterials and models currently used for bone regeneration. J Biomed Mater Res B Appl Biomater. 2013;101B:187–199. doi: 10.1002/jbm.b.32817. [DOI] [PubMed] [Google Scholar]
- 122.Zeng X, Zeng Y-s, Ma Y-h, Lu L-y, Du B-l, Zhang W, Li Y, Chan WY. Bone marrow mesenchymal stem cells in a three-dimensional gelatin sponge scaffold attenuate inflammation, promote angiogenesis, and reduce cavity formation in experimental spinal cord injury. Cell Transplant. 2011;20:1881–1899. doi: 10.3727/096368911X566181. [DOI] [PubMed] [Google Scholar]
- 123.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, López JA, Stroock AD. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci USA. 2012;109:9342–9347. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zimmermann G, Moghaddam A. Allograft bone matrix versus synthetic bone graft substitutes. Injury. 2011;42(Supplement 2):S16–S21. doi: 10.1016/j.injury.2011.06.199. [DOI] [PubMed] [Google Scholar]
- 125.Zioupos P, Currey JD. The extent of microcracking and the morphology of microcracks in damaged bone. J Mater Sci. 1994;29:978–986. [Google Scholar]