Abstract
Objective
Calcific aortic valve disease (CAVD) is a significant cause of morbidity and mortality, which affects approximately 1% of the US population and is characterized by calcific nodule formation and stenosis of the valve. Klotho-deficient mice were used to study the molecular mechanisms of CAVD as they develop robust aortic valve (AoV) calcification. Through microarray analysis of AoV tissues from klotho-deficient and wild type mice, increased expression of the gene encoding cyclooxygenase 2/COX2 (Ptgs2) was found. COX2 activity contributes to bone differentiation and homeostasis, thus the contribution of COX2 activity to AoV calcification was assessed.
Approach and Results
In klotho-deficient mice, COX2 expression is increased throughout regions of valve calcification and is induced in the valvular interstitial cells (VICs) prior to calcification formation. Similarly, COX2 expression is increased in human diseased AoVs. Treatment of cultured porcine aortic VICs with osteogenic media induces bone marker gene expression and calcification in vitro, which is blocked by inhibition of COX2 activity. In vivo, genetic loss of function of COX2 cyclooxygenase activity partially rescues AoV calcification in klotho-deficient mice. Moreover, pharmacologic inhibition of COX2 activity in klotho-deficient mice via celecoxib-containing diet reduces AoV calcification and blocks osteogenic gene expression.
Conclusions
COX2 expression is upregulated in CAVD and its activity contributes to osteogenic gene induction and valve calcification in vitro and in vivo.
Keywords: Calcific aortic valve disease, cyclooxygenase 2, valvular interstitial cell
Introduction
Calcific aortic valve disease (CAVD) is characterized by mineralized nodules on the valve cusps and typically results in progressive aortic valve stenosis (AS) (1, 2). CAVD is a significant cause of morbidity and mortality, thus research into the prevention or delay of CAVD may ultimately lead to improved clinical outcomes (3, 4). In the US, AS affects 0.4% of the total population, however the risk for developing AS due to CAVD increases with age, and the prevalence is estimated at 2.8% in the elderly (5). There are a number of factors that contribute to the development of CAVD, such as abnormal valve development, advanced age, end-stage kidney disease, and inflammation secondary to increased lipid deposition (3). Valve replacement surgery is the current standard of care, however, mechanical valves are associated with the risk of thromboembolism, and bioprosthetic valves have limited durability (4, 6, 7). Investigation into the cellular and molecular changes that occur at early stages of disease may lead to alternative therapies and alleviate the need for surgery.
During disease, valvular interstitial cells (VICs) from mineralized valves activate molecular pathways associated with valve development and bone differentiation (8, 9). Expression of the osteogenic gene markers osteocalcin (OCN), Runx2, osteopontin (OPN), alkaline phosphatase (ALP), and bone sialoprotein (BSP) is increased in human CAVD in comparison to control valves (8). Additional pathways involved in bone and cartilage formation, such as bone morphogenetic protein (BMP), Notch, and Wnt signaling, have also been shown to be involved in valve calcification (10-13). Together, the evidence suggests that an osteogenic-like mechanism is active in CAVD, although the contribution of such pathways may vary depending on the underlying cause of CAVD.
Klotho-deficient mice develop robust nodular aortic valve (AoV) calcification similar to that observed in human CAVD (14, 15). Klotho-deficient mice were originally described as a model of premature aging, as they have a shortened lifespan, but they also develop kidney disease and have increased serum phosphate levels, which are associated with an increased risk of CAVD in humans (16-19). In addition to exhibiting AoV calcification, klotho-deficient mice have increased osteogenic gene expression in the AoVs, similar to human CAVD (9, 14). Thus, klotho-deficient mice are useful for studying the cellular and molecular changes that occur during valve calcification in the early stages of CAVD, and, as they have little immune cell infiltration in the AoV, study of the VIC-intrinsic molecular changes are possible in this model (14).
Here we show that cyclooxygenase 2 (COX2) expression is increased in the mineralized AoVs of klotho-deficient mice and in human CAVD. COX2 has a key role in prostaglandin synthesis and is active during inflammation as well as in bone formation and repair (20-23). COX2-specific inhibitors are commonly used to treat pain and inflammation, but have an increased risk of cardiovascular side effects (24-27). In vitro, COX2 activity is necessary for osteogenic gene expression and VIC calcification. In klotho-deficient mice, genetic and pharmacologic manipulation of COX2 activity in vivo reduces AoV calcification. Thus COX2 activity contributes to osteogenic gene induction and calcification in AoVs in mice.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
COX2 expression is increased in regions of AoV calcification in klotho-deficient mice
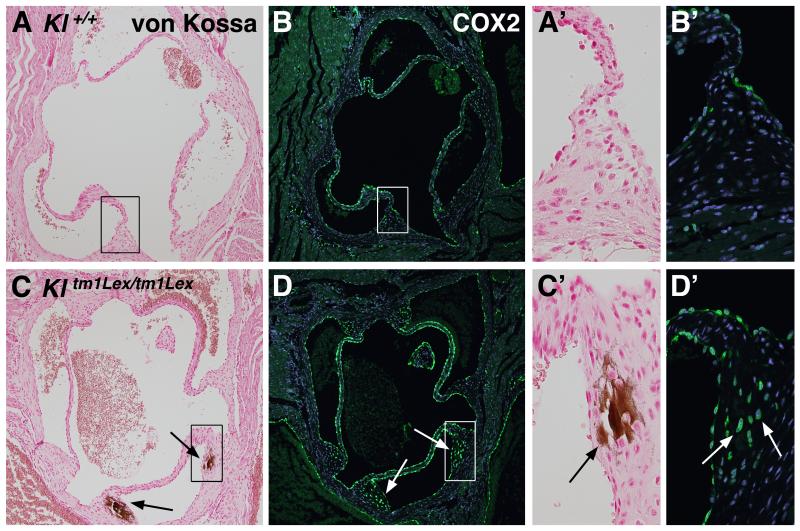
In order to identify novel molecular mechanisms involved in AoV calcification, gene expression in 6.5 week old klotho-deficient and wild type mice was evaluated by microarray. Among the genes with the most increased expression in klotho-deficient animals were Spp1 (osteopontin), previously reported to be increased in klotho-deficient valves (14), and Ptgs2 (Table SI, Table SII). Expression of Ptgs2 (prostaglandin-endoperoxide synthase 2), the gene encoding COX2 protein, was increased 4.3-fold in AoV tissues of klotho-deficient animals, and increased COX2 expression was confirmed by immunohistochemical analysis. Klotho-deficient mice develop calcification in the hinge region of the AoV, as apparent in von Kossa stained sections of hearts from 6-6.5 week old wild type (Kl+/+) and klotho-deficient (Kltm1Lex/tm1Lex) mice (Figure 1A, A’, C, C’) (14). In wild type mice, COX2 expression is limited to endothelial cells lining the AoV cusps and aortic wall (Figure 1B, B’). However, klotho-deficient mice have increased COX2 expression in the VICs of the AoV hinge region (Figure 1D, D’), in addition to the normal endothelial pattern. Importantly, COX2 expression in VICs spatially overlaps with the region of AoV calcification in klotho-deficient mice (Figure 1C-D’). These results demonstrate that increased COX2 protein expression is localized to regions of AoV calcification in klotho-deficient animals.
Figure 1. COX2 expression is increased and localized to the region of aortic valve calcification in klotho-deficient (Kl tm1Lex/tm1Lex) mice.
Positive von Kossa staining (black arrows) demonstrates AoV calcification in the hinge region of klotho-deficient mice (C, C’) compared to no observable calcification in wild type controls (Kl +/+) (A, A’). The boxed regions in panels A and C are magnified and shown in panels A’ and C’. Sections are counterstained with nuclear fast red (A, A’, C, C’). In wild type animals, immunofluorescence demonstrates that COX2 is primarily expressed in endothelial cells (green staining in B and B’). In contrast, Klotho-deficient mice have increased COX2 expression in VICs surrounding and throughout regions of valve calcification (green staining in panels D and D’ indicated by white arrows). Boxed regions in B and D are magnified and shown in panels B’ and D’. Nuclei are counterstained with ToPro3 (blue staining in B, B’, D, D’).
COX2 expression is increased in human CAVD
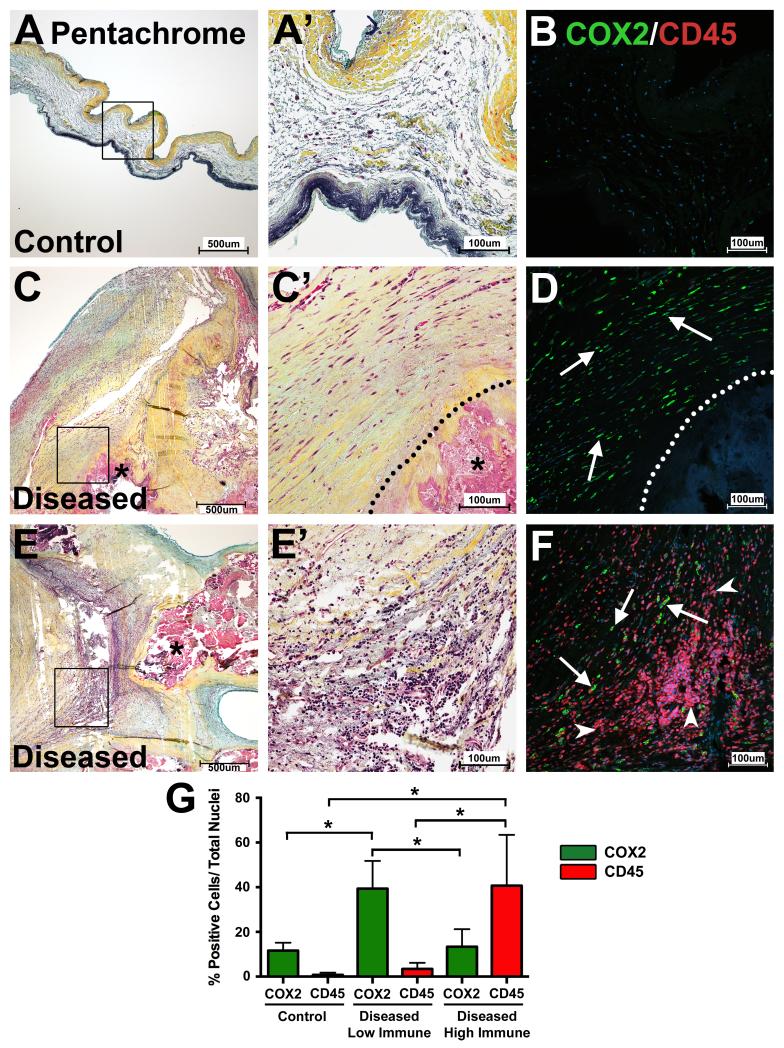
To examine whether COX2 expression is increased in human CAVD, explanted diseased and control AoV tissues were stained for COX2 by immunofluorescence. As COX2 is often associated with immune cell infiltration, COX2 protein expression was compared to the leukocyte/hematopoietic cell marker CD45. Pentachrome staining was used to examine the histology of control and diseased valves, particularly regions of immune cell infiltration and extracellular matrix thickening (Figure 2A, A’, C, C’, E, E’). Areas of intense COX2 protein expression are apparent in regions of the diseased valves surrounding calcification and, in comparison, little COX2 protein is observed in control AoVs (Figure 2B, D, F). COX2 is expressed in regions adjacent to calcific nodules where CD45 expression is absent (Figure 2D) but can also be readily observed in regions with intense CD45 expression (Figure 2F). Although COX2-positive and CD45-positive cells can be found in overlapping areas with immune cell infiltration, few cells co-express these proteins (Figure 2F). Control AoV tissues contain few COX2- or CD45-positive cells (Figure 2B). Quantification of COX2-positive, CD45-positive, and double-positive cells shows that the percentage of COX2 expressing cells is significantly increased in diseased tissues in regions of low immune cell infiltration when compared to control tissues (Figure 2G). As expected, regions of high immune cell infiltration in diseased AoV tissues have significantly higher numbers of CD45-positive cells compared to control valves (Figure 2G). Further, expression analysis of COX2 and smooth muscle alpha actin (alpha SMA) in human diseased and control AoVs demonstrated that the majority of COX2-expressing cells are not in close proximity to or co-localized with alpha SMA-expressing cells, although limited regions of co-expression were observed (Figure SI). Thus, COX2 expression is increased in human CAVD and, in some cases, is localized in proximity to calcified nodules in the absence of inflammation as occurs in the klotho-deficient mice.
Figure 2. COX2 expression is increased in human CAVD.
Movat’s pentachrome staining delineates the extracellular matrix of human control (A, boxed region magnified in A’; n = 5) and diseased (C and E, boxed regions magnified in C’ and E’; n = 7) AoV tissues. Co-immunofluorescence of COX2 (green) and CD45 (red) (B, D, F) shows increased COX2 expression in diseased AoVs (D, F) compared to controls (B). COX2 expression is low in human control AoVs (green staining in B) and few cells express the leukocyte/hematopoietic cell marker CD45 (red staining in B). In diseased tissues, COX2 expression is high in regions void of CD45-positive cells (“low immune” regions; green staining in D indicated by white arrows). COX2 expression also is observed in regions of diseased valves with high levels of CD45 expression (“high immune” regions), but co-staining of COX2 and CD45 is rarely observed in the same cell (CD45 = red staining indicated by white arrowheads (F); COX2 = green staining indicated by white arrows (F)). Quantification of COX2-positive cells (green bars) versus total nuclei demonstrates that COX2 expression is significantly increased in regions of few infiltrating immune cells in diseased AoVs when compared to controls (G). As expected, CD45 expression (red bars) was increased in diseased valves in regions of high immune cell infiltration when compared to controls (G). Dotted lines delineate calcified regions (C, D) and calcification is also marked by an asterisk (C, C’, E). Nuclei are counterstained with ToPro3 (blue in B, D, F). A one-way ANOVA test with a post-hoc multiple comparisons test was used to compare COX2 and CD45 expression between control and diseased valves with regions of low immune cell infiltration or regions of high immune cell infiltration; significant differences are indicated by asterisks (p<0.05) and error bars represent the SD (G).
Aortic VICs induce COX2 expression prior to the initiation of valve calcification
To determine the timing of COX2 induction in aortic VICs relative to the progression of CAVD, COX2 protein expression was examined in klotho-deficient mice (Kltm1Lex/tm1Lex) at 2, 3, 4, and 5 weeks of age. Although the timing is variable, Klotho-deficient mice typically develop AoV calcification between 4 and 5 weeks of age, as indicated by von Kossa staining (Figure SIIA-D). As expected, COX2 immunofluorescence is detected in the endothelial cells lining both klotho-deficient and wild type valves at all timepoints investigated (Figure 1B, Figure SIIE-H and data not shown). In addition, COX2 expression can be detected as early as 3 weeks of age in aortic VICs of klotho-deficient animals (Figure SIIF). Prior to calcification, the COX2-expressing VICs are in the AoV hinge region where calcific lesions ultimately form, and COX2 expression co-localizes with the region of calcification by 5 weeks of age (Figure SIIE-H). Thus, COX2 is expressed in the VICs of the AoV hinge region in klotho-deficient mice prior to the initiation of valve calcification.
Calcified VICs express COX2
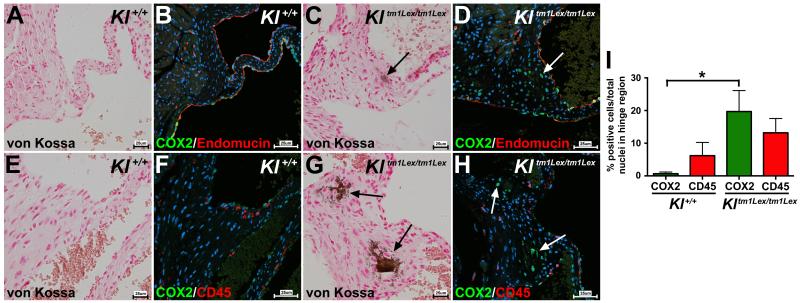
In wild type mice (Kl+/+) at 6-6.5 weeks of age, COX2 expression in the heart is limited to endothelial cells (Figure 1B, B’). To confirm that increased COX2 expression in the AoV hinge region of klotho-deficient mice (Kltm1Lex/tm1Lex) is separate from COX2 expression in endothelial cells, co-expression immunofluorescence of COX2 and endomucin was performed. Endomucin marks the endothelial cells lining the AoVs in both wild type and klotho-deficient mice, and a subset of endomucin-positive cells also expresses COX2 (Figure 3B, D). However, VICs that express COX2 in the calcified valve regions of klotho-deficient mice are separate from the valve endothelial cells, as they do not express endomucin (Figure 3C-D). COX2 expression is often associated with immune cell infiltration and an inflammatory response (24, 25). Few infiltrating immune cells or apoptotic cells, indicative of dystrophic calcification, are observed in the calcific lesions of klotho-deficient mice (Figure SIII) (14). To demonstrate that COX2 expressing cells in the valve interstitium are not leukocytes or other hematopoietic-derived cells, COX2 expression was compared to CD45 expression (Figure 3F, H) (28). No overlap between CD45 and COX2 expression was observed in the region of AoV calcification in klotho-deficient mice (Figure 3G-H). Similarly, there was no overlap between CD45 and COX2 expression in Kl+/+ mice (Figure 3E-F). Quantification of COX2 positive cells and CD45 positive cells revealed that COX2 expression is significantly increased in the VICs of the AoV hinge region in klotho-deficient mice, whereas the percentage of CD45-positive cells was similar to that of wild type controls (Figure 3I). Together, the data suggest that COX2-positive cells are not immune infiltrate nor cells of hematopoietic origin, but rather are resident VICs. To further support the idea that COX2 expressing cells are resident fibroblast-like VICs, expression of the fibroblast markers, type 1 collagen and vimentin, was analyzed in klotho-deficient and wild type mice. Calcified VICs in the AoVs of klotho-deficient mice express both type 1 collagen and vimentin, similar to surrounding non-calcified VICs and normal VICs in wild type mice (Figure SIV). Myofibroblast-like VICs, characterized by expression of alpha SMA, have been implicated in CAVD (3). In order to determine if there is a myofibroblast intermediate preceding VIC calcification, klotho-deficient mice were crossed with a tamoxifen-inducible alpha SMAcreER transgenic line with a cre-dependent ROSA membrane Tomato/membrane EGFP reporter. Mice were administered tamoxifen to induce SMAcreER activity prior to observable calcification. Analysis of recombined EGFP-positive cells revealed that calcified VICs did not arise from a myofibroblast intermediate nor do they have smooth muscle cell characteristics (Figure SV)(29). Thus, in klotho-deficient mice, COX2-expressing VICs are resident interstitial fibroblast-like cells localized to the calcified area.
Figure 3. VICs expressing COX2 do not co-express endothelial or leukocyte/hematopoietic cell markers in klotho-deficient mice.
COX2 (green staining) is co-expressed with the endothelial marker endomucin (red staining) in endothelial cells in wild type animals (Kl+/+) (B). In klotho-deficient mice (Kltm1Lex/tm1Lex), in addition to endothelial cell expression, COX2 is evident in VICs in regions of calcification (COX2 expression indicated by white arrow in D), which are not positive for endomucin. Kl+/+ mice exhibit dispersed expression of the leukocyte/hematopoietic cell marker CD45 (red staining in F). In Kltm1Lex/tm1Lex mice, co-immunofluorescent staining of CD45 (red staining) and COX2 (green staining) shows that COX2-expressing cells do not express CD45 (COX2 staining is indicated by white arrows in H). Quantification of COX2-positive (green bars) and CD45-positive (red bars) VICs in the AoV hinge region demonstrates a significant increase in COX2 expression in Kltm1Lex/tm1Lex compared to wild type animals, whereas CD45 expression was unchanged (I). AoV calcification is detected by von Kossa staining in adjacent sections of the same specimens (brown staining indicated by black arrows in A, C, E, G; sections are counterstained with nuclear fast red). Nuclei are counterstained with ToPro3 (blue staining in B, D, F, H). A one-way ANOVA with a post-hoc multiple comparisons test was used to compare expression of COX2 and CD45 in wild type and klotho-deficient mice, a significant difference is indicated by an asterisk (p<0.05) and error bars represent the SD (I).
COX2 inhibition reduces osteogenic gene induction and calcification in cultured porcine aortic VICs
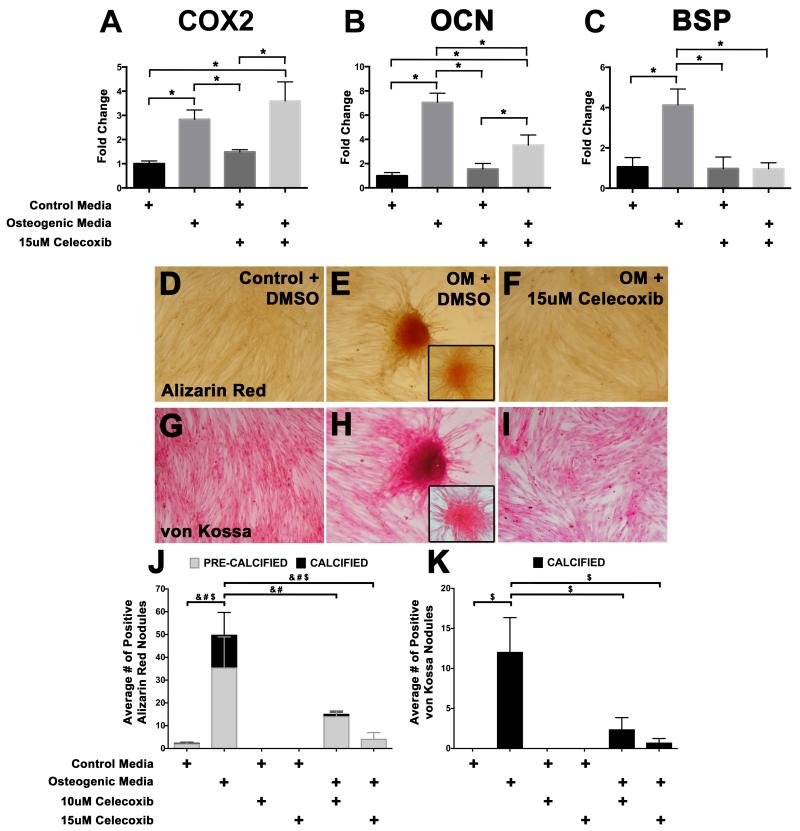
To examine whether COX2 activity is necessary for osteogenic gene induction and calcification in aortic VICs, a porcine VIC culture system was used. Upon treatment of VICs with osteogenic media, expression of the osteogenic genes OCN and BSP is increased, as detected by quantitative (q)PCR (Figure 4B, C). In addition, COX2 gene expression is also increased upon osteogenic media treatment, showing that COX2 mRNA expression is induced concomitant with osteogenic gene expression (Figure 4A). VICs were treated with the specific COX2 inhibitor celecoxib, which inhibits COX2 enzyme activity by binding to the active site of the protein, but does not affect COX2 gene expression (Figure 4A) (30). The requirement for COX2 activity in osteogenic gene induction was examined in VICs treated with osteogenic media in the presence of 15μM Celecoxib. In these experiments, expression levels of OCN and BSP were significantly reduced, compared to VICs treated with osteogenic media alone (Figure 4B, C). Therefore, COX2 inhibition reduces osteogenic gene induction in cultured VICs. In addition to gene expression, calcific nodule formation was assessed. VICs were treated with control or osteogenic media in low serum (2%), in combination with celecoxib treatment, and stained with alizarin red (Figure 4D-F) and von Kossa (Figure 4G-I) to detect calcification. The cellular response to osteogenic media and COX2 inhibition by celecoxib was measured by quantifying the number of pre-calcified (as detected by “orange” alizarin red staining) and calcified (as detected by “red” alizarin red staining and positive von Kossa staining) nodules (Figure 4D-K). In response to osteogenic media, VIC calcific nodule formation is increased (Figure 4E, H, J-K) when compared to cells treated with control media (Figure 4D, G, J-K). VICs treated with two different concentrations of celecoxib (10μM or 15μM) to inhibit COX2 activity had significantly fewer nodules (Figure 4F, I, J-K). Together, the data show that osteogenic media treatment stimulates COX2 mRNA induction, osteogenic gene expression, and calcific nodule formation. Thus, COX2 inhibition is sufficient to reduce osteogenic gene expression and cell calcification in isolated porcine VICs.
Figure 4. COX2 inhibition in porcine aortic VICs reduces osteogenic gene expression and cell calcification.
For gene expression studies, isolated VICs were cultured for 6 days in control or osteogenic media (OM) with vehicle (DMSO) or the COX2 inhibitor celecoxib (15μM) (A-C). Quantitative PCR analysis demonstrates that COX2 mRNA expression (A) and the osteogenic gene markers osteocalcin (OCN) (B) and bone sialoprotein (BSP) (C) are induced with OM treatment. COX2 inhibition by celecoxib reduces the expression of both OCN and BSP in cells induced with OM (B, C) whereas COX2 expression is unchanged (A). Statistical analysis was performed using ANOVA with a multiple comparison post-hoc test and significance was determined when p<0.05 (indicated by * in A-C). For calcification studies, VICs were cultured for 9 days in control or OM media with DMSO or two different concentrations of celecoxib (10μM or 15μM). Pre-calcified nodules (orange staining, inset in E) and calcified nodules (red staining, E) were identified by alizarin red staining in OM-treated cells (E), when compared to control cells (D). COX2 inhibition by celecoxib treatment reduces the number of alizarin red positive nodules upon OM treatment (F). A similar effect was observed in nodules stained by von Kossa to detect calcification (G-I; H indicates a positive von Kossa nodule (brown staining); the inset in H indicates a nodule that is not calcified and was excluded from the analysis). The average number of alizarin red pre-calcified (grey bars) and calcified (black bars) nodules (J), as well as average number of positive von Kossa nodules (K, black bars) in cultured VICs was quantified. Statistical significance as determined by ANOVA with post-hoc multiple comparisons tests (p<0.05) is indicated by & for the average number of pre-calcified nodules, # for the average number of alizarin red positive calcified nodules, and $ for the total nodules positive for alizarin red (J) and von Kossa staining (K).
Genetic COX2-deficiency reduces AoV calcification in klotho-deficient mice
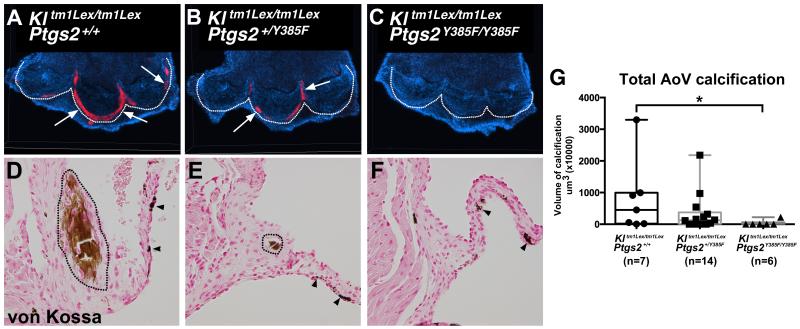
In order to determine if loss of COX2 function can inhibit AoV calcification in vivo, COX2 mutant mice (B6.129S6(FVB)-Ptgs2tm1.1Fun/J) were bred with klotho-deficient mice (31). COX2 mutant mice possess a point mutation in exon 8 of the Ptgs2 gene, which disrupts the cyclooxygenase activity, and mimics the effect of COX2 inhibition by selective COX2 inhibitor drugs (31). Klotho-deficient littermates with Ptgs2 wild type (Kltm1Lex/tm1Lex; Ptgs2+/+), heterozygous (Kltm1Lex/tm1Lex; Ptgs2+/Y385F), or homozygous (Kltm1Lex/tm1Lex; Ptgs2Y385F/Y385F) alleles were harvested at 6 weeks of age. AoV calcification was assessed by whole mount alizarin red staining, and positive staining was quantified for each genotype to obtain AoV calcification volumes (Figure 5A-C, G). Aortic wall calcification was excluded from this analysis. Although the extent of calcification is variable, Kltm1Lex/tm1Lex; Ptgs2+/+ mice with intact Ptgs2 alleles demonstrate the highest level of calcification (Figure 5A, G; n=7). In comparison, mice with two mutated copies of the Ptgs2 gene (Kltm1Lex/tm1Lex; Ptgs2Y385F/Y385F) have significantly lower amounts of AoV calcification, and 67% of animals had no detectable AoV calcification (Figure 5C, G; n=6, p=0.039). There was no statistically significant difference in the amount of AoV calcification observed in Kltm1Lex/tm1Lex; Ptgs2+/Y385F mice (Figure 5B, G; n=14). Histological assessment of the AoV calcification by von Kossa staining confirms the reduced calcification in Kltm1Lex/tm1Lex; Ptgs2Y385F/Y385F mice (Figure 5D-F). Together, these findings show that genetic loss of COX2 cyclooxygenase activity in vivo prevents AoV calcification in klotho-deficient mice.
Figure 5. Genetic loss of COX2 activity in klotho-deficient mice reduces AoV calcification.
Whole mount alizarin red staining reveals AoV calcification in klotho-deficient mice (Kl tm1Lex/tm1Lex; Ptgs2+/+) (n=7) (A), klotho-deficient mice with one mutated allele of the COX2 gene (Kl tm1Lex/tm1Lex; Ptgs2 +/Y385F) (n=14) (B) and klotho-deficient mice with 2 mutated alleles of the COX2 gene (Kl tm1Lex/tm1Lex; Ptgs2Y385F/Y385F) (n=6) (C). Alizarin red staining is indicated by white arrows, nuclei are counterstained with ToPro3 (blue), and the base of the AoV cusps is outlined with dotted lines. Von Kossa staining confirms valve calcification in Kl tm1Lex/tm1Lex; Ptgs2+/+ (D), and Kl tm1Lex/tm1Lex; Ptgs2+/Y385F (E) but not Kl tm1Lex/tm1Lex; Ptgs2Y385F/Y385F (F) mice (calcific regions outlined by black dotted lines in D and E, dark melanocytes are indicated by black arrowheads). AoV calcification, exclusive of aortic wall calcification, was quantified from alizarin red whole mount stained specimens and volumes are represented in the graph where the horizontal bar represents the median values and the error bars represent the minimum and maximum values obtained (G). The Kruskal-Wallis statistical test reveals significantly lower volumes of AoV calcification in Kl tm1Lex/tm1Lex; Ptgs2Y385F/Y385F mice compared to Kl tm1Lex/tm1Lex; Ptgs2+/+ mice (G, p=0.039).
Inhibition of COX2 activity by celecoxib treatment reduces AoV calcification and osteogenic gene expression in klotho-deficient mice
To determine if pharmacological inhibition of COX2 activity is sufficient to reduce AoV calcification in vivo, klotho-deficient mice were treated with the selective COX2 inhibitor celecoxib. Increased COX2 expression is present in aortic VICs as early as 3 weeks of age in klotho-deficient mice (Figure SIIF). Therefore animals were started on celecoxib diet at this stage. Klotho-deficient mice were split into two groups; the control group was fed a normal chow diet, whereas the treatment group was fed chow containing 1000ppm celecoxib ad libitum from 3 to 6 weeks of age. Animals were harvested at 6 weeks of age, and whole mount alizarin red staining was performed to obtain AoV calcification volumes, excluding aortic wall calcification (Figure 6A-C). Although variable, klotho-deficient mice fed normal chow diet have obvious AoV calcification (Figure 6A, C; n=12). In comparison, klotho-deficient mice fed celecoxib-containing diet have significantly reduced levels of AoV calcification (Figure 6B-C; n=12, p=0.038). Furthermore, celecoxib diet treatment significantly reduced mRNA expression of the osteogenic gene markers OPN and Runx2 in the AoVs of klotho-deficient mice (Figure 6E-F). Thus, pharmacologic inhibition of COX2 activity in vivo is sufficient to reduce osteogenic gene expression, in addition to valve calcification. As expected, COX2 mRNA expression is increased in klotho-deficient mice but is not affected by celecoxib treatment (Figure 6D). Vascular calcification in the valve sinus and ascending aorta was unchanged between animals treated with normal diet versus celecoxib-containing diet, suggesting that vascular calcification is relatively COX2-independent (Figure SVI). Serum phosphate levels were comparable in untreated and celecoxib-treated animals (Figure SVII), demonstrating that reduced AoV calcification by COX2 inhibition is not the result of normalized serum phosphate levels in klotho-deficient mice. Thus, COX2 inhibition by administration of celecoxib in vivo specifically reduces AoV calcification and osteogenic gene induction in klotho-deficient mice.
Figure 6. COX2 inhibition with celecoxib treatment reduces AoV calcification and osteogenic gene expression in klotho-deficient mice.
Klotho-deficient mice (Kl tm1Lex/tm1Lex) were fed either normal chow diet (A, n = 12) or diet containing 1000 ppm celecoxib (B, n = 12). AoV calcification was detected by whole mount alizarin red staining (A-B, valve annulus calcification is indicated by white arrows; nuclei are counterstained with ToPro3 in blue). The white * indicates aortic wall calcification in the valve sinus that was excluded from analysis (A-B). AoV calcification was quantified and plotted with the horizontal bar representing the median values and the error bars represent the minimum and maximum values (C). A Mann-Whitney test determined that treatment with celecoxib-containing diet significantly reduces the amount of AoV hinge region calcification in klotho-deficient mice (C, p=0.038, indicated by *). COX2 mRNA expression is increased in klotho-deficient mice and its expression is not changed with celecoxib treatment (D). mRNA expression of the osteogenic genes OPN (E) and Runx2 (F) is significantly reduced, and ALP (G) is trending downward by treatment with celecoxib diet in klotho-deficient mice (E, F). Statistical analysis was performed using ANOVA with post-hoc multiple comparisons test and p<0.05 was considered significant (designated by *).
Discussion
Here we demonstrate that COX2 inhibition reduces osteogenic gene induction and AoV calcification in a mouse model of CAVD. COX2 expression is increased in calcified AoVs of klotho-deficient mice and is localized to the VICs prior to the formation of AoV calcification, which occurs in the context of minimal inflammation. In human CAVD, COX2 expression is increased and is present in regions where the valve leaflet is calcified. In porcine VICs, COX2 mRNA expression is induced upon treatment with osteogenic media, and COX2 inhibition reduces osteogenic gene induction and calcification, supporting a direct role for COX2 in VIC mineralization. Further, COX2 inhibition through genetic or pharmacologic manipulation is sufficient to reduce AoV calcification and osteogenic gene expression in klotho-deficient mice. Thus, inhibition of COX2 activity is sufficient to reduce AoV calcification in vivo in mice.
COX2 has not previously been associated with the process of calcification in CAVD, but it is necessary for bone homeostasis and fracture healing. COX2 knockout mice display abnormal bone density and reduced ability to heal after fracture, and nonselective COX inhibitors are associated with delayed or inhibited bone fracture healing in humans (20, 22, 23, 32). Conversely, lentivirus-mediated COX2 overexpression has been used to promote bone fracture healing in mice (33). COX2 activity and its prostaglandin metabolites play a key role in osteogenic gene induction during bone formation and maintenance. In osteoblast cultures, prostaglandin treatment induces expression of the osteogenic genes BMP2, OCN and Runx2, while promoting matrix mineralization, and injection of prostaglandins in mice and rats induces bone formation in vivo (23, 34-38). Osteogenic gene induction also occurs during valve calcification in human diseased valves and in klotho-deficient mice (8, 9, 14). In the current study, we show for the first time that COX2 inhibition reduces the expression of OCN and BSP in cultured porcine VICs stimulated with osteogenic media and also reduces the expression of Runx2 and OPN in klotho-deficient mice treated with celecoxib diet. Furthermore, we show that COX2 expression is induced in the interstitial cells of klotho-deficient mice prior to the initiation of valve calcification. Thus, COX2 is activated prior to valve calcification and is required for osteogenic gene expression in the valves, as COX2 inhibition is able to block the expression of these factors and reduce valve calcification.
CAVD can result from abnormal valve development, inflammation due to increased lipid deposition, or hyperphosphatemia secondary to chronic kidney disease, and the cellular processes that lead to valve calcification may differ depending on the underlying factors of the disease (16, 19, 39, 40). Klotho-deficient mice develop hyperphosphatemia secondary to kidney failure, and valve calcification in these animals is likely related to chronic high serum phosphate (17, 18). Similarly, humans with chronic kidney disease or elevated serum phosphate levels have a higher risk for developing AoV calcification (16, 19). Valve endothelial injury, leading to the recruitment of infiltrating immune cells and subsequent VIC myofibroblast activation, has been implicated in the initial stages of CAVD (3). In the current study, we show that there is little evidence of macrophage infiltration in the aortic valves of klotho-deficient mice, and COX2 expression in VICs does not overlap with CD45 expression, suggesting that COX2-expressing cells are not leukocytes (Fig 3 and SIII). In addition, calcified VICs do not transition through a myofibroblast intermediate prior to calcifying in klotho-deficient mice, as indicated by lack of SMACreER lineage-positive calcified cells (Figure SV and SVI). Likewise, alpha SMA (ACTA2) gene expression was not increased in cultured porcine VICs treated with osteogenic media, consistent with published studies (data not shown and (41). Human diseased aortic valves also exhibit calcification and COX2 expression in regions with little immune infiltration or myofibroblast activation, suggesting that valve calcification can occur by multiple cellular mechanisms (Figure 2 and SI). Thus, there may be distinctive differences in the process of aortic valve calcification depending on the underlying factors contributing to the disease.
Non-selective COX1/COX2 inhibitors are non-steroidal anti-inflammatory drugs (NSAIDs) commonly used to treat inflammation associated with joint and/or muscle pain (24). The only currently FDA approved selective COX2 inhibitor, celecoxib (Celebrex), is used to treat pain associated with osteoarthritis, but is contraindicated for patients with heart disease risk factors (26, 27, 42). The current study demonstrates that COX2 inhibition via celecoxib treatment reduces calcification associated with CAVD in klotho-deficient mice and also reduces osteogenic gene induction and mineralization in cultured porcine VICs. This approach may not be feasible in human patients due to the increased cardiovascular risk associated with the use of COX2 inhibitors (26, 27). A recent observational study in the Multi-Ethnic Study of Atherosclerosis (MESA) participants showed that use of specific or non-specific COX inhibitors was not associated with lower AoV calcification scores (43). However this was not a placebo-controlled trial and additional research is necessary to determine the effectiveness of COX inhibitors in human CAVD.
Supplementary Material
Significance.
CAVD is a significant cause of morbidity and mortality. Understanding the molecular mechanisms that contribute to the formation and progression of valve calcification may lead to new pharmacotherapies as alternatives to surgery. In the klotho-deficient mouse model of CAVD, we show that COX2 expression is increased in valvular interstitial cells (VICs) at the hinge region of the aortic valves and localizes to the region of calcification once the calcific lesions have formed. We also show that human explanted CAVD tissues have increased COX2 expression when compared to healthy controls. Blockade of COX2 activity in vitro blocks osteogenic gene induction and mineralization in VICs. Further, genetic mutation or pharmacologic inhibition of COX2 activity in vivo is sufficient to reduce AoV calcification in the klotho-mouse model of CAVD. This study shows for the first time that COX2 inhibition reduces osteogenic gene induction and AoV calcification in vivo.
Acknowledgments
The authors would like to acknowledge Jonathan Cheek and Christina Alfieri for their technical support.
Sources of Funding
Funding for this project was provided by NIH 5F32-HL110390 and the American Heart Association 11POST7360040 (E.E.W.), NIH K23 HL085122 and Cincinnati Children’s Research Foundation (R.B.H.), NIH R01 HL094319 and NIH R01 HL114682 (K.E.Y).
Abbreviations
- CAVD
calcific aortic valve disease
- AoV
aortic valve
- AS
aortic valve stenosis
- COX2
cyclooxygenase 2
- Ptgs2
prostaglandin-endoperoxide synthase 2
- VIC
valvular interstitial cell
Footnotes
Disclosures
None.
References
- 1.Gohlke-Barwolf C, Minners J, Jander N, Gerdts E, Wachtell K, Ray S, Pedersen TR. Natural history of mild and of moderate aortic stenosis-new insights from a large prospective European study. Curr Probl Cardiol. 2013;38:365–409. doi: 10.1016/j.cpcardiol.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol. 2011;8:162–172. doi: 10.1038/nrcardio.2010.202. [DOI] [PubMed] [Google Scholar]
- 3.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O'Brien KD, et al. Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 6.Brennan JM, Edwards FH, Zhao Y, O'Brien S, Booth ME, Dokholyan RS, Douglas PS, Peterson ED, Team DEAR Long-term safety and effectiveness of mechanical versus biologic aortic valve prostheses in older patients: results from the Society of Thoracic Surgeons Adult Cardiac Surgery National Database. Circulation. 2013;127:1647–1655. doi: 10.1161/CIRCULATIONAHA.113.002003. [DOI] [PubMed] [Google Scholar]
- 7.Pibarot P, Dumesnil JG. Prosthetic heart valves: selection of the optimal prosthesis and long-term management. Circulation. 2009;119:1034–1048. doi: 10.1161/CIRCULATIONAHA.108.778886. [DOI] [PubMed] [Google Scholar]
- 8.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wirrig EE, Hinton RB, Yutzey KE. Differential expression of cartilage and bone-related proteins in pediatric and adult diseased aortic valves. J Mol Cell Cardiol. 2011;50:561–569. doi: 10.1016/j.yjmcc.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 11.Rajamannan NM. The role of Lrp5/6 in cardiac valve disease: experimental hypercholesterolemia in the ApoE−/− /Lrp5−/− mice. J Cell Biochem. 2011;112:2987–2991. doi: 10.1002/jcb.23221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu S, Gotlieb AI. Wnt3a/beta-catenin increases proliferation in heart valve interstitial cells. Cardiovasc Pathol. 2013;22:156–166. doi: 10.1016/j.carpath.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Meng X, Su X, Mauchley DC, Ao L, Cleveland JC, Jr., Fullerton DA. Bone morphogenic protein 2 induces Runx2 and osteopontin expression in human aortic valve interstitial cells: role of Smad1 and extracellular signal-regulated kinase 1/2. J Thorac Cardiovasc Surg. 2009;138:1008–1015. doi: 10.1016/j.jtcvs.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Cheek JD, Wirrig EE, Alfieri CM, James JF, Yutzey KE. Differential activation of valvulogenic, chondrogenic, and osteogenic pathways in mouse models of myxomatous and calcific aortic valve disease. J Mol Cell Cardiol. 2012;52:689–700. doi: 10.1016/j.yjmcc.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thubrikar MJ, Aouad J, Nolan SP. Patterns of calcific deposits in operatively excised stenotic or purely regurgitant aortic valves and their relation to mechanical stress. Am J Cardiol. 1986;58:304–308. doi: 10.1016/0002-9149(86)90067-6. [DOI] [PubMed] [Google Scholar]
- 16.Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol. 2009;20:381–387. doi: 10.1681/ASN.2008040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuro-o M. A potential link between phosphate and aging--lessons from Klotho-deficient mice. Mech Ageing Dev. 2010;131:270–275. doi: 10.1016/j.mad.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 19.Linefsky JP, O'Brien KD, Katz R, de Boer IH, Barasch E, Jenny NS, Siscovick DS, Kestenbaum B. Association of serum phosphate levels with aortic valve sclerosis and annular calcification: the cardiovascular health study. J Am Coll Cardiol. 2011;58:291–297. doi: 10.1016/j.jacc.2010.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Q, Rho JY, Fan Z, Laulederkind SJ, Raghow R. Congenital lack of COX-2 affects mechanical and geometric properties of bone in mice. Calcif Tissue Int. 2003;73:387–392. doi: 10.1007/s00223-002-0009-x. [DOI] [PubMed] [Google Scholar]
- 21.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 22.Robertson G, Xie C, Chen D, Awad H, Schwarz EM, O'Keefe RJ, Guldberg RE, Zhang X. Alteration of femoral bone morphology and density in COX-2−/− mice. Bone. 2006;39:767–772. doi: 10.1016/j.bone.2006.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, O'Keefe RJ. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109:1405–1415. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grosser T. Variability in the response to cyclooxygenase inhibitors: toward the individualization of nonsteroidal anti-inflammatory drug therapy. J Investig Med. 2009;57:709–716. doi: 10.2310/JIM.0b013e3181b04d1f. [DOI] [PubMed] [Google Scholar]
- 25.Narumiya S. Prostanoids and inflammation: a new concept arising from receptor knockout mice. J Mol Med (Berl) 2009;87:1015–1022. doi: 10.1007/s00109-009-0500-1. [DOI] [PubMed] [Google Scholar]
- 26.Salvo F, Fourrier-Reglat A, Bazin F, Robinson P, Riera-Guardia N, Haag M, Caputi AP, Moore N, Sturkenboom MC, Pariente A, et al. Cardiovascular and gastrointestinal safety of NSAIDs: a systematic review of meta-analyses of randomized clinical trials. Clin Pharmacol Ther. 2011;89:855–866. doi: 10.1038/clpt.2011.45. [DOI] [PubMed] [Google Scholar]
- 27.Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 28.Hajdu Z, Romeo SJ, Fleming PA, Markwald RR, Visconti RP, Drake CJ. Recruitment of bone marrow-derived valve interstitial cells is a normal homeostatic process. J Mol Cell Cardiol. 2011;51:955–965. doi: 10.1016/j.yjmcc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wendling O, Bornert JM, Chambon P, Metzger D. Efficient temporally-controlled targeted mutagenesis in smooth muscle cells of the adult mouse. Genesis. 2009;47:14–18. doi: 10.1002/dvg.20448. [DOI] [PubMed] [Google Scholar]
- 30.Hood WF, Gierse JK, Isakson PC, Kiefer JR, Kurumbail RG, Seibert K, Monahan JB. Characterization of celecoxib and valdecoxib binding to cyclooxygenase. Mol Pharmacol. 2003;63:870–877. doi: 10.1124/mol.63.4.870. [DOI] [PubMed] [Google Scholar]
- 31.Yu Y, Fan J, Chen XS, Wang D, Klein-Szanto AJ, Campbell RL, FitzGerald GA, Funk CD. Genetic model of selective COX2 inhibition reveals novel heterodimer signaling. Nat Med. 2006;12:699–704. doi: 10.1038/nm1412. [DOI] [PubMed] [Google Scholar]
- 32.Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br. 2003;85:700–705. [PubMed] [Google Scholar]
- 33.Lau KH, Kothari V, Das A, Zhang XB, Baylink DJ. Cellular and molecular mechanisms of accelerated fracture healing by COX2 gene therapy: studies in a mouse model of multiple fractures. Bone. 2013;53:369–381. doi: 10.1016/j.bone.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Arikawa T, Omura K, Morita I. Regulation of bone morphogenetic protein-2 expression by endogenous prostaglandin E2 in human mesenchymal stem cells. J Cell Physiol. 2004;200:400–406. doi: 10.1002/jcp.20031. [DOI] [PubMed] [Google Scholar]
- 35.Choudhary S, Alander C, Zhan P, Gao Q, Pilbeam C, Raisz L. Effect of deletion of the prostaglandin EP2 receptor on the anabolic response to prostaglandin E2 and a selective EP2 receptor agonist. Prostaglandins Other Lipid Mediat. 2008;86:35–40. doi: 10.1016/j.prostaglandins.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao Q, Xu M, Alander CB, Choudhary S, Pilbeam CC, Raisz LG. Effects of prostaglandin E2 on bone in mice in vivo. Prostaglandins Other Lipid Mediat. 2009;89:20–25. doi: 10.1016/j.prostaglandins.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Jee WS, Ueno K, Kimmel DB, Woodbury DM, Price P, Woodbury LA. The role of bone cells in increasing metaphyseal hard tissue in rapidly growing rats treated with prostaglandin E2. Bone. 1987;8:171–178. doi: 10.1016/8756-3282(87)90017-2. [DOI] [PubMed] [Google Scholar]
- 38.Minamizaki T, Yoshiko Y, Kozai K, Aubin JE, Maeda N. EP2 and EP4 receptors differentially mediate MAPK pathways underlying anabolic actions of prostaglandin E2 on bone formation in rat calvaria cell cultures. Bone. 2009;44:1177–1185. doi: 10.1016/j.bone.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of 'degenerative' valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 40.Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol. 1970;26:72–83. doi: 10.1016/0002-9149(70)90761-7. [DOI] [PubMed] [Google Scholar]
- 41.Monzack EL, Masters KS. Can valvular interstitial cells become true osteoblasts? A side-by-side comparison. J Heart Valve Dis. 2011;20:449–463. [PMC free article] [PubMed] [Google Scholar]
- 42.Singh G, Fort JG, Goldstein JL, Levy RA, Hanrahan PS, Bello AE, Andrade-Ortega L, Wallemark C, Agrawal NM, Eisen GM, et al. Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS-I Study. Am J Med. 2006;119:255–266. doi: 10.1016/j.amjmed.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 43.Delaney JA, Lehmann N, Jockel KH, Elmariah S, Psaty BM, Mahabadi AA, Budoff M, Kronmal RA, Nasir K, O'Brien KD, et al. Associations between aspirin and other non-steroidal anti-inflammatory drugs and aortic valve or coronary artery calcification: the Multi-Ethnic Study of Atherosclerosis and the Heinz Nixdorf Recall Study. Atherosclerosis. 2013;229:310–316. doi: 10.1016/j.atherosclerosis.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.