Abstract
Introduction
Activating mutations in the epidermal growth factor receptor (EGFR) predict for prolonged progression-free survival in patients with advanced non-small cell lung cancer (NSCLC) treated with EGFR-tyrosine kinase inhibitors (EGFR-TKIs) versus chemotherapy. Long-term survival outcomes, however, remain undefined. The objective of this study was to determine the 5-year survival in these patients, and identify clinical factors associated with overall survival (OS).
Methods
Patients with EGFR-mutant metastatic lung adenocarcinoma treated with erlotinib or gefitinib at Dana-Farber Cancer Institute between 2002 and 2009 were included. OS was analyzed.
Results
Among 137 patients, median PFS and OS were 12.1 months (95% CI, 10.2-13.5 months) and 30.9 months (95% CI, 28.2-35.7 months), respectively. Twenty patients (14.6%) were 5-year survivors. In multivariate analysis, exon 19 deletions (hazard ratio [HR], 0.63; 95% CI, 0.44-0.91; P = 0.01), absence of extrathoracic (HR 0.62; 95% CI, 0.41-0.93; P = 0.02) or brain metastasis (HR 0.48; 95% CI, 0.30-0.77, P = 0.002), and not a current smoker (HR 0.23; 95% CI, 0.09-0.59; P = 0.002) were associated with prolonged OS. Age, gender, stage at diagnosis, liver or bone or adrenal metastasis, specific TKI, and line of TKI therapy were not associated with OS.
Conclusions
Our data suggest that the prevalence of 5-year survival among EGFR-mutant metastatic lung adenocarcinoma patients treated with erlotinib or gefitinib is 14.6%. Exon 19 deletions and absence of extrathoracic or brain metastasis are associated with prolonged survival. Based on our findings, clinicians can gain an enhanced estimation of long-term outcomes in this population.
Keywords: Non-small cell lung cancer, EGFR, TKI, long-term survival
Introduction
Non-small cell lung cancer (NSCLC) typically presents in patients at an advanced stage, with a poor prognosis. Combination chemotherapy in advanced NSCLC results in a median overall survival (OS) of 8 to 12 months and a median progression-free survival (PFS) of 5 to 6 months.1-3 The development of epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) gefitinib and erlotinib and the systematic identification of EGFR mutations heralded the advent of targeted therapy in lung cancer, transforming the landscape of its treatment and prognosis. When used in trials as a first-line treatment for patients with advanced NSCLC harboring activating EGFR mutations, gefitinib and erlotinib led to response rates of 56 to 74%, median PFS of 10 to 14 months, and OS of 20 to 30 months.4-8 These results propelled the TKIs into first-line use for patients with advanced EGFR-mutant NSCLC.
Despite the data available from published trials and prospective studies on the median survival, data on 5-year survival for metastatic NSCLC patients treated with EGFR-TKIs has been lacking. Five-year survival serves as the traditional surrogate for cure in many cancers.9 The SEER program has reported a 5-year relative survival for unselected patients with distant-stage NSCLC as 4.5%.10 Prior lung cancer studies available for review have similarly suggested an estimate of <5%.11,12 To the best of our knowledge, no study to date has defined the rate of 5-year survival among patients with metastatic lung adenocarcinoma harboring sensitizing EGFR mutations who are then treated with EGFR-TKIs as targeted therapy.
Furthermore, outcomes in patients treated with EGFR-TKIs vary widely, suggesting heterogeneity in the underlying clinical or genetic parameters that may further modify patient response and determine the ultimate course of disease. Initial studies on the clinical predictors of EGFR-TKI responsiveness in unselected NSCLC patients identified individuals with adenocarcinomas, non-smoking history, East-Asian origin, and female gender as those likely to derive a greater benefit.13-16 Later studies elucidated that activating EGFR mutations were predominantly present in the aforementioned patients, representing the unifying molecular mechanism underlying their sensitivity to EGFR inhibition.17-24 Defining the clinical factors associated with the outcome within the specific EGFR-mutant metastatic adenocarcinoma population may similarly help identify those more likely to achieve the 5-year survival endpoint.
Herein, we sought to determine the prevalence of 5-year actual survival in patients with EGFR-mutant metastatic lung adenocarcinoma treated with an FDA-approved EGFR-TKI, and identify the clinical features associated with this outcome. We hypothesized based on clinical experiences that our study would ascertain a higher percentage of 5-year survivors than previously defined in the literature and in the SEER data for NSCLC patients.
Materials and Methods
Study Design and Population
Patients were eligible if they had (a) stage IV lung adenocarcinoma or stage I-III lung adenocarcinoma with subsequent systemic relapse, diagnosed at the Dana-Farber Cancer Institute (DFCI) between January 1, 2002 and September 31, 2009, (b) activating somatic EGFR mutations, and (c) treatment with gefitinib or erlotinib. Afatinib was not included as it was approved later and not routinely available to patients during this study time frame. Patients were identified by querying two databases at DFCI that store the clinico-pathologic information for prospectively enrolled patients (Supplemental Methods, Supplemental Digital Content): Clinical Research Information System (CRIS) and Thoracic Oncology Basic Assessment of Cancer and Clinical Outcomes (TOBACCO). The information from these databases has been used for multiple prior publications.25-28
A total of 942 patients were identified who had metastatic lung adenocarcinoma within the study period. Of these, 668 patients (71%) were tested for EGFR mutations. The proportion of patients tested for EGFR mutations increased towards the later years of the study time frame, as the EGFR mutation testing became more embedded in clinical practice. The never smokers in the study cohort were also more likely to be tested than the former smokers and current smokers (85% vs 70.1% vs 50%, respectively; P < 0.001), based on the published literature.13-16 Among the 668 tested patients, 248 (37.1% of those tested) were found to have an EGFR mutation. Thirty-two of the 668 patients (4.8%) failed testing. In these patients who failed testing, the decision to pursue further diagnostic procedures to obtain additional tissue was left at the discretion of the clinician.
Of the 248 patients found to have EGFR mutations, 60 were excluded as they had been treated at our partner institution Massachusetts General Hospital Cancer Center rather than at DFCI, but enrolled in our databases for other studies. Subsequently, 51 patients were excluded because they were not eligible secondary to the following: non-sensitizing EGFR mutations, diagnosis prior to the date cut-off on further review, presence of a concurrent malignancy, no documented exposure to TKI or chemotherapy, seen only once in consultation or incomplete medical records, missing identifier, or failure to meet the requirement for a minimum 5-year follow-up if alive at the time of analysis (Supplemental Methods, Supplemental Digital Content; Supplemental Figure, Supplemental Digital Content). Ultimately, 137 patients were included in this analysis. All patients provided written informed consent for the collection of baseline clinical parameters and outcome, and collection and analysis of their tumor specimens.
Mutation Analyses
The EGFR mutation status for each patient was obtained using tumor specimens from diagnostic or surgical procedures. Patients were prospectively genotyped in CLIA laboratory starting in 2004. Those starting treatment between 2002 and 2004 were sequenced when the technology became available later in their clinical course. Sequencing of exons 18 to 21 was performed per the institutional pathology lab protocol by Sanger technique as described.22 Sensitizing EGFR mutations were defined as exon 19 deletions and missense mutations of L858R or involving L861 or G719 as previously reported.28
Statistical Methods
From the information collected in CRIS and TOBACCO (Supplemental Methods, Supplemental Digital Content), the following baseline patient or tumor parameters were analyzed for this study: age at the diagnosis of metastatic disease, gender, race, self-reported smoking status prospectively collected, initial staging, presence of extrathoracic tumor at the diagnosis of metastatic disease, metastatic site(s) (characterized at up to 1 month within start of systemic therapy to the liver, adrenals, bone, brain, and leptomeninges), type of sensitizing EGFR mutation, and line of EGFR-TKI therapy. Smoking status was classified as never (<100 lifetime cigarettes), former (quit ≥1 year before start of therapy), and current (active or quit within 1 year prior to start of therapy).
OS was calculated from the date of start of the first-line systemic treatment for metastatic disease until death from any cause. Patients still alive were censored at their last follow-up visit. PFS was defined as months from the date of initiation on TKIs to clinically determined disease progression or death, whichever occurred first. Here, the date of “clinically determined disease progression” was defined as the date of radiographic imaging which demonstrated progression deemed clinically significant by the physician—whether due to resultant patient symptoms, or due to a radiographic change that was significant enough to warrant a discussion of change in therapy.29,30 Patients who remained alive and progression-free at the time of analysis were censored at the date of their last disease assessment. Kaplan-Meier curves were utilized to estimate survival distributions. Time-to-event comparisons were made using log-rank tests. Univariate and multivariate analyses were performed using the Fisher's exact test or Kruskal-Wallis test and Cox's proportional hazards regression models. All reported P values are two-sided and no adjustments have been made for multiple comparisons. All analyses were performed using R version 2.10.0 (R Found Stat Comput).
Results
Patient Characteristics
We identified 137 patients who developed EGFR-mutant metastatic lung adenocarcinoma and were treated with gefitinib or erlotinib within the study period. Table 1 summarizes the baseline characteristics of these patients. Consistent with observations in major randomized trials of EGFR-mutant advanced NSCLC patients, most of our patients were female with a median age of 60 years, and more than half of the cohort were never-smokers.4-8 The distribution of activating EGFR mutations was also similar to what was reported in prior studies.4-8 The most predominant activating mutation was the exon 19 deletion, followed by the L858R mutation. In comparison, the L861Q/R and G719X mutations were relatively less common (Table 1). Notably, a tumor harboring coexisting sensitizing mutations in L861 and G719 were diagnosed in two patients, and a tumor bearing concomitant L858R and T790M mutations in the pre-TKI treatment specimen was diagnosed in another patient. Finally, approximately 68% of the cohort received an EGFR-TKI as the first-line therapy, and 28% as second-line (Table 1). The information on salvage therapies administered after progression on an EGFR-TKI was not consistently collected in the databases, and therefore, this was not analyzed in the study.
Table 1. Baseline Patient Characteristics.
| Characteristic | n | % |
|---|---|---|
| Gender | ||
| Men | 31 | 22.6 |
| Women | 106 | 77.4 |
| Race | ||
| White | 118 | 86.1 |
| Asian | 13 | 9.5 |
| Black | 4 | 2.9 |
| Hispanic | 0 | 0.0 |
| Other/Unknown | 2 | 1.5 |
| Smoking status | ||
| Never | 77 | 56.2 |
| Former | 55 | 40.1 |
| Current | 5 | 3.6 |
| Median agea (range), years | 60 (35-84) | |
| Stageb | ||
| Relapsedc | 31 | 22.6 |
| IVAb | 42 | 30.7 |
| IVBb | 64 | 46.7 |
| Presence of Extrathoracic Metastasis | ||
| Yes | 79 | 57.7 |
| No (without/with pleural effusion) | 58 (29/29) | 42.3 |
| Involved Metastatic Sites | ||
| Liver | 21 | 15.3 |
| Adrenal | 4 | 2.9 |
| Bone | 59 | 43.1 |
| Brain | 30 | 21.9 |
| EGFR Mutation | ||
| Exon 19 Deletion | 76 | 55.5 |
| L858R | 47 | 34.3 |
| L861 | 7 | 5.1 |
| G719 | 4 | 2.9 |
| L861+G719 | 2 | 1.5 |
| L858R+T790M | 1 | 0.7 |
| EGFR-TKI | ||
| Erlotinib | 121 | 88.3 |
| Gefitinib | 16 | 11.7 |
| Line of EGFR TKI | ||
| First-Line | 93 | 67.9 |
| Second-Line | 38 | 27.7 |
| Third- or Greater-Line | 6 | 4.4 |
Age at diagnosis of metastatic disease.
American Joint Committee on Cancer (AJCC) Staging System, 7th edition.
Patients with stages I-III with systemic relapse following definitive therapy.
Five-Year Survivors
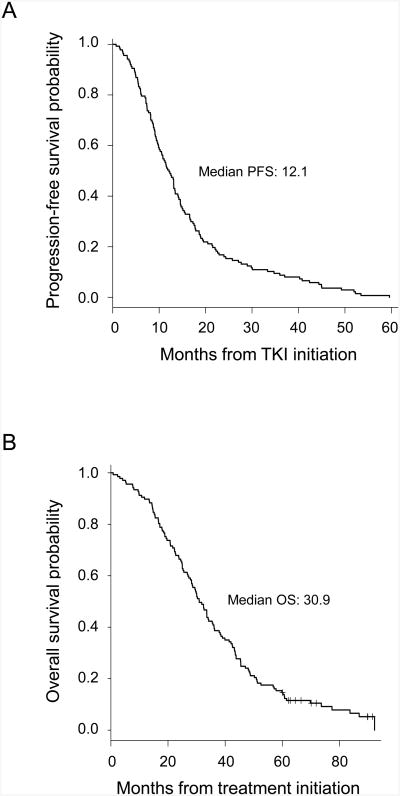
The median PFS and OS for the cohort were 12.1 months (95% CI, 10.2-13.5 months) and 30.9 months (95% CI, 28.2-35.7 months), respectively (Figure 1). Ninety-one patients (66.4%) survived for ≥2 years, and 20 (14.6%) survived for ≥5 years (range, 5.0-7.7 years). The 95% CI for the 5-year survivor rate (14.6%) ranged from 9.7-21.9%. Ten of the 5-year survivors (50.0%, or 7.3% of the entire cohort) remained alive at the data collection cut-off date, and the median duration of follow-up on these patients was 89.8 months (range, 60.1-91.6 months). Notably, the patient whose tumor harbored both the L858R and T790M mutations in the pre-treatment specimen demonstrated evidence of rapid disease progression after only 44 days of treatment with erlotinib and had a short OS of 7.7 months, consistent with the observation that T790M renders resistance to EGFR inhibition.31
Figure 1.
Kaplan-Meier plot of (A) progression-free survival and (B) overall survival for all patients in the study cohort. Median survivals are annotated in months.
Factors Associated with Prolonged Survival
The 137 patients were categorized into 3 subsets based on their OS (<2 years vs ≥2 to <5 years vs ≥5 years). Among these groups, clinical parameters associated with the prolonged survival category were intrathoracic disease (P = 0.01) and the absence of bone (P = 0.02) and brain (P = 0.003) metastasis at the time of diagnosis of metastatic disease (Table 2). The type of EGFR mutation additionally showed a marginal association with OS (P = 0.06). There were no significant differences in OS according to age, gender, smoking status, initial staging, liver or adrenal metastasis, specific EGFR-TKI (erlotinib or gefitinib), line of EGFR-TKI therapy, or specific EGFR mutation at the amino acid level.
Table 2. Clinical Subsets of EGFR-Mutated Patients by Survival Group.
| Characteristic | Category | No. (%) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| <2 years n = 46 | 2 - <5 years n = 71 | 5+ years n = 20 | Total n = 137 | P Value | ||
| Gender | Female | 35 (76) | 54 (76) | 17 (85) | 106 (77) | 0.75 |
| Male | 11 (24) | 17 (24) | 3 (15) | 31 (23) | ||
|
| ||||||
| Race | White | 41 (89) | 62 (87) | 15 (75) | 118 (86) | 0.14 |
| Asian | 3 (7) | 6 (8) | 4 (20) | 13 (9) | ||
| Black | 0 (0) | 3 (4) | 1 (5) | 4 (3) | ||
| Other/Missing | 2 (4) | 0 (0) | 0 (0) | 2 (1) | ||
|
| ||||||
| Smoking Statusa | Never | 25 (54) | 41 (58) | 11 (55) | 77 (56) | 0.38 |
| Former | 17 (37) | 29 (41) | 9 (45) | 55 (40) | ||
| Current | 4 (9) | 1 (1) | 0 (0) | 5 (4) | ||
|
| ||||||
| Stageb | I | 2 (4) | 5 (7) | 4 (20) | 11 (8) | 0.26 |
| II | 2 (4) | 5 (7) | 1 (5) | 8 (6) | ||
| III | 7 (15) | 4 (6) | 1 (5) | 12 (9) | ||
| IV | 35 (76) | 57 (80) | 14 (70) | 106 (77) | ||
|
| ||||||
| Age at Diagnosis of Metastatic Disease | Mean (SD) | 60.8 (11.8) | 58.5 (10.4) | 59.2 (11.1) | 59.4 (10.9) | 0.48 |
| Median (Q1, Q3) | 63 (53, 70) | 58 (50, 66) | 62 (54, 64) | 60 (51, 67) | ||
|
| ||||||
| Presence of Extrathoracic Metastasisc | No | 13 (28) | 31 (44) | 14 (70) | 58 (42) | 0.01 |
| Yes | 33 (72) | 40 (56) | 6 (30) | 79 (58) | ||
|
| ||||||
| Liver Metastasisc | No | 36 (78) | 61 (86) | 19 (95) | 116 (85) | 0.22 |
| Yes | 10 (22) | 10 (14) | 1 (5) | 21 (15) | ||
|
| ||||||
| Adrenal Metastasisc | No | 44 (96) | 69 (97) | 20 (100) | 133 (97) | 0.81 |
| Yes | 2 (4) | 2 (3) | 0 (0) | 4 (3) | ||
|
| ||||||
| Bone Metastasisc | No | 19 (41) | 44 (62) | 15 (75) | 78 (57) | 0.02 |
| Yes | 27 (59) | 27 (38) | 5 (25) | 59 (43) | ||
|
| ||||||
| Brain Metastasisc | No | 30 (65) | 57 (80) | 20 (100) | 107 (78) | 0.003 |
| Yes | 16 (35) | 14 (20) | 0 (0) | 30 (22) | ||
|
| ||||||
| Exon of EGFR Mutation | 18 | 2 (4) | 2 (3) | 0 (0) | 4 (3) | 0.06 |
| 19 | 19 (41) | 41 (59) | 16 (80) | 76 (55) | ||
| 21 | 24 (52) | 26 (38) | 4 (20) | 54 (39) | ||
| 18, 21d | 0 (0) | 2 (3) | 0 (0) | 2 (1) | ||
| 20, 21d | 1 (2) | 0 (0) | 0 (0) | 1 (1) | ||
|
| ||||||
| EGFR AA Change | Exon 19 Deletion | 19 (41) | 41 (58) | 16 (80) | 76 (55) | 0.09 |
| L858R | 19 (41) | 24 (34) | 4 (20) | 47 (34) | ||
| L861Q/R | 5 (11) | 2 (3) | 0 (0) | 7 (5) | ||
| G719 | 2 (4) | 2 (3) | 0 (0) | 4 (3) | ||
| L861+G719d | 0 (0) | 2 (3) | 0 (0) | 2 (1) | ||
| L858R+T790Md | 1 (2) | 0 (0) | 0 (0) | 1 (1) | ||
|
| ||||||
| EGFR-TKI | Erlotinib | 43 (93) | 61 (86) | 17 (85) | 121 (88) | 0.38 |
| Gefitinib | 3 (7) | 10 (14) | 3 (15) | 16 (12) | ||
|
| ||||||
| Line of TKI | First-Line | 31 (67) | 46 (65) | 16 (80) | 93 (68) | 0.74 |
| Second-Line | 14 (30) | 21 (30) | 3 (15) | 38 (28) | ||
| Third-Line | 1 (2) | 3 (4) | 1 (5) | 5 (4) | ||
| Fourth-Line | 0 (0) | 1 (1) | 0 (0) | 1 (1) | ||
Abbreviations: OS, overall survival; SD, standard deviation; Q1, first quartile; Q3, third quartile; AA, amino acid.
Never: <100 lifetime cigarettes; Former: quit ≥1 year before therapy; Current: active or quit within 1 year prior to therapy.
Initial staging at time of diagnosis:AJCC Staging System, 7th edition.
Presence or absence of metastasis at the specified site, characterized at up to 1 month within start of therapy.
Outlier, removed from analysis for the computation of P value.
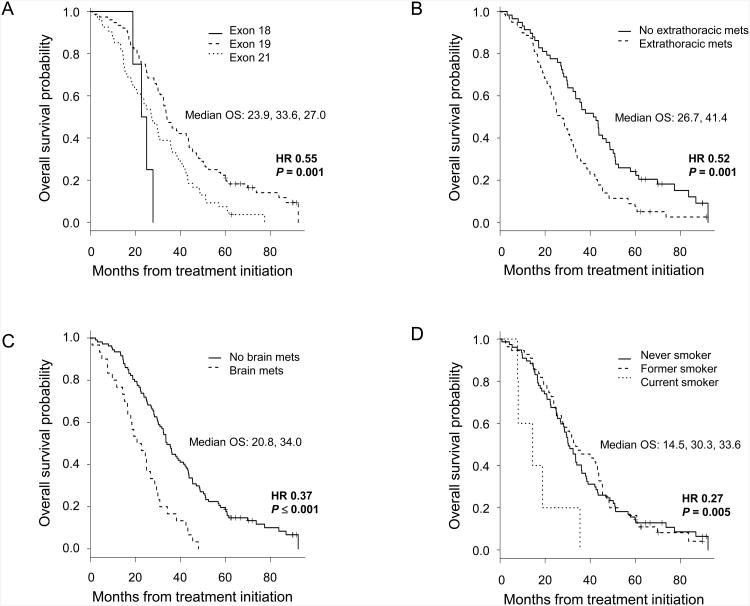
The univariate analyses of OS were estimated using the Kaplan-Meier methodology. The presence of an EGFR mutation in exon 19, vs 18 or 21, was associated with longer survival (HR 0.55; 95% CI, 0.38-0.79; P = 0.001). The absence of extrathoracic (HR 0.52; 95% CI, 0.36-0.75; P = 0.001), brain (HR 0.37; 95% CI, 0.24-0.57; P ≤ 0.001), and bone (HR 0.66; 95% CI, 0.47-0.95; P = 0.02) metastasis was also associated with extended survival (Figure 2). Finally, the former or never (vs current) smoking status was correlated with prolonged OS (HR 0.27; 95% CI, 0.11-0.66; P = 0.005). By comparison, there was no significant survival difference between former vs never smokers (HR 0.96; 95% CI, 0.67-1.37; P = 0.81). No difference in survival was seen among patients who received EGFR-TKI treatment as first-, second-, or third- or greater-line (median OS, 30.9 months, 30.9 months, 37.2 months, respectively; P = 0.74).
Figure 2.
Kaplan-Meier plots of overall survival according to the parameters of: the (A) exon of EGFR mutation (exon 19: n = 76; exon 21: n = 54; exon 18: n = 4), (B) presence of extrathoracic metastasis (yes: n = 79; no: n = 58), (C) presence of brain metastasis (yes: n = 30; no: n = 107), and (D) smoking status (never: n = 77; former: n = 55; current: n = 5). Median survival values are annotated in months. Hazard ratio (HR) according to the univariate analysis is as listed. For (A), HR is calculated for exon 19 vs either exon 18 or 21 EGFR mutation.
In multivariate analysis after adjusting for all of the factors above, the variables independently associated with prolonged survival were the exon 19 EGFR mutation, absence of extrathoracic metastasis, absence of brain metastasis, and non-current smoking status (Table 3). Bone metastasis was no longer statistically significantly associated with OS. Interestingly, 20 of the 30 patients with brain metastasis at the time of diagnosis also had bone metastasis, suggesting a statistically significant association (P = 0.004). Adrenal or liver metastasis was not associated with brain metastasis (P = 0.21 and P = 0.40, respectively).
Table 3. Multivariate Cox Regression Analysis of OS.
| Characteristic | OS | ||
|---|---|---|---|
|
|
|||
| HR | 95% CI | P Value | |
| Exon 19 Deletion | 0.63 | 0.44-0.91 | 0.01 |
| No Extrathoracic Metastasis | 0.62 | 0.41-0.93 | 0.02 |
| No Brain Metastasis | 0.48 | 0.30-0.77 | 0.002 |
| Not a Current Smoker | 0.23 | 0.09-0.59 | 0.002 |
Discussion
We analyzed the 5-year survival among EGFR-mutant metastatic lung adenocarcinoma patients treated with an EGFR-TKI and determined the rate of 14.6% (95% CI, 9.7-21.9%), in contrast to the <5% that has previously been reported for unselected patients with distant-stage NSCLC.10-12,32-38 To our knowledge, this is the first published study reporting on the outcome of 5-year survival within this patient population. Our center initiated systematic genotyping of patients with advanced NSCLC in 2004 (including those started on treatment between 2002 and 2004), allowing for prospective characterization of a cohort of patients with a minimum of a 5-year follow-up. Thus far, the median duration of follow-up in the major randomized clinical trials addressing the first-line use of erlotinib or gefitinib in EGFR-mutant advanced NSCLC has ranged 13-19 months.4-8,39
Previously, the SEER Program published a relative 5-year survival rate of 4.5% for unselected patients with distant-stage NSCLC.9 A few prior studies in the literature also examined long-term survival in advanced NSCLC patients,11-12,32-38 yet these patients were predominantly treated with chemotherapy and not exposed to an EGFR-TKI. Although Nishino et al. analyzed 5-year survival among advanced NSCLC patients treated with gefitinib (reporting a rate of 8.4%),33 they also did not restrict the analysis to patients with sensitizing EGFR mutations, such that the presence of an activating EGFR mutation alone (in 37.9% of all patients and 14.3% of the 5-year survivors) emerged as a variable affecting survival.33 Our literature review, in sum, identified 8 published studies since 2000 that reported on long-term survivors with advanced NSCLC; of these, 6 specified the % of 5-year survivors (Supplemental Table, Supplemental Digital Content).11-12,32-38 Analysis of the 6 studies revealed that, in aggregate, 3.9% of patients (or 46 of a total of 1176) with advanced or metastatic NSCLC have been reported as 5-year survivors (Supplemental Table, Supplemental Digital Content).11-12,32-38 The analysis is certainly limited by the caveat that the studies were not controlled for disparate patient demographics. Furthermore, although the analysis attempted to be comprehensive and capture all relevant studies, it is likely that some studies were inevitably overlooked because the information was not readily available in the title or the abstract. Despite these limitations, the ∼4% is an estimate for the historically reported % of 5-year survivors in advanced NSCLC, and is consistent with the rate presented by the SEER Program.10
We speculate that the 2-year survival rate of 66.4% and 5-year survival rate of 14.6% observed in our study (vs 7-16% and <5%, respectively, reported for unselected advanced NSCLC patients in the literature; Supplemental Table, Supplemental Digital Content) likely reflects both the patient characteristics and the early and now prevalent use of EGFR-TKIs. Although clinicians generally acknowledge that TKIs have transformed the outcome of some patients with NSCLC, there has been a paucity of data to objectively demonstrate the impact of EGFR-TKIs on the long-term survival of patients with sensitizing mutations treated with these agents. Multiple studies previously showed a marked PFS benefit delivered by EGFR-TKIs,4-8,39-42 but data on OS advantage was absent until the recent LUX-Lung 3 and LUX-Lung 6 trials with afatinib, in which median survival was prolonged by 12-13 months versus with chemotherapy for patients with exon 19 deletions (but not for the entire populations in the trials).39-45 It has been proposed that this lack of OS advantage in pertinent clinical trials might be attributable to high crossover rates from chemotherapy to EGFR-TKIs.41-43 Our findings provide support for this notion and suggest that the proportion of patients surviving beyond 5 years is significantly higher compared to that observed in unselected patients prior to the discovery of EGFR mutations.
We additionally demonstrated here that outcomes in our examined population remain heterogeneous despite the mutual presence of both major activating EGFR mutations and adenocarcinoma histology, highlighting the importance of elucidating other reliable predictors of survival. In our multivariate analysis, the clinical factors independently associated with prolonged OS were the presence of an exon 19 mutation in EGFR, absence of extrathoracic or brain metastasis, and absence of current smoking status. The association between exon 19 deletions and prolonged survival in advanced NSCLC patients treated with EGFR-TKIs (including the more recently approved afatinib) has been reported by several groups.6,23,24,43-45 Biological mechanisms to explain the apparent differences in outcome rendered by these EGFR mutations remain an area of investigation. In vitro, growth of cell lines harboring exon 19 deletions or L858R is inhibited similarly by erlotinib or gefitinib,17,46 urging the question of whether, in vivo, tumors with discrete sensitizing EGFR mutations bear unique propensities to acquire concomitant resistance mutations within EGFR (such as T790M) or other driver mutations beyond those in EGFR which then ultimately alter disease behavior and clinical outcomes. The association between extrathoracic metastasis and survival has been suggested by Park et al. and Lee et al.47,48 Both groups examined clinical features associated with survival in EGFR-mutant advanced NSCLC patients treated with an EGFR-TKI and identified tumor burden as one such feature.47,48 However, these studies defined tumor burden as the number of metastatic sites (1-2 vs ≥3), whereas our study instead analyzed the dichotomous variable of the presence of extrathoracic metastasis. Notably, brain metastasis in particular was associated with shortened OS in our study. The central nervous system involvement of lung cancer has long been recognized as a factor predictive of worse outcome.49,50 This association may be due to the incomplete penetration of the blood-brain barrier by the EGFR-TKIs which, nonetheless, have induced improved partial treatment responses compared to that achieved by chemotherapy.25,26
The limitations of our study include: the retrospective design wherein undefined biases may have existed and influenced clinical outcomes; the relatively small number of patients (for example, only 5 patients belonging to the active smoker group in our analysis); the data collection and analysis performed at a single tertiary academic center imposing a possible selection bias; and the restriction of the analysis to clinical parameters as well as exclusion of certain clinical parameters that may be relevant to survival, such as salvage therapies or performance status (due to inconsistent collection of this information in our database). The findings reported herein will therefore need validation in larger patient cohorts. A follow-up study is warranted to elucidate genetic alterations that may co-occur with the sensitizing EGFR mutations and further impact survival. In this regard, our group is working to retrieve tumor samples of the long-term vs short-term survivors, in order to characterize these at a molecular level and identify genetic predictors of survival beyond the activating somatic mutations in EGFR. As the sample number is limited to allow for meaningful analysis, we will be working to form a consortium to study this further.
In summary, our findings estimate a 5-year survival rate of 14.6% in patients with EGFR-mutant metastatic lung adenocarcinoma treated with erlotinib or gefitinib. We demonstrated through multivariate analysis that exon 19 deletions are independently associated with prolonged OS, whereas the presence of extrathoracic or brain metastasis and current smoking status are associated with shortened OS. These observations help delineate subsets of patients who might benefit from EGFR inhibition to reach a 5-year survival endpoint. Future studies designed to investigate the molecular and genetic factors that impact survival should help further tailor our understanding of heterogeneous outcomes in these patients.
Supplementary Material
Supplemental Figure. Patient flow through the eligibility criteria in the study.
Supplemental Table. Literature series on long-term survivors with advanced NSCLC.
Acknowledgments
Grant Support: Dana-Farber/Harvard Cancer Center Lung Cancer Specialized Program in Research Excellence (SPORE) P50 CA090578, and NIH grant 5R01-CA114465 (Drs. Jänne and Johnson).
Funding Source: National Institutes of Health and Dana-Farber/Harvard Cancer Center Lung Cancer Specialized Program in Research Excellence (SPORE).
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 2.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 3.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naïve patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 5.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 6.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 7.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 8.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 9.Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howlader N, Noone AM, Krapcho M, et al., editors. National Cancer Institute; Bethesda, MD: SEER Cancer Statistics Review, 1975-2011. http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014. [Google Scholar]
- 11.Van Damme V, Govaerts E, Nackaerts K. Clinical factors predictive of long-term survival in advanced non-small cell lung cancer. Lung Cancer. 2013;79:73–76. doi: 10.1016/j.lungcan.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Ozkaya S, Findik S, Dirican A, et al. Long-term survival rates of patients with stage IIIB and IV non-small cell lung cancer treated with cisplatin plus vinorelbine or gemcitabine. Exp Ther Med. 2012;4:1035–1038. doi: 10.3892/etm.2012.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 14.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 trial) J Clin Oncol. 2003;21:2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 15.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 16.Mitsudomi T, Kosaka T, Endoh H, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005;23:2513–2520. doi: 10.1200/JCO.2005.00.992. [DOI] [PubMed] [Google Scholar]
- 17.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 18.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 19.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 20.Kosaka T, Yatabe Y, Endoh H, et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 21.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 22.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 23.Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:3908–3914. doi: 10.1158/1078-0432.CCR-06-0462. [DOI] [PubMed] [Google Scholar]
- 24.Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:839–844. doi: 10.1158/1078-0432.CCR-05-1846. [DOI] [PubMed] [Google Scholar]
- 25.Heon S, Yeap BY, Lindeman NI, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 2012;18:4406–4414. doi: 10.1158/1078-0432.CCR-12-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010;16:5873–5882. doi: 10.1158/1078-0432.CCR-10-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costa DB, Nguyen KS, Cho BC, et al. Effects of erlotinib in EGFR mutated non-small cell lung cancers with resistance to gefitinib. Clin Cancer Res. 2008;14:7060–7067. doi: 10.1158/1078-0432.CCR-08-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010;28:357–360. doi: 10.1200/JCO.2009.24.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park K, Ahn M, Yu C, et al. ASPIRATION: first-line erlotinib (E) until and beyond RECIST progression (PD) in Asian patients (pts) with EGFR mutation-positive (mut+) NSCLC. Ann Oncol. 2014;25:iv426–iv470. [Google Scholar]
- 30.Lo PC, Dahlberg SE, Nishino M, et al. Delay of treatment change after objective progression on first-line erlotinib in epidermal growth factor receptor-mutant lung cancer. Cancer. 2015;121:2570–2577. doi: 10.1002/cncr.29397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su KY, Chen HY, Li KC, et al. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol. 2012;30:433–440. doi: 10.1200/JCO.2011.38.3224. [DOI] [PubMed] [Google Scholar]
- 32.Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29:2866–2874. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 33.Nishino K, Imamura F, Morita S, et al. A retrospective analysis of 335 Japanese lung cancer patients who responded to initial gefitinib treatment. Lung Cancer. 2013;82:299–304. doi: 10.1016/j.lungcan.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Okamoto T, Maruyama R, Shoji F, et al. Long-term survivors in stage IV non-small cell lung cancer. Lung cancer. 2005;47:85–91. doi: 10.1016/j.lungcan.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Satoh H, Ishikawa H, Ohara G, et al. Long-term survivors after chemotherapy in advanced non-small cell lung cancer. Anticancer Res. 2007;27:4457–4460. [PubMed] [Google Scholar]
- 36.Giroux Leprieur E, Lavole A, Ruppert AM, et al. Factors associated with long-term survival of patients with advanced non-small cell lung cancer. Respirology. 2012;17:134–142. doi: 10.1111/j.1440-1843.2011.02070.x. [DOI] [PubMed] [Google Scholar]
- 37.Kaira K, Takahashi T, Murakami H, et al. Long-term survivors of more than 5 years in advanced non-small cell lung cancer. Lung Cancer. 2010;67:120–123. doi: 10.1016/j.lungcan.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 38.Chen YZ, Feng XB, Li ZD, et al. Clinical study on long-term overall survival of advanced non-small-cell lung cancer patients treated with Chinese medicine and Western medicine. Chin J Integr Med. 2014;20:179–183. doi: 10.1007/s11655-014-1770-6. [DOI] [PubMed] [Google Scholar]
- 39.Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002) Ann Oncol. 2013;24:54–59. doi: 10.1093/annonc/mds214. [DOI] [PubMed] [Google Scholar]
- 40.Yoshioka H, Mitsudomi T, Morita S, et al. Final overall survival results of WJTOG 3405, a randomized phase III trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first-line treatment for patients with non-small cell lung cancer harboring mutations of the epidermal growth factor receptor (EGFR) J Clin Oncol. 2014;32:8117. abstr. [Google Scholar]
- 41.Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst. 2013;105:595–605. doi: 10.1093/jnci/djt072. [DOI] [PubMed] [Google Scholar]
- 42.Cardarella S, Johnson BE. Meta-analysis of EGFR kinase inhibitors: not always greater than the sum of its parts. J Natl Cancer Inst. 2013;105:589–590. doi: 10.1093/jnci/djt085. [DOI] [PubMed] [Google Scholar]
- 43.Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–151. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 44.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 45.Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 46.Mukohara T, Engelman JA, Hanna NH, et al. Differential effects of gefitinib and cetuximab on non-small-cell lung cancers bearing epidermal growth factor receptor mutations. J Natl Cancer Inst. 2005;97:1185–1194. doi: 10.1093/jnci/dji238. [DOI] [PubMed] [Google Scholar]
- 47.Park JH, Kim TM, Keam B, et al. Tumor burden is predictive of survival in patients with non-small-cell lung cancer and with activating epidermal growth factor receptor mutations who receive gefitinib. Clin Lung Cancer. 2013;14:383–389. doi: 10.1016/j.cllc.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Lee JY, Lim SH, Kim M, et al. Is there any predictor for clinical outcome in EGFR mutant NSCLC patients treated with EGFR TKIs? Cancer Chemother Pharmacol. 2014;73:1063–1070. doi: 10.1007/s00280-014-2442-8. [DOI] [PubMed] [Google Scholar]
- 49.Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol. 2005;23:6207–6219. doi: 10.1200/JCO.2005.03.145. [DOI] [PubMed] [Google Scholar]
- 50.Berger LA, Riesenberg H, Bokemeyer C, et al. CNS metastases in non-small-cell lung cancer: current role of EGFR-TKI therapy and future perspectives. Lung Cancer. 2013;80:242–248. doi: 10.1016/j.lungcan.2013.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Patient flow through the eligibility criteria in the study.
Supplemental Table. Literature series on long-term survivors with advanced NSCLC.