Abstract
Importance
Intravitreous injections of melphalan are increasingly used in the treatment of vitreous seeding of retinoblastoma. While this technique can save eyes otherwise destined for enucleation, ocular salvage may come at the price of local toxicity. Posterior segment toxicity in this context is well-established. This report focuses on toxicity to the anterior segment following intravitreous melphalan.
Observations
Our clinic cohort includes 76 patients treated with intravitreous injections of melphalan at Memorial Sloan Kettering Cancer Center treated from September 2012 through April 2015. We here report a series of five patients from this cohort who developed anterior segment toxicity. These abnormalities were found at the injection site or within the meridian of the injection and included: a traumatic cataract following an injection at an outside hospital, iris depigmentation and thinning, iris recession with hypotony, a filtering conjunctival bleb, and focal scleromalacia with localized pigmentation.
Conclusions and Relevance
Intravitreous melphalan injection may result in toxicity to the anterior segment of the eye, in addition to retinal toxicity; and appears to be more common in the meridian of the injection where the drug concentration is highest.
Intravitreous melphalan injection is an effective means of treating vitreous seeding for retinoblastoma, and this technique now saves many eyes that once would have been enucleated1–3. However, each injection of intravitreous melphalan results in decrement of approximately 5% in retinal function as measured by electroretinogram4. Other groups have confirmed the toxic effects of intravitreous melphalan on the posterior segment of the eye3,5–7. In this report, we discuss the previously unrecognized topic of anterior segment toxicity to the eye following intravitreous melphalan. These findings are particularly pertinent as more groups are using this treatment technique.
All injections were performed using a 33 gauge, ½ inch needle with a tri-beveled point and siliconized shaft. The Institutional Review Board of Memorial Sloan Kettering Cancer Center approved this study.
Case 1
A 22-month-old child with bilateral retinoblastoma was previously treated at an outside institution with systemic chemotherapy, laser and cryotherapy. The left eye received four infusions of ophthalmic artery chemosurgery (OAC) and three injections of intravitreous melphalan 20mcg. He was referred to our institution for second opinion regarding persistent disease in the left eye. During our initial examination, a needle tract site from a prior intravitreous injection was identified in the lens and appeared as three linear speckled white punctate lesions (Figure 1). The eyes remain stable and free of tumor and at 14 months follow up.
Figure 1. Lens opacity and conjunctival bleb.
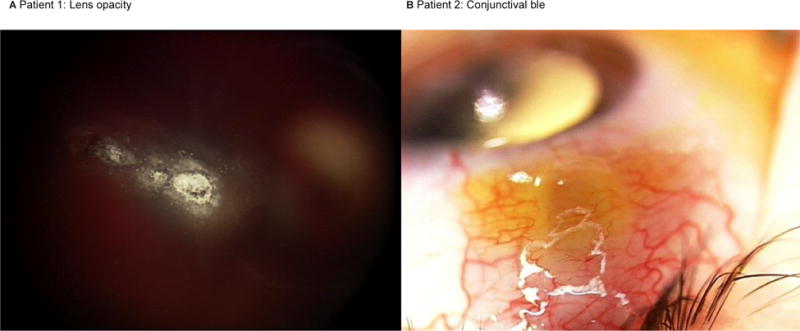
Color photograph of patient 1 demonstrating punctate, linear lenticular opacities after injection at an outside hospital (A). Following an intravitreous injection of melphalan, a Seidel-negative conjunctival bleb was noted at the injection site of patient 2, and resolved by four weeks (B).
Case 2
A 22 month old diagnosed with unilateral retinoblastoma of the right eye (Reese-Ellsworth Class VB, International Classification Group D) was treated with 4 cycles of OAC. Persistent dust (type I) vitreous seeds were noted overlying the main calcified tumor and the eye received a single injection of intravitreous melphalan (30 mcg). The following week, a conjunctival bleb, Seidel-negative, was noted in the location of the intravitreous injection (figure 1). At the one-month follow up, the bleb had resolved. The vitreous seeds regressed and at 22 months follow up the eye continues to be tumor free.
Case 3
An 11-week-old child with unilateral retinoblastoma of the right eye (Reese-Ellsworth Class VB, International Classification Group C) was treated with one cycle of systemic carboplatin, three OAC infusions and laser. Persistent vitreous seeds (resembling “dust” – type I)8 were identified and the eye received five intravitreous melphalan (30mcg) injections, the last two of which were accompanied by periocular topotecan (1mg). All injections at our institution were performed in a manner that has previously been reported4. Following the second-to-last injection, iris depigmentation and poor dilation was noted spanning the quadrant where the intravitreous injection was administered (figure 2). An anterior segment optical coherence tomography (OCT) revealed thinning of the iris and loss of crypts compared to the normal iris in the opposite meridian. The child’s disease is inactive and stable at 13 months follow up.
Figure 2. Iris and ciliary body toxicity.
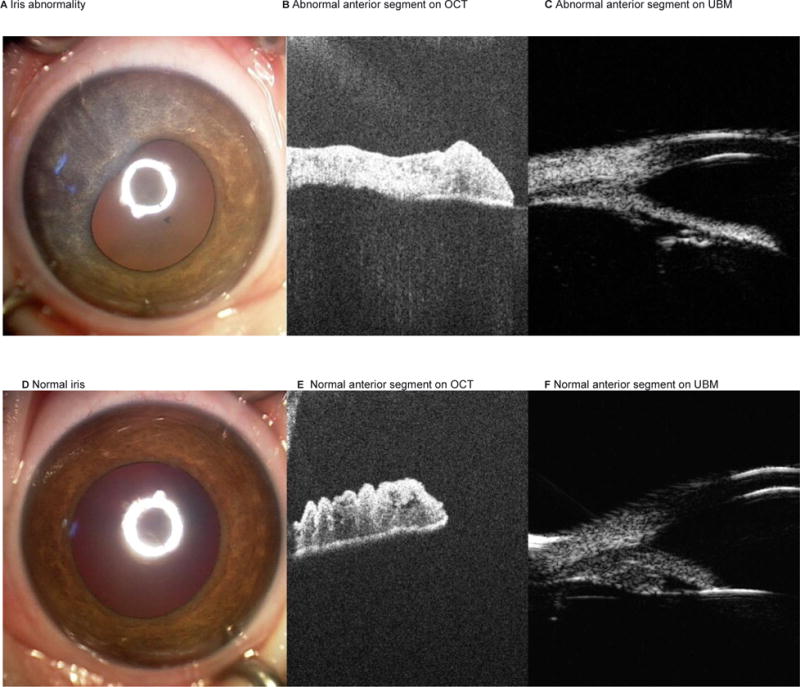
Patient 3 with iris abnormality in the meridian of the intravitreous injection (A). Anterior segment OCT and UBM images demonstrate synechae, iris thinning and loss of iris crypts, and ciliary body atrophy (B and C). By comparison, the unaffected normal left iris is shown (D, E and F).
Case 4
A 43 month old diagnosed with unilateral retinoblastoma in the left eye (Reese-Ellsworth Class VB, International Classification Group D) was treated with two cycles of OAC. Due to a cloud (type III) of vitreous seeds, the eye was treated with four injections of intravitreous melphalan (30mcg). Persistent disease was noted and the eye was further treated with three injections of intravitreous melphalan (25mcg) and concomitant intravitreous topotecan (20mcg). Following the second injection of intravitreous melphalan and topotecan, retinal necrosis (mimicking a retinotomy), choroidal atrophy, retinal pigment epithelium (RPE) disturbance, iris recession by ultrasonic biomicroscopy, and a hypotensive pressure (5mg) were noted (figure 3). The tumor responded and the eye remains stable at 4 months follow up.
Figure 3. Iris and scleral toxicity.
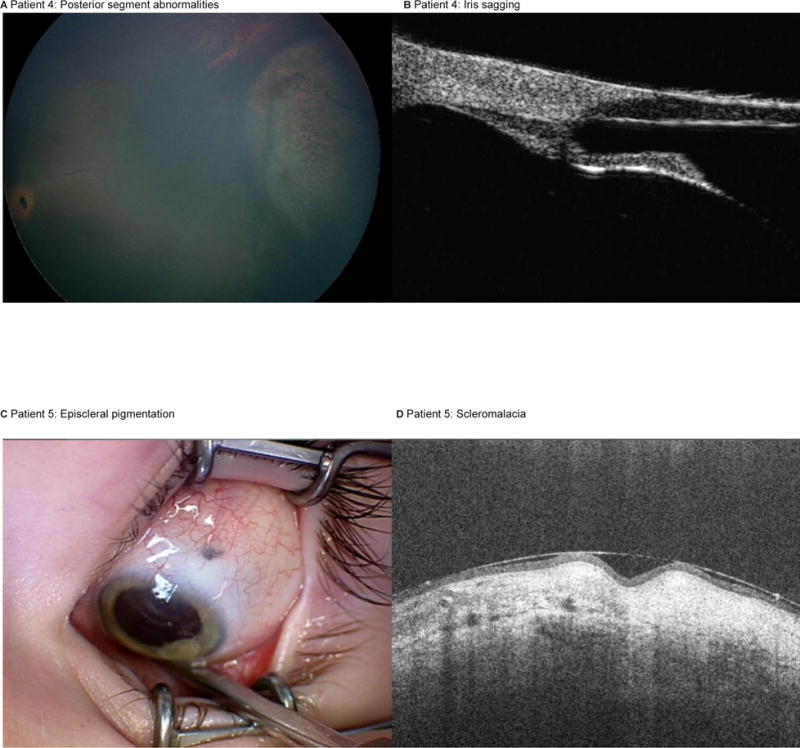
Patient 4 with posterior segment findings at the meridian of the injection site (A), ultrasonic biomicroscopy demonstrating sagging of the iris (B). Patient 5 with geographic area of episcleral pigmentation at the previous injection site (C); and anterior segment OCT depicting scleromalacia at the needle tract (D).
Case 5
A 36 month old boy was diagnosed with unilateral retinoblastoma in the left eye (RE VB/ IC E) was treated with 7 cycles of OAC, and 7 intravitreous injections of topotecan (20mcg), the final three of which were combined with intravitreous injections of melphalan (30mcg). One month following the final intravitreous injections, a geographic area of scleral pigmentation was noted at the injection site. It measured 1mm by 0.8mm with mobile, non-pigmented conjunctiva overlying it; and an anterior segment OCT revealed focal indentation of the sclera (figure 3). The tumor is inactive and the eye is stable at 5 months follow up.
Discussion
A number of studies, in both humans and adults, demonstrate posterior segment toxicity from intravitreous melphalan. For instance, disturbance of the retinal pigment epithelium (salt and pepper retinopathy) is reported to occur in approximately 43–50% of patients and has been significantly associated with more pronounced degradation of electroretinogram responses1,4.
Anterior segment abnormalities have been described extensively following intravitreous injections of anti-vascular endothelial growth factor agents. These findings include scleral thinning after repeat injections, episcleral cystoid cavities9, intraocular inflammation10, corneal edema, descemets folds11, cataract,12 and ocular surface foreign bodies13. In our cohort we observed anterior abnormalities in five patients receiving intravitreous melphalan for retinoblastoma, including a traumatic cataract following injection at an outside hospital, iris depigmentation and thinning, iris recession (in association with retinal necrosis and hypotony), a conjunctival bleb one month following the injection and focal scleromalacia with localized pigmentation. All of these abnormalities were found at the injection site or within the meridian of the injection.
The lens opacity induced at the outside hospital can be explained by the surgical technique and has the potential of occurring irrespective of the drug being injected. However, the other findings and the extent of their damage can to be correlated to melphalan toxicity. The iris depigmentation and thinning can be likened to the salt and pepper retinopathy that is seen in the fundus, and which is thought to occur from the proximity of the drug to the disturbed surface1,4. In this case, one may speculate whether drug escaped to the anterior chamber, making contact with and damaging the iris stroma. Similarly, the iris recession and hypotony appeared to be an extension of the posterior segment finding of retinal necrosis, choroidal atrophy and RPE disturbance, and are likely related to elevated drug concentration in the location of the injection. Temporary blebs are often observed immediately following intravitreous injections and presumably result from reflux of intraocular contents into the subconjunctival space. However, in this instance, the bleb was most apparent at 1 week following the injection, perhaps suggesting the refluxed melphalan allowed for continued patency of a potential trans-scleral fistula, resulting in a filtering bleb. Finally, while scleral thinning following bevacizumab has been visualized with OCT9, it seldom involves a rarity of scleral tissue with visualization of the underlying uvea as was seen in our case. It is possible that the melphalan contributed to atrophy/necrosis of the ocular surface, in a similar manner that has been found with the iris, choroid, RPE and retina1,4,14,15. On the other hand, the scleral pigmentation may simply represent migrated deposition of released pigments from nearby retina, ciliary body or iris.
In summary, while intravitreous melphalan has allowed ophthalmic oncologists to salvage eyes that, up until recent years, would have been enucleated, this does not come without both anterior and posterior segment toxicity. These anterior segment findings are of importance when guiding parents through the intravitreous technique, particularly since many of these features have the potential of being easily observed by the parents without the need for sophisticated equipment.
Acknowledgments
This work was supported by the Fund for Ophthalmic Knowledge who contributed to the design and conduct of the study.
Footnotes
Jasmine H. Francis had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Authors contribution: JHF- design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript, BPM- review of manuscript, SB- review of manuscript, DHA- design and conduct of the study, interpretation of the data, review of manuscript
None of the authors have any financial disclosures or conflicts.
References
- 1.Munier FL, Gaillard M-C, Balmer A, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol. 2012 doi: 10.1136/bjophthalmol-2011-301450. [DOI] [PubMed] [Google Scholar]
- 2.Shields CL, Manjandavida FP, Arepalli S, Kaliki S, Lally SE, Shields JA. Intravitreal melphalan for persistent or recurrent retinoblastoma vitreous seeds: preliminary results. JAMA Ophthalmol. 2014;132(3):319–325. doi: 10.1001/jamaophthalmol.2013.7666. [DOI] [PubMed] [Google Scholar]
- 3.Ghassemi F, Shields CL. Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol. 2012;130(10):1268–1271. doi: 10.1001/archophthalmol.2012.1983. [DOI] [PubMed] [Google Scholar]
- 4.Francis JH, Schaiquevich P, buitrago E, et al. Local and systemic toxicity of intravitreal melphalan for vitreous seeding in retinoblastoma: a preclinical and clinical study. Ophthalmology. 2014;121(9):1810–1817. doi: 10.1016/j.ophtha.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 5.Ghassemi F, Amoli FA. Pathological findings in enucleated eyes after intravitreal melphalan injection. Int Ophthalmol. 2013 doi: 10.1007/s10792-013-9851-2. [DOI] [PubMed] [Google Scholar]
- 6.Shimoda Y, Hamano R, Ishihara K, et al. Effects of intraocular irrigation with melphalan on rabbit retinas during vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2008;246(4):501–508. doi: 10.1007/s00417-007-0685-3. [DOI] [PubMed] [Google Scholar]
- 7.Ueda M, Tanabe J, Inomata M, Kaneko A, Kimura T. [Study on conservative treatment of retinoblastoma – effect of intravitreal injection of melphalan on the rabbit retina] Nippon Ganka Gakkai Zasshi. 1995;99(11):1230–1235. [PubMed] [Google Scholar]
- 8.Francis JH, Abramson DH, Gaillard M-C, Marr BP, Beck-Popovic M, Munier FL. The Classification of Vitreous Seeds in Retinoblastoma and Response to Intravitreal Melphalan. Ophthalmology. 2015 doi: 10.1016/j.ophtha.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Zinkernagel MS, Schorno P, Ebneter A, Wolf S. Scleral thinning after repeated intravitreal injections of antivascular endothelial growth factor agents in the same quadrant. Invest Ophthalmol Vis Sci. 2014;56(3):1894–1900. doi: 10.1167/iovs.14-16204. [DOI] [PubMed] [Google Scholar]
- 10.Sato T, Emi K, Ikeda T, et al. Severe intraocular inflammation after intravitreal injection of bevacizumab. Ophthalmology. 2010;117(3):512–6– 516.e1–2. doi: 10.1016/j.ophtha.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 11.Bayar SA, Altinors DD, Kucukerdonmez C, Akova YA. Severe corneal changes following intravitreal injection of bevacizumab. Ocul Immunol Inflamm. 2010;18(4):268–274. doi: 10.3109/09273948.2010.490630. [DOI] [PubMed] [Google Scholar]
- 12.Meyer CH, Rodrigues EB, Michels S, et al. Incidence of damage to the crystalline lens during intravitreal injections. J Ocul Pharmacol Ther. 2010;26(5):491–495. doi: 10.1089/jop.2010.0045. [DOI] [PubMed] [Google Scholar]
- 13.Jørgensen JS, Kemp H, Sørensen TL. Identification of foreign bodies on the ocular surface after uneventful intravitreal injections. Acta Ophthalmol. 2012;90(8):e646–7. doi: 10.1111/j.1755-3768.2012.02405.x. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki S, Aihara Y, Fujiwara M, Sano S, Kaneko A. Intravitreal injection of melphalan for intraocular retinoblastoma. Jpn J Ophthalmol. 2015 doi: 10.1007/s10384-015-0378-0. [DOI] [PubMed] [Google Scholar]
- 15.Smith SJ, Smith BD, Mohney BG. Ocular side effects following intravitreal injection therapy for retinoblastoma: a systematic review. Br J Ophthalmol. 2014;98(3):292–297. doi: 10.1136/bjophthalmol-2013-303885. [DOI] [PubMed] [Google Scholar]


