Abstract
Decellularized tissues have become a common regenerative medicine platform with multiple materials being researched in academic laboratories, tested in animal studies, and used clinically. Ideally, when a tissue is decellularized the native cell niche is maintained with many of the structural and biochemical cues that naturally interact with the cells of that particular tissue. This makes decellularized tissue materials an excellent platform for providing cells with the signals needed to initiate and maintain differentiation into tissue-specific lineages. The extracellular matrix (ECM) that remains after the decellularization process contains the components of a tissue specific microenvironment that is not possible to create synthetically. The ECM of each tissue has a different composition and structure and therefore has unique properties and potential for affecting cell behavior. This review describes the common methods for preparing decellularized tissue materials and the effects that decellularized materials from different tissues have on cell phenotype.
Keywords: decellularization, differentiation, extracellular matrix, tissue engineering, stem cell
1. Introduction
Decellularized tissues are widely used clinically for tissue repair and regeneration. A few tissues such as dermis, small intestinal submuscosa, urinary bladder, and pericardium from humans, pigs, and cows have been decellularized to create commercially available scaffolds such as AlloDerm, CuffPatch™, MatriStem, Pelvicol, and Dura-Guard® [1]. However, in basic research and preclinical studies, numerous tissues and organs have now been decellularized and used in various regenerative medicine applications [2]. The extracellular matrix (ECM), which remains after decellularization, plays a crucial role as a structural support for tissue as well as a source of biochemical and biophysical cues for the cells that reside within it. Through these two roles the ECM directs cell proliferation, migration, differentiation, and behavior [3]. The ECM of each tissue provides a unique tissue specific microenvironment for resident cells. This cell niche has been adapted by nature to provide the cells with the structure and biochemical cues that are required for their function [4]. It has therefore been hypothesized that decellularized tissue materials should have distinct effects on cell differentiation based on which tissue the material was sourced. Using nature’s tailored cell niches to direct cells to differentiate towards specific cell lineages has become a popular avenue for tissue engineering studies. By understanding decellularized tissues’ effects on cell differentiation more thoroughly, this technology could provide a useful platform for controlling cell fate and generating regenerative therapies.
This manuscript will review the different methods for creating decellularized tissue materials and the studied effects of these materials on cell behavior, with a focus on differentiation. These materials can be in the form of entire organ scaffolds, slices or blocks of ECM, hydrogels, and coatings, and have been derived from the following tissues: lung, liver, kidney, heart, central nervous system, adipose, tendon, skeletal muscle, cartilage, bone, nucleus pulposus, uterus, corneal stroma, musculofascia, trachea, and dermis.
2. Preparation of Decellularized Materials
2.1. Decellularization Methods
The goal of decellularizing tissues is to maintain all of the structural and biochemical cues in the ECM, but remove the cellular components that cause an adverse inflammatory response in the host and hinder regeneration [2]. The different methods used for decellularization utilize mechanical, chemical, enzymatic, or detergent approaches [2, 3]. The protocols for decellularizing different tissues have been reviewed thoroughly, but there is no consensus for the best protocols to use with each tissue [2, 5, 6]. When choosing decellularization protocols it is important to realize that one method does not work for every tissue, since tissues vary in ECM density, cell density, and basic morphology [2]. Other reviews have more thoroughly examined different decellularization protocols and the effects of the commonly used methods. The reader is referred to several excellent reviews in this area [1, 2, 7]. Briefly, freeze-thawing can affect the ultrastructure of the ECM, pressure techniques may affect mechanical properties of the ECM fibers, alkaline and acidic solutions can degrade components of the ECM, ionic detergents (like sodium dodecyl sulfate) disrupt non-covalent bonds between proteins and can be problematic to remove from the matrix, and alcohol has been shown to crosslink collagen in the ECM increasing its stiffness [1, 2, 6–8]. All of these factors can also affect how cells interact with the materials because they change the environment with which the cells are in direct contact. Therefore, care must be taken to choose the ideal decellularization protocol for each application because it can affect the success of directing cells towards a specific lineage [9]. In short, considerations must be made in designing and selecting the optimal decellularization process before the materials are manipulated into their final form.
2.2. Material Preparation
While decellularized tissue materials can be left as an intact whole organ, they can also be further processed by cutting into small sections or blocks, or digesting into a liquid to form a hydrogel or coat a substrate. Each of these categories of materials has been used with multiple tissues and may provide different benefits. Keeping the whole organ intact during the decellularization process is especially useful when the goal is to engineer a transplantable organ. Decellularizing cadaveric organs maintains the macro and micro architectures of the organ along with the natural ECM composition, thereby providing a platform for recellularization and regeneration of the organ [1]. Perfusion decellularization has been used on lungs, livers, kidneys, and hearts from rats, mice, pigs, and humans to create acellular organ scaffolds [10–19]. The process of perfusion decellularization requires flowing the decellularizing reagents through native arteries at physiological pressures in order to enter the innate blood vessel system and reach every cell [20]. Thin tissues, like mouse tibialis anterior, and ones that do not have easily accessible blood vessel systems, like cartilage, may use submersion and agitation techniques [21, 22].
If an entire organ is not required for a study, then thin sheets of the decellularized organ can be used as a more convenient method for testing the ECM’s effect on cells while still maintaining the tissue’s architecture and biochemical composition. The two main approaches for creating these slices are either to first decellularize the entire organ then cut it into slices or do the opposite by sectioning the unprocessed organ and then decellularizing the slices. To section the organ after decellularization, the organ must first be stiffened either by embedding the organ in agarose, OCT freezing medium, or paraffin [9–12, 23, 24]. However, the most common approach to create decellularized sections is to first cut the tissue into the desired thickness and then decellularize the slices of tissue. This approach has been used for tendon, brain, kidney, lung, liver, corneal stroma, and dermis [25–30]. Decellularized materials can also be created with sizes between whole organ decellularization and thin slices, for example 4 × 4 × 3 mm cubes of bone [31] or 4 × 2 × 0.5 cm pieces of musculofascial tissue [32].
Decellularized tissue can also be processed further into hydrogels. Hydrogels are commonly used with the goal of injecting into sites of injury to promote regeneration, but are also a favorable platform for cell culture because they can be made in larger quantities and designed to retain cells more easily than ECM sections. The methods of preparation vary greatly depending on the application and the characteristics that need to be modified for an experiment. The strategies include using decellularized matrix as the only component of the hydrogel, using cross-linking agents, or creating composites with additional materials. A common method for making hydrogels with ECM as the only element in the material requires lyophilizing, milling, and digesting the ECM with pepsin. At this point the ECM can be brought to body temperature to form a hydrogel [30, 33–37]. These materials have been used alone in vivo [37–40] or to create a 2D and 3D culture environments in vitro [36, 40–42]. Another approach is to continue manipulating the hydrogel after it is created and make it into a composite material [40–44]. Composite hydrogels can be fabricated by using photo-crosslinking [40, 41], chemical crosslinking [43], or addition of collagen with the goal of improving cell delivery ability and/or altering mechanical properties and degradation rate of the material [42, 44]. Another modification to hydrogels is the use of cross-linking within the ECM alone, which can increase the stiffness of the material and increase degradation times [45, 46]. Table 1 summarizes the hydrogel chemistries of the reviewed manuscripts.
Table 1.
Hydrogel Formation
| Hydrogel Formation | Organ/Tissue Type | Composite? | Citations |
|---|---|---|---|
| Self-Assembly | Adipose, Heart, Kidney, Liver, Tendon, Urinary Bladder | No | [33],[37],[35],[47],[36], [30],[48], [34] |
| Self-Assembly | Heart, Tendon |
Yes Collagen |
[42],[44] |
| Photo-Crosslinked (Methacrylation) | Adipose, Cartilage, Meniscus, Tendon |
Yes Glycol Chitosan, Chondroitin Sulfate, Gelatin |
[40],[41] |
| Chemically Crosslinked (NHS) | Heart |
Yes Polyacrylamide |
[43] |
| Chemically Crosslinked (EDC and NHS) | Heart, Nucleus Pulposus | No | [46],[45] |
The final category of ECM materials is a substrate coating. A liquid form of the ECM material is first created with a digestion process similar to making hydrogels, and then the ECM proteins are adsorbed onto a tissue culture surface [49–52]. Cells are then seeded onto the coated plates or wells and can interact with the specific biochemical cues. A polyacrylamide gel with various degrees of stiffness may be used beneath the coating to alter the mechanical cues of the cell niche [53, 54]. Coatings are comparable to thin decellularized tissue slices, in that they create a 2D surface to study; however, they no longer maintain the native architecture of the tissue.
3. ECM Effects on Cell Differentiation
Each tissue has a unique ECM composition that affects cell differentiation in a variety of ways. A representation of differences in tissue architectures can be found in Figure 2. In addition to the architecture of the tissue, the biochemical cues play a significant role in determining cell fate. A variety of experiments have been completed to study this interaction with various tissues, cell types, and conditions. Table 2 summarizes the tissues, materials, and cell types used in the reviewed studies.
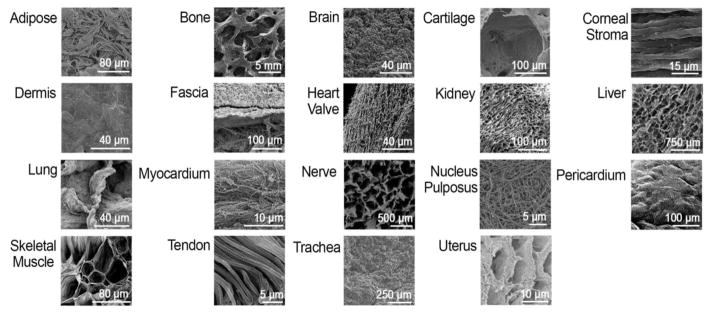
Figure 2.
Representative scanning electron microscopy (SEM) images for different decellularized tissues. These images demonstrate the vast differences in microenvironment architecture that are found in different tissues. These architectures make up the local niche that cells interact with and are therefore understood to play a significant role in shaping cell differentiation into corresponding cell lineages. SEM images reprinted with permission from [23, 31, 32, 36, 55–68].
Table 2.
Cell and ECM Materials
| Organ/Tissue | Cell Types | Material Types | Citations |
|---|---|---|---|
| Adipose | ASC, MSC, Host Cell | Hydrogel, Coating | [53], [33], [40], [80], [79] |
| Central Nervous System | iPSC derived nueron, PC12, MSC, NSC | Section, Coating | [77], [78], [56], [25] |
| Cartilage | ASC, MSC | Hydrogel | [57], [79], [41], [21] |
| Heart | MSC, ESC, ESC derived cardiomyocyte, cardiac progenitor, iPSC derived MSC, neonatal ventricular myocyte | Hydrogel, Composite Hydrogel, Entire Organ, Coating | [75], [51], [72], [42], [43], [49], [46], [54], [76], [73] |
| Kidney | ESC, Kidney Stem Cell, Osteoblast | Hydrogel, Entire Organ, Section | [17], [36], [29], [18], [19] |
| Liver | hepatocyte, progenitor hepatocyte, fetal hepatocyte, fetal satellite, ASC, MSC | Hydrogel, Entire Organ, Section, Coating | [30], [71], [16], [50], [52] |
| Lung | ESC, MSC, Fibroblast, lung epithelial cell, endothelial progenitor cell, ESC derived endoderm cell, iPSC derived alveolar epithelial cell and lung progenitor | Entire Organ, Section | [69], [71], [10], [23], [29], [11], [12], [13], [14], [15], [70] |
| Skeletal Muscle | C2C12, Host Cell, ASC | Entire Organ, Coating, Section, Scaffold | [51], [22], [83], [32], [82] |
| Tendon | MSC, ASC, Tendon Stem Cell, Progenitor Tendon Cell | Hydrogel, Section | [24], [48], [44], [81], [27], [26] |
3.1. Lung
Many studies have shown that decellularized lung matrices are able to encourage lung specific cell differentiation of more mature cell types such as progenitor cells, however other more naïve cell types require additional cues to initiate differentiation into lung specific lineages. Cortiella et al. seeded murine embryonic stem cells (mESCs) onto a small block of acellular rat lung matrix with lung cell culture media. The study showed an increase in lung epithelial and endothelial cell markers on cells cultured on the acellular lung compared to cells cultured on Gelfoam, Matrigel, and Collagen 1 [69]. However, the differences in porosity and stiffness of the lung ECM, compared to the controls used, do not allow for the conclusion that the effects are due to the ECM itself and not the mechanical and transport property variability of the materials. Increased lung epithelial cell markers were also seen with human derived mesenchymal stem cells (hMSCs) cultured in a whole decellularized rat lung with small airway epithelial growth medium in a perfusion bioreactor [13]. These previous studies used supplemented media to achieve their results. Further studies also suggest that media supplements are required and lung ECM cannot induce lung specific differentiation of MSCs with regular growth media alone [10]. Zhou et al. showed that the mouse induced pluripotent stem cells (iPSCs) required initial differentiation in order to become integrated into a whole decellularized mouse lung. The undifferentiated cells formed clusters and did not show differentiated phenotypes when grown on lung sections [11]. However, when human iPSCs were differentiated into lung progenitor cells before seeding onto decellularized human lung slices, there was increased lung specific differentiation into alveolar epithelial cells compared to non-ECM cultures [12]. An additional study demonstrated that mouse embryonic stem cell (ESC) derived endoderm cells differentiated into multiple mature lung epithelium cell types with no additional growth factors or serum in the growth medium when cultured on thick sections of decellularized rat lung [70]. This study also concluded that the differentiation required heparan sulfate proteoglycans [70]. These studies showed that lung ECM had a beneficial effect on differentiation into lung specific lineages, but it did not provide all of the cues necessary to initiate the differentiation.
Lung derived materials have also been shown to have tissue specific effects. When mouse alveolar and hepatocyte progenitor cells were grown on thick slices of decellularized rat lung and liver, the tissue-specific material best guided differentiation of the corresponding progenitor cell [71]. The influence of these tissue specific cues on differentiation depends on which cells and tissues are being compared. When human embryonic stem cells (hESCs) were cultured on decellularized rhesus monkey kidney and lung sections, both materials led to an increase in kidney and lung markers. It was hypothesized that the tissue-specific materials did not improve differentiation into their corresponding cell lineages because of their similarity in architecture [29]. Additionally, ESCs are in a less differentiated state than the progenitor cells and therefore may require more cues to determine their cell lineage. There are still many unknowns about how lung matrices affect different cells. For example, when rat alveolar type II cells were cultured on a whole decellularized rat lung they lost all of their epithelial markers and began expressing mesenchymal markers [15].
Another area of interest is not only the extent of differentiation that the ECM can direct, but also how differences in the matrix can affect stem cell behavior. While few studies to date have investigated the effect of diseased lung matrices on cell differentiation, proliferation and viability assessments showed that the intact 3D architecture of a decellularized emphysematous human lung was not able to sustain cell growth of human bronchial epithelial cells, endothelial progenitor cells, hMSCs, and lung fibroblasts [14]. One study also showed that primary human fibroblasts grown on fibrotic human lung matrix slices pushed them to a myofibroblast lineage [23]. These studies are part of the ever-growing body of knowledge about how lung ECM can be used for tissue engineering.
3.2. Liver
Multiple groups have demonstrated that liver ECM is able to direct cells towards or maintain a hepatocyte phenotype. Initial studies determined that primary human hepatocytes seeded between two layers of decellularized porcine liver hydrogel maintained their hepatocyte phenotype as successfully as when cultured on matrigel when looking at functional qualities like albumin secretion, transport ability, and ammonia metabolism [30]. A later study completed a thirteen day perfusion of human fetal hepatocytes and human fetal satellite cells into an entire decellularized porcine liver and showed a trend towards full maturation of the cells into epithelial cells according to phenotype and gene analysis [16]. More recently, groups have investigated the potential of decellularized liver to differentiate cells into hepatocytes. Lee et al. studied human adipose-derived stem cell (hASC) differentiation on rat liver ECM versus collagen in both coating and hydrogel forms. They observed a significant increase in differentiation on the ECM materials compared to collagen [50]. However, the study was done with hepatocyte culture media, so it did not fully characterize the liver ECM’s potential alone. Zhang et al. further studied a decellularized rat liver coating in relation to collagen, Matrigel, and fibronectin with and without growth factors. In all cases mouse adipose derived MSCs had improved hepatic differentiation on the liver ECM [52]. Rat liver ECM has also shown that is has organ specific characteristics by successfully guiding differentiation of mouse hepatocyte progenitor cells but not mouse alveolar progenitors [71]. Overall it has been demonstrated that liver ECM has the potential to maintain and guide hepatocyte cell lineages.
3.3. Kidney
There have been a variety of experiments performed with decellularized kidney materials. Perfusion of an entire decellularized kidney with cells has demonstrated great promise for the use of the kidney matrix for tissue engineering. When mESCs were incorporated into a whole decellularized rat kidney, epithelial differentiation was seen on the cells lining the native vasculature in the matrix [17]. Similarly, a different study observed that when mESCs were perfused into an intact decellularized rat kidney the cells in peritubular capillaries and tubular compartments began to differentiate into meso-endodermal lineages [18]. These studies both suggested that the kidney matrix has region specific effects on differentiation. O’Neil et al. examined the effects of kidney regions directly by creating ECM slices, hydrogels, and media supplements from the medulla, papilla, and cortex of porcine kidneys. They saw that mouse kidney stem cells had different metabolic activity, proliferation, and morphology in each kidney region [36]. Although differentiation was not directly measured, the differences indicate that there may also be region-specific effects on stem cell differentiation due to different mechanical or biochemical cues. It is important to note that none of these studies directly concluded that the kidney caused kidney specific cell differentiation. As mentioned above, a study that compared the effect of rhesus monkey kidney and lung matrix slices on hESCs showed that both matrices caused an increase in lung and kidney markers, with no significant differences between each other [29]. Burgkart et al. had a unique experimental set up where they showed that a decellularized kidney could be used for bone engineering because the intact vasculature provided nutrients to the osteoblasts. In this study they perfused a whole decellularized rat kidney with primary human osteoblasts using osteogenic media and saw that the osteoblasts kept their phenotype and reconstructed the matrix [19]. These results highlight that there is still little understood about the kidney ECM niche and how it can affect differentiation.
3.4. Heart
Three cardiac tissues have been decellularized and studied to show how they affect stem cell differentiation: myocardium, heart valve, and pericardium. Several groups have looked at the effect of myocardium ECM on cell lineage. The general conclusion was that the decellularized material alone was able to guide differentiation and maturation of cells into a cardiac cell fate. This was demonstrated with hESCs, hESC derived cardiomyocytes, human mesendodermal cells, rat cardiac progenitor cells, and neonatal rat ventricular myocytes on entire decellularized hearts, hydrogels, collagen composite hydrogels, and 2D coatings [42, 49, 51, 72–74]. One of these studies encapsulated hESC embryoid bodies into hydrogels with varying percentages of porcine heart ECM and observed that the higher percentage of ECM correlated with increased cardiac markers and increased number of contracting cells with larger contraction amplitudes [42]. The studies mentioned above all used healthy adult hearts to create the decellularized materials. When fetal, neonatal and adult rat heart ECMs were compared, the neonatal cardiac ECM hydrogel was able to promote Nkx2.5 and GATA4 expression in rat MSCs better than the others [43]. Comparing rat MSCs cultured on an ECM coating from infarcted rat hearts and healthy rat hearts showed that ECM from infarcted tissue caused MSCs to release proangiogenic, anti-fibrotic, and cytoprotective factors and did not lead to mesenchymal stem cell (MSC) differentiation into cardiomyocytes [54].
A study of porcine heart valve ECM demonstrated that the ECM from the ventricularis and fibrosa regions both guided hMSCs toward valve interstitial cell lineages, with the ventricularis being more significant [75]. There was also location specific differentiation when human iPSC derived MSCs were seeded onto human pulmonary valve leaflets. Cells on the surface of the valve expressed more α-smooth muscle actin compared to cells deeper in the tissue [76]. In addition to studying the heart valve, people have looked at the pericardium. It was observed that making a decellularized hydrogel from human pericardium and then lyophilizing it created a successful platform to increase the expression of early cardiac markers in human cardiac progenitor cells compared to collagen and non-lyophilized decellularized pericardium [46]. They showed that the lyophilization increased the interconnectivity of the pores in the material and therefore improved cell migration and nutrient transportation [46]. This suggests there may be benefits of testing alternative processing procedures for decellularized ECM materials.
3.5. Central Nervous System
Research on the effect of decellularized tissue from the central nervous system is more limited compared to other tissues. A porcine brain-derived ECM coating was used to culture human iPSC derived neurons and increased dendritic branching was observed compared to a Matrigel coating, suggesting improved neural maturation on the matrix [77]. A different study looked at the effect of ECM solubilized and incorporated into the media for culturing PC12 cells, a cell line that can differentiate into neural-like cells. Matrix from porcine optic nerve, spinal cord, brain, and urinary bladder were compared and all four matrices promoted neural differentiation while the central nervous system materials also promoted cell migration [78]. Further, an electrospun gelatin material incorporating rat brain ECM showed successful cell adhesion, proliferation, survival, and preliminary differentiation of rat MSCs towards neural progenitors [56]. These studies showed potential for the use of decellularized central nervous system tissue for neural cell differentiation, although they do not confirm that the tissue specific matrix is necessary. Conversely, one study looked at the opposite effect, namely maintenance of an undifferentiated phenotype. Waele et al. used decellularized mouse brain sections as a platform to culture rat neural stem cells (NSCs). They were able to maintain the NSC phenotype without further differentiation with media supplements such as epidermal growth factor and human basic fibroblast growth factor [25]. This may suggest that cues from nervous system ECM that were shown to promote differentiation in the previously mentioned studies can be overridden simply with media supplements.
3.6. Adipose
Adipose tissue is uniquely accessible from living human donors because of the prevalence of liposuction surgeries and has been shown to create a matrix that directs adipogenic differentiation. Studies of the effect of decellularized adipose tissue on cell differentiation have focused both on in vitro and in vivo studies. One in vitro experiment studied the combination of biochemical and mechanical cues of the native cell niche by coating various polyacrylamide gels with a decellularized human adipose tissue coating. Increased adipogenic markers were observed on hASCs cultured on gels of lower stiffness, confirming that this engineered environment provided the necessary cues to direct differentiation [53]. Another in vitro study compared a composite hydrogel, made of decellularized adipose tissue with methacrylated glycol chitosan or methacrylated chondroitin sulfate, that incorporated decellularized human adipose tissue to a hydrogel with no ECM and showed that there was increased glyrcerol-3-phosphate dehydrogenase activity (an important enzyme in lipid metabolism) and adipogenesis in the hASCs on the composite hydrogel [40]. A third group created a 3D printed bioink from porcine adipose matrix and encapsulated hASCs in the gel before forming the scaffold. They observed that hASCs cultured in the material successfully committed to adipogenic lineages [79]. The results described all show how decellularized adipose tissue was capable of providing the necessary biochemical cues to influence hASCs to differentiate down adipogenic lineages. When a human adipose matrix hydrogel combined with human ASCs or a cross-linker was injected into mice subcutaneously there was increased adipogenesis in the material compared to Juvederm injections, which is currently the clinical standard for filling adipose deficits [33]. However, the adipogenesis occurred through endogenous cells rather than the transplanted hASCs. Another group injected mouse adipose ECM with basic fibroblast growth factor into mice subcutaneously and saw adipogenesis in the material along with host tissue infiltration [80]. Together, these studies demonstrate the ability of decellularized adipose tissue materials to promote adipogenic differentiation.
3.7. Tendon
Differentiation of stem cells into tenocytes using decellularized tendon scaffolds has been studied with an assortment of approaches with varying success. One study found that the cues from porcine tendon ECM sections were able to cause human tendon progenitor cells to differentiate towards tendon lineage even in the presence of osteogenic growth factors [27]. In contrast, Zhang et al. observed that rabbit tendon stem cells were able to proliferate and remain undifferentiated on rabbit tendon ECM films. However, when the same cells were injected into the back of rats with a rabbit tendon ECM hydrogel, tendon-like tissue formed, a result not observed following cell only injections [48]. This implies that additional cues were required in order to continue differentiation from the tendon stem cell stage.
Many groups incorporated mechanical and architectural cues to the decellularized tissue constructs to further control differentiation and were more successful in directing tenogenesis. When canine ASCs were grown in 3D culture in a collagen and human tendon ECM composite hydrogel with static tension, the cells underwent differentiation towards tenocytes and showed increased alignment and tenomodulin expression [44]. One group observed improved horse MSC integration and differentiation into thick horse tendon ECM sections with 3% strain compared to lesser values [26]. To increase tendon-like architecture of the material, one study took canine MSC-seeded tendon sections and sutured them together to create bundles that were cultured in suspension. This led to cell alignment and an increase in tenomodulin expression compared to day zero [24]. Additionally, a study from Tong et al. argued that the process of decellularizing tendon tissue changes its crucial tissue architecture, thus instead of decellularization they cultured human MSCs directly onto histological sections of bovine tendon [81]. To further test their hypothesis they used their sections as templates and molded polydimethylsiloxane (PDMS) replicas of the topography of the slices. When hMSCs were seeded on collagen coated PDMS replicas they elongated like tendon cells and expressed tenomodulin [81]. Though the decellularized material showed positive results, there is continued investigation necessary to completely understand the mechanisms that factor into tenocyte differentiation and how best to recreate its microenvironment.
3.8. Skeletal Muscle
Studies into the effects of decellularized muscle on cell differentiation are limited. C2C12s, a mouse myoblast cell line, cultured on a coating of decellularized porcine muscle ECM exhibited increased muscle differentiation compared to a collagen coating [51]. Additionally, when a decellularized mouse muscle scaffold graft was transplanted into a mouse there was successful host cell infiltration and multinucleated myofibers were seen in the scaffold [22]. Similar results were however also seen when a decellularized porcine urinary bladder scaffold was transplanted into mice [82]. Wang et al. sought to replicate the musculofascial tissue environment by using decellularized porcine muscle and decellularized porcine fascia seeded with hASCs. They determined that the muscle scaffold had myogenic and angiogenic potential while the decellularized fascia provided more mechanical support [32]. The effects of muscle ECM scaffolds may however not dominate over soluble differentiation cues. Perniconi et al. observed that although mouse muscle ECM can provide the necessary cues for differentiation along a muscle lineage, it does not prevent differentiation to other lineages when cells are exposed to specific growth factors [83]. While some of these results are promising, further research is still needed on decellularized skeletal muscle materials for tissue engineering.
3.9. Cartilage
Decellularized cartilage has been tested in many different forms and shown to direct chondrocyte specific differentiation to a certain extent. When differentiation media was added to canine MSCs cultured on a human cartilage ECM porous disc-shaped scaffold, the cells proliferated and differentiated into chondrocytes. The same material implanted in vivo led to cartilage-like tissue formation [57]. Another group 3D printed a porcine cartilage ECM bioink with a polycaprolactone frame and observed higher expression of chondrocyte specific genes in hMSCs on the ECM material than on collagen [79]. A third approach was to decellularize an entire bovine ear and culture hMSCs on small punches from the decellularized organ. The cells expressed more chondrogenic-specific genes when cultured on the scaffold compared to a monolayer [21]. Finally, Visser et al. created a composite hydrogel with the equine cartilage ECM and gelatin methacrylamide but did not see clear improvements between composites with and without the ECM [41]. Compared to several tissues mentioned above, there are currently limited studies with cartilage ECM, including those with conflicting results, and therefore further investigation is needed.
3.10. Other Tissues
Several studies have observed benefits of using decellularized tissue materials for cell differentiation in tissues besides those mentioned above. One group decellularized blocks of porcine bone and observed rat MSC differentiation towards osteogenic lineage without added dexamethasone [31]. Another study created a decellularized porcine nucleus pulposus hydrogel and observed nucleus pulposus positive cell markers in hASCs cultured on the hydrogel with no differentiation media added [45]. A decellularized rat uterus that was recellularized with a mixture of rat neonatal uterine cells, adult uterine cells, and MSCs formed endometrium-like tissue in vitro and successfully supported pregnancy in a grafted uterus [84]. Also, hASCs grown on decellularized human corneal stroma sections differentiated into keratocytes [28]. Finally, a study showed that the biochemical cues from decellularized porcine dermis and mesothelium can be overridden by using differentiation media to differentiate hMSCs down adipose or osteoblast lineages [85]. The success of decellularized materials in these various tissues demonstrates that this approach can be used for even more tissues to direct cell differentiation.
4. Current Challenges and Future Opportunities
There are common themes explored within each tissue type that provide their own sets of challenges moving forward. A common experimental approach was exploring the role of biochemical and mechanical cues of the decellularized ECM on stem cell differentiation. These studies provide important insight into the effects of these cues, but are often limited in how many combinations of mechanical and biochemical factors can be probed. The ECM itself is a complex combination of biochemical cues and therefore it can be difficult to determine which specific components or combinations thereof are causing certain cell behaviors. Additionally, the majority of studies that included stiffness in their design were limited to 2D cultures, as opposed to 3D cultures, which are arguably more physiologically relevant. The combination of more quantitative analysis of the components of the ECM [86, 87] along with high throughput cell-biomaterial interaction screening [88, 89], which can simultaneously examine multiple biochemical cues and mechanical properties, could enable more mechanistic future studies.
Another common question guiding these studies was whether there were tissue or region specific effects on differentiation. These results varied based on which tissues were being compared. This suggests that the ECM of some tissues are more similar than others and studies of this nature should compare a large variety ECMs in order to investigate this theme more thoroughly. The region specific effects on differentiation in highly complex organs, such as the kidney, provide an interesting consideration for all decellularized tissue studies. Even studies that do not look at region specificity must still be cautious in creating samples due to possible regional variations in composition and therefore varied effects on differentiation.
The effects of alternative processing methods on the ECM are also clearly important based on several of the studies reviewed. This provides an additional challenge to researchers probing ECM effects because the material preparation itself can alter the outcomes of the experiment. It is therefore important to continue working towards improved processing, but to also keep the processing consistent when comparing results. Furthermore, more quantitative analysis of the ECM, such as QconCat based mass spectrometry [86, 87], is needed to more rigorously assess differences in the ECM and efficiency of decellularization after processing.
The last theme that was repeated with many tissues is the effect of diseased decellularized ECM on the material’s ability to guide differentiation. This is both important for understanding disease mechanisms, but also for looking forward towards scaling up the production of decellularized ECM materials. Many of the available human samples that could be used for decellularized materials are either aged or diseased. In order to ensure consistent materials are being created, the variables (including the health and age of the donor) that may affect the material quality must be thoroughly understood.
Addressing these challenges will provide an avenue to further expand the use of decellularized ECM as a basic research platform for studying stem cell biology, as well in preclinical studies with the goal of moving towards clinical translation. To date, clinical application with decellularized ECM has included acellular administration, mainly in the form of ECM sheets as surgical patches [90, 91], and recently the initiation of a clinical trial with an injectable cardiac ECM derived hydrogel (clinicaltrials.gov identifier NCT02305602). The acellular route is undoubtedly easier in terms of translation and has the potential to modulate the fate of endogenous progenitor cells. However, the studies reviewed herein also suggest that ECM scaffolds have potential as cell delivery scaffolds, although this significantly increases treatment costs and has a more difficult regulatory pathway as a combination product. More long term development could result in the use of decellularized ECM for whole organ engineering [92–95]; however, this increase in complexity creates numerous more challenges that must be overcome, including creating fully functional constructs and preventing thrombosis in the vasculature [96, 97].
5. Conclusion
The use of decellularized tissue materials has had success in many different applications. The current designs of these materials include different types of entire organ matrices, sections, blocks, hydrogels and coatings. There is room to expand on the materials and methods for creating decellularized ECM in order to maintain the native architecture and incorporate all of the cues that are beneficial for cell differentiation [1–3]. Each tissue has its own obstacles when decellularizing and processing the material, and as new tissues are used for decellularization experiments there will be new challenges that will have to be overcome. Each material category also has its own benefits for specific applications. Entire organ matrices can be used for larger transplants and organ replacements. Hydrogels are best for creating 3D environments and can be used as injectable therapies. Sections and coatings are useful for discovering the effects of the matrix components on cell differentiation directly.
Overall, there are clear effects of decellularized materials on cell differentiation. To date, lung, liver, kidney, heart, central nervous system, adipose, tendon, muscle, cartilage, and other decellularized tissues have shown signs of positive impact on cell differentiation in laboratory settings. However, the extent to which each tissue can direct cell lineage differs. Heart, liver, and adipose tissue all demonstrate the ability to direct differentiation with the ECM alone and no addition treated media [40, 49, 52]. The other reviewed tissues had an effect on stem cell behavior, but required additional cues in order to control stem cells into the tissue specific lineages. This may be due to unique tissue microarchitecture, use of cell lineages that are more easily directed, variations in the decellularization process that altered the ECM’s structure and composition, or other unknown factors. For this reason, further studies must be conducted to understand these details more fully.
It is important to remember this variation among tissue and material types when designing decellularized tissue materials. Though it is generally understood that the ECM contains necessary cues for directing tissue specific gene expression, there are many factors that are needed for differentiation and the ECM alone may not be sufficient for every tissue engineering purpose. However, the papers reviewed demonstrate that decellularized tissue materials can be a powerful tool by harnessing what nature has already designed. With future research and development, this field has excellent potential to provide a greater impact in both basic and translational tissue engineering studies.
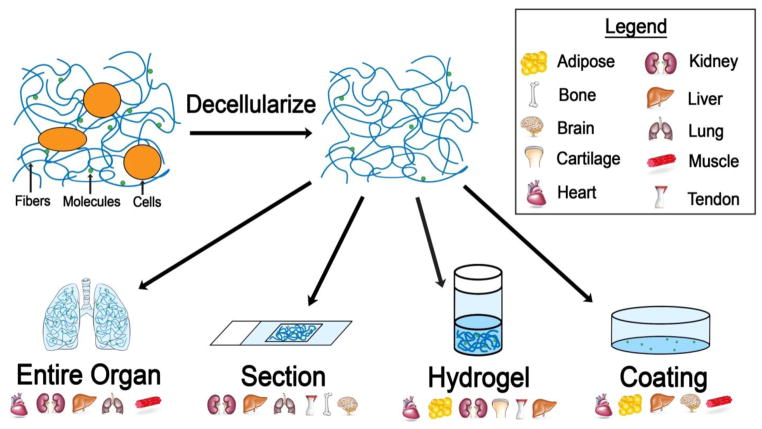
Figure 1.
Decellularized tissue materials made from various tissues. After removing the cellular contents of the native tissue, the extracellular matrix remains and can be left unprocessed as an entire organ, or be processed into a section, hydrogel, or coating. The tissues that have been processed for the purpose of testing cell differentiation effects are shown below each material category.
Highlights.
Different methods of ECM material preparation provide various benefits
ECM of each tissue has a unique architecture and biochemical composition
ECM materials have tissue specific effects that can direct cell differentiation
Decellularized tissues are a promising platform for tissue engineering applications
Acknowledgments
We thank Sophia Suarez and Jessica Ungerleider for their critical reading of the manuscript. This work was funded in part by NIH NHLBI (1R01HL113468). Dr. Christman is co-founder, board member, and holds equity interest in Ventrix, Inc.
Abbreviations
- ASC
Adipose Derived Stem Cell
- ECM
Extracellular Matrix
- EDC
1-ethyl-3-[3-dimethylaminopropyl] carbodiimide
- ESC
Embryonic Stem Cell
- hASC
Human Adipose Derived Stem Cell
- hESC
Human Embryonic Stem Cell
- hMSC
Human Mesenchymal Stem Cell
- iPSC
Induced Pluripotent Stem Cell
- mESC
Murine Embryonic Stem Cell
- MSC
Mesenchymal Stem Cell
- NHS
N-hydroxysulfosuccinimide
- NSC
Neural Stem Cell
- PDMS
Polydimethylsiloxane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Faulk DM, Johnson SA, Zhang L, Badylak SF. Role of the extracellular matrix in whole organ engineering. J Cell Physiol. 2014;229:984–9. doi: 10.1002/jcp.24532. [DOI] [PubMed] [Google Scholar]
- 2.Keane TJ, Swinehart IT, Badylak SF. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods. 2015;84:25–34. doi: 10.1016/j.ymeth.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Hoshiba T, Lu H, Kawazoe N, Chen G. Decellularized matrices for tissue engineering. Expert Opin Biol Ther. 2010;10:1717–28. doi: 10.1517/14712598.2010.534079. [DOI] [PubMed] [Google Scholar]
- 4.Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 2002;13:377–83. doi: 10.1016/s1084952102000940. [DOI] [PubMed] [Google Scholar]
- 5.Moroni F, Mirabella T. Decellularized matrices for cardiovascular tissue engineering. Am J Stem Cells. 2014;3:1–20. [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Q, Li L, Li J. Stem cells with decellularized liver scaffolds in liver regeneration and their potential clinical applications. Liver Int. 2015;35:687–94. doi: 10.1111/liv.12581. [DOI] [PubMed] [Google Scholar]
- 7.Arenas-Herrera JE, Ko IK, Atala A, Yoo JJ. Decellularization for whole organ bioengineering. Biomed Mater. 2013;8:014106. doi: 10.1088/1748-6041/8/1/014106. [DOI] [PubMed] [Google Scholar]
- 8.Fu R-h, Wang Y-c, Liu S-p, Shih T-r, Lin H-l, Chen Y-m, et al. Decellularization and Recellularization Technologies in Tissue Engineering. Cell Transplantation. 2014;23:621–30. doi: 10.3727/096368914X678382. [DOI] [PubMed] [Google Scholar]
- 9.Ofenbauer A, Sebinger DD, Prewitz M, Gruber P, Werner C. Dewaxed ECM: A simple method for analyzing cell behaviour on decellularized extracellular matrices. J Tissue Eng Regen Med. 2015;9:1046–55. doi: 10.1002/term.1658. [DOI] [PubMed] [Google Scholar]
- 10.Daly AB, Wallis JM, Borg ZD, Bonvillain RW, Deng B, Ballif BA, et al. Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng Part A. 2012;18:1–16. doi: 10.1089/ten.tea.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Q, Ye X, Sun R, Matsumoto Y, Moriyama M, Asano Y, et al. Differentiation of mouse induced pluripotent stem cells into alveolar epithelial cells in vitro for use in vivo. Stem Cells Transl Med. 2014;3:675–85. doi: 10.5966/sctm.2013-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilpin SE, Ren X, Okamoto T, Guyette JP, Mou H, Rajagopal J, et al. Enhanced lung epithelial specification of human induced pluripotent stem cells on decellularized lung matrix. Ann Thorac Surg. 2014;98:1721–9. doi: 10.1016/j.athoracsur.2014.05.080. discussion 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendez JJ, Ghaedi M, Steinbacher D, Niklason LE. Epithelial cell differentiation of human mesenchymal stromal cells in decellularized lung scaffolds. Tissue Eng Part A. 2014;20:1735–46. doi: 10.1089/ten.tea.2013.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner DE, Bonenfant NR, Parsons CS, Sokocevic D, Brooks EM, Borg ZD, et al. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials. 2014;35:3281–97. doi: 10.1016/j.biomaterials.2013.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calle EA, Mendez JJ, Ghaedi M, Leiby KL, Bove PF, Herzog EL, et al. Fate of distal lung epithelium cultured in a decellularized lung extracellular matrix. Tissue Eng Part A. 2015 doi: 10.1089/ten.tea.2014.0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barakat O, Abbasi S, Rodriguez G, Rios J, Wood RP, Ozaki C, et al. Use of decellularized porcine liver for engineering humanized liver organ. J Surg Res. 2012;173:e11–25. doi: 10.1016/j.jss.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 17.Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, et al. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol. 2009;20:2338–47. doi: 10.1681/ASN.2008111196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonandrini B, Figliuzzi M, Papadimou E, Morigi M, Perico N, Casiraghi F, et al. Recellularization of well-preserved acellular kidney scaffold using embryonic stem cells. Tissue Eng Part A. 2014;20:1486–98. doi: 10.1089/ten.tea.2013.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgkart R, Tron A, Prodinger P, Culmes M, Tuebel J, van Griensven M, et al. Decellularized kidney matrix for perfused bone engineering. Tissue Eng Part C Methods. 2014;20:553–61. doi: 10.1089/ten.TEC.2013.0270. [DOI] [PubMed] [Google Scholar]
- 20.Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends Mol Med. 2011;17:424–32. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Utomo L, Pleumeekers MM, Nimeskern L, Nurnberger S, Stok KS, Hildner F, et al. Preparation and characterization of a decellularized cartilage scaffold for ear cartilage reconstruction. Biomed Mater. 2015;10:015010. doi: 10.1088/1748-6041/10/1/015010. [DOI] [PubMed] [Google Scholar]
- 22.Perniconi B, Costa A, Aulino P, Teodori L, Adamo S, Coletti D. The pro-myogenic environment provided by whole organ scale acellular scaffolds from skeletal muscle. Biomaterials. 2011;32:7870–82. doi: 10.1016/j.biomaterials.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186:866–76. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omae H, Zhao C, Sun YL, An KN, Amadio PC. Multilayer tendon slices seeded with bone marrow stromal cells: a novel composite for tendon engineering. J Orthop Res. 2009;27:937–42. doi: 10.1002/jor.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Waele J, Reekmans K, Daans J, Goossens H, Berneman Z, Ponsaerts P. 3D culture of murine neural stem cells on decellularized mouse brain sections. Biomaterials. 2015;41:122–31. doi: 10.1016/j.biomaterials.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 26.Youngstrom DW, Rajpar I, Kaplan DL, Barrett JG. A bioreactor system for in vitro tendon differentiation and tendon tissue engineering. J Orthop Res. 2015 doi: 10.1002/jor.22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin Z, Chen X, Zhu T, Hu JJ, Song HX, Shen WL, et al. The effect of decellularized matrices on human tendon stem/progenitor cell differentiation and tendon repair. Acta Biomater. 2013;9:9317–29. doi: 10.1016/j.actbio.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Alio Del Barrio JL, Chiesa M, Garagorri N, Garcia-Urquia N, Fernandez-Delgado J, Bataille L, et al. Acellular human corneal matrix sheets seeded with human adipose-derived mesenchymal stem cells integrate functionally in an experimental animal model. Exp Eye Res. 2015;132:91–100. doi: 10.1016/j.exer.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Nakayama KH, Lee CC, Batchelder CA, Tarantal AF. Tissue specificity of decellularized rhesus monkey kidney and lung scaffolds. PLoS One. 2013;8:e64134. doi: 10.1371/journal.pone.0064134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sellaro TL, Ranade A, Faulk DM, McCabe GP, Dorko K, Badylak SF, et al. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng Part A. 2010;16:1075–82. doi: 10.1089/ten.tea.2008.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto Y, Funamoto S, Kimura T, Nam K, Fujisato T, Kishida A. The effect of decellularized bone/bone marrow produced by high-hydrostatic pressurization on the osteogenic differentiation of mesenchymal stem cells. Biomaterials. 2011;32:7060–7. doi: 10.1016/j.biomaterials.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Johnson JA, Chang DW, Zhang Q. Decellularized musculofascial extracellular matrix for tissue engineering. Biomaterials. 2013;34:2641–54. doi: 10.1016/j.biomaterials.2012.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adam Young D, Bajaj V, Christman KL. Award winner for outstanding research in the PhD category, 2014 Society for Biomaterials annual meeting and exposition, Denver, Colorado, April 16–19, 2014: Decellularized adipose matrix hydrogels stimulate in vivo neovascularization and adipose formation. J Biomed Mater Res A. 2014;102:1641–51. doi: 10.1002/jbm.a.35109. [DOI] [PubMed] [Google Scholar]
- 34.Freytes DO, Martin J, Velankar SS, Lee AS, Badylak SF. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 2008;29:1630–7. doi: 10.1016/j.biomaterials.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409–16. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Neill JD, Freytes DO, Anandappa AJ, Oliver JA, Vunjak-Novakovic GV. The regulation of growth and metabolism of kidney stem cells with regional specificity using extracellular matrix derived from kidney. Biomaterials. 2013;34:9830–41. doi: 10.1016/j.biomaterials.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeQuach JA, Lin JE, Cam C, Hu D, Salvatore MA, Sheikh F, et al. Injectable skeletal muscle matrix hydrogel promotes neovascularization and muscle cell infiltration in a hindlimb ischemia model. Eur Cell Mater. 2012;23:400–12. doi: 10.22203/ecm.v023a31. discussion 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, et al. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J Am Coll Cardiol. 2012;59:751–63. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci Transl Med. 2013;5:173ra25. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheung HK, Han TT, Marecak DM, Watkins JF, Amsden BG, Flynn LE. Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials. 2014;35:1914–23. doi: 10.1016/j.biomaterials.2013.11.067. [DOI] [PubMed] [Google Scholar]
- 41.Visser J, Levett PA, Te Moller NC, Besems J, Boere KW, van Rijen MH, et al. Crosslinkable Hydrogels Derived from Cartilage, Meniscus, and Tendon Tissue. Tissue Eng Part A. 2015 doi: 10.1089/ten.tea.2014.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duan Y, Liu Z, O’Neill J, Wan LQ, Freytes DO, Vunjak-Novakovic G. Hybrid gel composed of native heart matrix and collagen induces cardiac differentiation of human embryonic stem cells without supplemental growth factors. J Cardiovasc Transl Res. 2011;4:605–15. doi: 10.1007/s12265-011-9304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gershlak JR, Resnikoff JI, Sullivan KE, Williams C, Wang RM, Black LD., 3rd Mesenchymal stem cells ability to generate traction stress in response to substrate stiffness is modulated by the changing extracellular matrix composition of the heart during development. Biochem Biophys Res Commun. 2013;439:161–6. doi: 10.1016/j.bbrc.2013.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang G, Rothrauff BB, Lin H, Gottardi R, Alexander PG, Tuan RS. Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials. 2013;34:9295–306. doi: 10.1016/j.biomaterials.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mercuri JJ, Patnaik S, Dion G, Gill SS, Liao J, Simionescu DT. Regenerative potential of decellularized porcine nucleus pulposus hydrogel scaffolds: stem cell differentiation, matrix remodeling, and biocompatibility studies. Tissue Eng Part A. 2013;19:952–66. doi: 10.1089/ten.TEA.2012.0088. [DOI] [PubMed] [Google Scholar]
- 46.Rajabi-Zeleti S, Jalili-Firoozinezhad S, Azarnia M, Khayyatan F, Vahdat S, Nikeghbalian S, et al. The behavior of cardiac progenitor cells on macroporous pericardium-derived scaffolds. Biomaterials. 2014;35:970–82. doi: 10.1016/j.biomaterials.2013.10.045. [DOI] [PubMed] [Google Scholar]
- 47.Johnson TD, Dequach JA, Gaetani R, Ungerleider J, Elhag D, Nigam V, et al. Human versus porcine tissue sourcing for an injectable myocardial matrix hydrogel. Biomater Sci. 2014;2014:60283D. doi: 10.1039/C3BM60283D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Li B, Wang JH. The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo. Biomaterials. 2011;32:6972–81. doi: 10.1016/j.biomaterials.2011.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.French KM, Boopathy AV, DeQuach JA, Chingozha L, Lu H, Christman KL, et al. A naturally derived cardiac extracellular matrix enhances cardiac progenitor cell behavior in vitro. Acta Biomater. 2012;8:4357–64. doi: 10.1016/j.actbio.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JS, Shin J, Park HM, Kim YG, Kim BG, Oh JW, et al. Liver extracellular matrix providing dual functions of two-dimensional substrate coating and three-dimensional injectable hydrogel platform for liver tissue engineering. Biomacromolecules. 2014;15:206–18. doi: 10.1021/bm4015039. [DOI] [PubMed] [Google Scholar]
- 51.DeQuach JA, Mezzano V, Miglani A, Lange S, Keller GM, Sheikh F, et al. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS One. 2010;5:e13039. doi: 10.1371/journal.pone.0013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X, Dong J. Direct comparison of different coating matrix on the hepatic differentiation from adipose-derived stem cells. Biochem Biophys Res Commun. 2015;456:938–44. doi: 10.1016/j.bbrc.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Young DA, Choi YS, Engler AJ, Christman KL. Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials. 2013;34:8581–8. doi: 10.1016/j.biomaterials.2013.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sullivan KE, Quinn KP, Tang KM, Georgakoudi I, Black LD., 3rd Extracellular matrix remodeling following myocardial infarction influences the therapeutic potential of mesenchymal stem cells. Stem Cell Res Ther. 2014;5:14. doi: 10.1186/scrt403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flynn L, Semple JL, Woodhouse KA. Decellularized placental matrices for adipose tissue engineering. J Biomed Mater Res A. 2006;79:359–69. doi: 10.1002/jbm.a.30762. [DOI] [PubMed] [Google Scholar]
- 56.Baiguera S, Del Gaudio C, Lucatelli E, Kuevda E, Boieri M, Mazzanti B, et al. Electrospun gelatin scaffolds incorporating rat decellularized brain extracellular matrix for neural tissue engineering. Biomaterials. 2014;35:1205–14. doi: 10.1016/j.biomaterials.2013.10.060. [DOI] [PubMed] [Google Scholar]
- 57.Yang Q, Peng J, Guo Q, Huang J, Zhang L, Yao J, et al. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials. 2008;29:2378–87. doi: 10.1016/j.biomaterials.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 58.Zhao Y, Zhang Z, Wang J, Yin P, Zhou J, Zhen M, et al. Abdominal hernia repair with a decellularized dermal scaffold seeded with autologous bone marrow-derived mesenchymal stem cells. Artif Organs. 2012;36:247–55. doi: 10.1111/j.1525-1594.2011.01343.x. [DOI] [PubMed] [Google Scholar]
- 59.Lang R, Stern MM, Smith L, Liu Y, Bharadwaj S, Liu G, et al. Three-dimensional culture of hepatocytes on porcine liver tissue-derived extracellular matrix. Biomaterials. 2011;32:7042–52. doi: 10.1016/j.biomaterials.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 60.Oberwallner B, Brodarac A, Choi YH, Saric T, Anic P, Morawietz L, et al. Preparation of cardiac extracellular matrix scaffolds by decellularization of human myocardium. J Biomed Mater Res A. 2014;102:3263–72. doi: 10.1002/jbma.35000. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Luo H, Zhang Z, Lu Y, Huang X, Yang L, et al. A nerve graft constructed with xenogeneic acellular nerve matrix and autologous adipose-derived mesenchymal stem cells. Biomaterials. 2010;31:5312–24. doi: 10.1016/j.biomaterials.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 62.Chan LK, Leung VY, Tam V, Lu WW, Sze KY, Cheung KM. Decellularized bovine intervertebral disc as a natural scaffold for xenogenic cell studies. Acta Biomater. 2013;9:5262–72. doi: 10.1016/j.actbio.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 63.Deeken CR, White AK, Bachman SL, Ramshaw BJ, Cleveland DS, Loy TS, et al. Method of preparing a decellularized porcine tendon using tributyl phosphate. J Biomed Mater Res B Appl Biomater. 2011;96:199–206. doi: 10.1002/jbm.b.31753. [DOI] [PubMed] [Google Scholar]
- 64.Hellstrom M, El-Akouri RR, Sihlbom C, Olsson BM, Lengqvist J, Backdahl H, et al. Towards the development of a bioengineered uterus: comparison of different protocols for rat uterus decellularization. Acta Biomater. 2014;10:5034–42. doi: 10.1016/j.actbio.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 65.Dong J, Li Y, Mo X. The study of a new detergent (octyl-glucopyranoside) for decellularizing porcine pericardium as tissue engineering scaffold. J Surg Res. 2013;183:56–67. doi: 10.1016/j.jss.2012.11.047. [DOI] [PubMed] [Google Scholar]
- 66.Baiguera S, Del Gaudio C, Jaus MO, Polizzi L, Gonfiotti A, Comin CE, et al. Long-term changes to in vitro preserved bioengineered human trachea and their implications for decellularized tissues. Biomaterials. 2012;33:3662–72. doi: 10.1016/j.biomaterials.2012.01.064. [DOI] [PubMed] [Google Scholar]
- 67.Dai Y, Chen J, Li H, Li S, Chen J, Ding Y, et al. Characterizing the effects of VPA, VC and RCCS on rabbit keratocytes onto decellularized bovine cornea. PLoS One. 2012;7:e50114. doi: 10.1371/journal.pone.0050114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ye X, Wang H, Zhou J, Li H, Liu J, Wang Z, et al. The effect of Heparin-VEGF multilayer on the biocompatibility of decellularized aortic valve with platelet and endothelial progenitor cells. PLoS One. 2013;8:e54622. doi: 10.1371/journal.pone.0054622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16:2565–80. doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- 70.Shojaie S, Ermini L, Ackerley C, Wang J, Chin S, Yeganeh B, et al. Acellular Lung Scaffolds Direct Differentiation of Endoderm to Functional Airway Epithelial Cells: Requirement of Matrix-Bound HS Proteoglycans. Stem Cell Reports. 2015;4:419–30. doi: 10.1016/j.stemcr.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shamis Y, Hasson E, Soroker A, Bassat E, Shimoni Y, Ziv T, et al. Organ-specific scaffolds for in vitro expansion, differentiation, and organization of primary lung cells. Tissue Eng Part C Methods. 2011;17:861–70. doi: 10.1089/ten.tec.2010.0717. [DOI] [PubMed] [Google Scholar]
- 72.Ng SL, Narayanan K, Gao S, Wan AC. Lineage restricted progenitors for the repopulation of decellularized heart. Biomaterials. 2011;32:7571–80. doi: 10.1016/j.biomaterials.2011.06.065. [DOI] [PubMed] [Google Scholar]
- 73.Pok S, Benavides OM, Hallal P, Jacot JG. Use of myocardial matrix in a chitosan-based full-thickness heart patch. Tissue Eng Part A. 2014;20:1877–87. doi: 10.1089/ten.tea.2013.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gaetani R, Yin C, Srikumar N, Braden R, Doevendans PA, Sluijter JP, et al. Cardiac derived extracellular matrix enhances cardiogenic properties of human cardiac progenitor cells. Cell Transplant. 2015 doi: 10.3727/096368915X689794. In Press. [DOI] [PubMed] [Google Scholar]
- 75.Iop L, Renier V, Naso F, Piccoli M, Bonetti A, Gandaglia A, et al. The influence of heart valve leaflet matrix characteristics on the interaction between human mesenchymal stem cells and decellularized scaffolds. Biomaterials. 2009;30:4104–16. doi: 10.1016/j.biomaterials.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 76.Simpson DL, Wehman B, Galat Y, Sharma S, Mishra R, Galat V, et al. Engineering patient-specific valves using stem cells generated from skin biopsy specimens. Ann Thorac Surg. 2014;98:947–54. doi: 10.1016/j.athoracsur.2014.04.075. [DOI] [PubMed] [Google Scholar]
- 77.DeQuach JA, Yuan SH, Goldstein LS, Christman KL. Decellularized porcine brain matrix for cell culture and tissue engineering scaffolds. Tissue Eng Part A. 2011;17:2583–92. doi: 10.1089/ten.tea.2010.0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Crapo PM, Medberry CJ, Reing JE, Tottey S, van der Merwe Y, Jones KE, et al. Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials. 2012;33:3539–47. doi: 10.1016/j.biomaterials.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pati F, Jang J, Ha DH, Won Kim S, Rhie JW, Shim JH, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu Q, Li M, Zou Y, Cao T. Delivery of basic fibroblast growth factors from heparinized decellularized adipose tissue stimulates potent de novo adipogenesis. J Control Release. 2014;174:43–50. doi: 10.1016/j.jconrel.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 81.Tong WY, Shen W, Yeung CW, Zhao Y, Cheng SH, Chu PK, et al. Functional replication of the tendon tissue microenvironment by a bioimprinted substrate and the support of tenocytic differentiation of mesenchymal stem cells. Biomaterials. 2012;33:7686–98. doi: 10.1016/j.biomaterials.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 82.Sicari BM, Rubin JP, Dearth CL, Wolf MT, Ambrosio F, Boninger M, et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci Transl Med. 2014;6:234ra58. doi: 10.1126/scitranslmed.3008085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perniconi B, Coletti D, Aulino P, Costa A, Aprile P, Santacroce L, et al. Muscle acellular scaffold as a biomaterial: effects on C2C12 cell differentiation and interaction with the murine host environment. Front Physiol. 2014;5:354. doi: 10.3389/fphys.2014.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miyazaki K, Maruyama T. Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials. 2014;35:8791–800. doi: 10.1016/j.biomaterials.2014.06.052. [DOI] [PubMed] [Google Scholar]
- 85.Hoganson DM, Meppelink AM, Hinkel CJ, Goldman SM, Liu XH, Nunley RM, et al. Differentiation of human bone marrow mesenchymal stem cells on decellularized extracellular matrix materials. J Biomed Mater Res A. 2014;102:2875–83. doi: 10.1002/jbm.a.34941. [DOI] [PubMed] [Google Scholar]
- 86.Johnson TD, Hill RC, Dzieciatkowska M, Nigam V, Behfar A, Christman KL, et al. Quantification of decellularized human myocardial matrix: A comparison of six patients. Proteomics Clin Appl. 2015 doi: 10.1002/prca.201500048. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hill RC, Calle EA, Dzieciatkowska M, Niklason LE, Hansen KC. Quantification of extracellular matrix proteins from a rat lung scaffold to provide a molecular readout for tissue engineering. Mol Cell Proteomics. 2015;14:961–73. doi: 10.1074/mcp.M114.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ranga A, Gobaa S, Okawa Y, Mosiewicz K, Negro A, Lutolf MP. 3D niche microarrays for systems-level analyses of cell fate. Nat Commun. 2014;5:4324. doi: 10.1038/ncomms5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gobaa S, Hoehnel S, Lutolf MP. Substrate elasticity modulates the responsiveness of mesenchymal stem cells to commitment cues. Integr Biol (Camb) 2015;7:1135–42. doi: 10.1039/c4ib00176a. [DOI] [PubMed] [Google Scholar]
- 90.Turner NJ, Badylak SF. The Use of Biologic Scaffolds in the Treatment of Chronic Nonhealing Wounds. Adv Wound Care (New Rochelle) 2015;4:490–500. doi: 10.1089/wound.2014.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Badylak SF, Freytes DO, Gilbert TW. Reprint of: Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2015;23(Suppl):S17–26. doi: 10.1016/j.actbio.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 92.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 93.Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19:646–51. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–41. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jank BJ, Xiong L, Moser PT, Guyette JP, Ren X, Cetrulo CL, et al. Engineered composite tissue as a bioartificial limb graft. Biomaterials. 2015;61:246–56. doi: 10.1016/j.biomaterials.2015.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim IH, Ko IK, Atala A, Yoo JJ. Whole kidney engineering for clinical translation. Curr Opin Organ Transplant. 2015;20:165–70. doi: 10.1097/MOT.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 97.Stabler CT, Lecht S, Mondrinos MJ, Goulart E, Lazarovici P, Lelkes PI. Revascularization of Decellularized Lung Scaffolds: Principles and Progress. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1273–L85. doi: 10.1152/ajplung.00237.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]