Abstract
Objectives
This study was undertaken to determine which symptoms are perceived to be most problematic for patients with ALS and how their severity changes over time.
Methods
A retrospective study was performed of data from a randomized, double-blind, placebo-controlled trial of ceftriaxone in ALS. Participants completed the ALS Specific Quality of Life Instrument (ALSSQOL) at baseline and at intervals up to 96 weeks. Ten ALSSQOL items ask participants to rate how problematic symptoms are (the subjective feeling of burden of these symptoms), ranging from 0 (no problem) to 10 (tremendous problem). Six are non-bulbar (pain, fatigue, breathing, strength and ability to move, sleep, and bowel and bladder) and four are bulbar (eating, speaking, excessive saliva, and mucus).
Results
There were 82 subjects (56% men, mean age 53 ± 10.3 years) with ALSSQOL data for weeks 0 and 96. All 10 symptoms became more problematic over time. For non-bulbar symptoms, strength/ability to move and fatigue were the most problematic. Speaking was the most problematic bulbar symptom.
Conclusions
Although all the symptoms in the ALSSQOL were acknowledged as problematic, some had greater impact than others. All became more problematic over time. This should help prioritize research into symptom management, and assist individual clinicians in their approach to patient care.
Keywords: amyotrophic lateral sclerosis, quality of life, fatigue, strength, speech
Introduction
The management of patients with amyotrophic lateral sclerosis (ALS) is aimed at providing optimal supportive care(1), most often in a multidisciplinary setting based on guidelines such as those of the American Academy of Neurology and the EFNS Task Force (2–5). This form of care has been shown to prolong lifespan and to positively impact quality of life (QOL) in patients with ALS in most(6–10), but not all (11,12), comparative studies of multidisciplinary care versus other forms of care. A key part of the supportive care is the management of a wide variety of symptoms. An attempt to identify a core group of symptoms that are of importance to this patient group was undertaken during the development of the ALS Specific Quality of Life Instrument (ALSSQOL), which includes 6 non-bulbar and 4 bulbar symptoms relevant to ALS (13). The perception by patients of the magnitude of the problem posed by each of these 10 items has not been assessed. Knowledge of the relative importance of these items should permit researchers to better prioritize the development of interventions for symptom management. An understanding of the changes in these problems over time should aid clinicians as they address symptom management and attempt to optimize QOL in individual patients. The objectives of this study were to determine which symptoms are perceived to be the most problematic for patients with ALS and how the severity of those problems changes over time.
Subjects and Methods
This was a retrospective study using a limited data set from a multi-center, three-stage randomized, double-blind, placebo-controlled study on ceftriaxone in ALS, conducted between Sept 4, 2006, and July 30, 2012 (ClinicalTrials.gov NCT00349622) (14), a study that did not show efficacy. The main inclusion and exclusion criteria were an El Escorial diagnosis(15) of possible ALS or higher, vital capacity of more than 60% of predicted, and symptom duration of less than 3 years. Participants completed the ALS Specific Quality of Life Instrument(13) and the ALS Functional Rating Scale-Revised(16), and performed spirometry at baseline, 4 weeks, 16 weeks, and every 16 weeks thereafter throughout the trial, for a total of 8 assessments. For inclusion in the data set, participants must have completed a minimum of 96 weeks in the trial. The study was approved by our Institutional Review Board.
Instruments
The ALS Specific Quality of Life Instrument: The ALSSQOL (13) is an ALS-specific measure of overall QOL, assessing health-related and non-health-related factors. It assesses overall QOL via six specific domains, and has been validated on a national US sample of adult men and women who were receiving care for their disease in ALS multidisciplinary clinics. In addition to its suitability for use in a multidisciplinary ALS clinic, the instrument has been used to assess patients not receiving multidisciplinary care(12). Each of its 59 items uses a 0–10 point Likert scale, with 0 being the least desirable situation, and 10 the most desirable. The instrument contains 6 domains of QOL: Negative Emotion, Interaction with People and the Environment, Intimacy, Religiosity, Physical Symptoms, and Bulbar Function. An Average Total Score is obtained by adding the individual item scores and dividing by the total number of items, resulting in a score that varies from 0 (worst QOL) to 10 (best QOL). The first 10 items of the ALSSQOL ask participants to rate how problematic a variety of symptoms have been in the last 7 days, ranging from 0 (no problem) to 10 (tremendous problem). Six of the items are non-bulbar symptoms (pain, fatigue, breathing, strength and ability to move, sleep, and bowel and bladder) and the other four are bulbar (eating, speaking, excessive saliva, and mucus).
The ALS Functional Rating Scale-Revised: The ALSFRS-R(16) is a widely-used, 12-item, ALS-specific questionnaire assessing physical function in the bulbar, upper limb, lower limb, and respiratory domains. Each item is scored from 0 (poorest function) to 4 (normal function), and the scores are added to produce a total score from 0 (worst) to 48 (normal).
Statistical Methods
Only subjects with non-missing data at weeks 0 and 96 for ALSSQOL average were included in the analyses. The 10 individual symptoms of interest from the ALSSQOL (ranging from 0–10) were also categorized in terms of severity (0=None, 1–3=Mild, 4–6=Moderate, and 7–10=severe). All variables were summarized with means and standard deviations for continuous variables and frequencies and percentages for categorical variables. The means for ALSFRSR total score, average % predicted FVC, ALSSQOL, and the 10 individual symptoms of interest were plotted over time. The distribution of the continuous outcome variables was evaluated prior to analysis using histograms and box plots. A normal distribution was found. Comparisons were made between week 0 and week 96 for ALSFRSR total score, average % predicted FVC, and ALSSQOL using a paired t-test, for the individual symptoms in their original format (0–10) using a Wilcoxon signed rank test, and for the individual symptoms categorized as severity using Bowker’s test of symmetry. Statistical significance was taken as p < 0.05. Additionally, we analyzed the differences from week 0 to the other seven time points leading up to week 96 by using either a linear mixed effects model for repeated measures applied to all time points at once or by using individual Wilcoxon signed rank tests at each time point. The choice of the applied method depended on the distribution of the outcome variable, but for both methods we adjusted the p-values for the seven comparisons made for each outcome variable using the Bonferroni correction for multiple comparisons. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
There were 82 subjects for whom there was complete ALSSQOL data for week 0 and week 96. Fifty-four (56%) of the subjects were men. The mean age was 53 ± 10.3 years. Physical function (ALSFRSR), respiratory function (FVC) and QOL decreased over the course of the trial (See Table 1).
Table 1.
Physical and Functional Measures
| Measure | N | Week 0 | Week 96 | P-value* |
|---|---|---|---|---|
| Mean ± SD (range) | Mean ± SD (range) | |||
| ALSFRSR total score (0–48) | 81 | 38.5 ± 6.0 (20–48) | 24.3 ± 9.2 (3–45) | <0.001 |
| % predicted FVC (0–100) | 69 | 89.9 ± 15.5 (62.0–133.3) | 57.1 ± 25.5 (6.7–113.0) | <0.001 |
| ALSSQOL average (0–10) | 82 | 7.5 ± 1.1 (4.6–9.3) | 6.6 ± 1.3 (3.6–9.8) | <0.001 |
Paired t-test
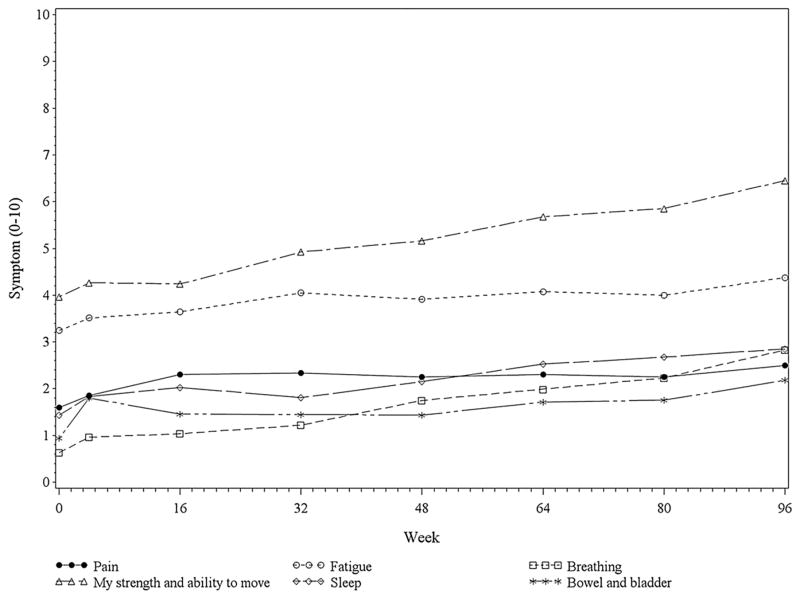
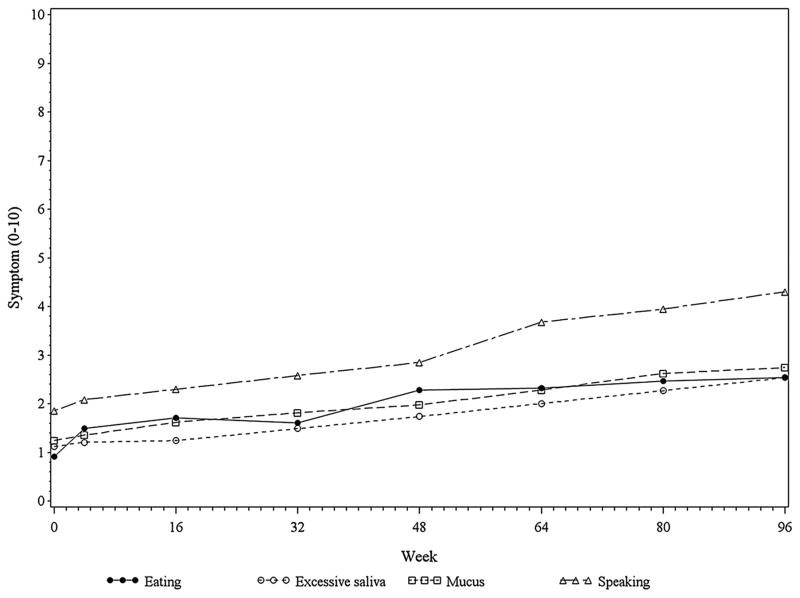
The average item scores of the 6 non-bulbar symptoms and 4 bulbar symptoms over the course of the 96 weeks of the trial are plotted in Figures 1 and 2 respectively. All 10 of the symptoms demonstrated an increase over time in patients’ perception of the degree to which they were problems (Tables 2 and 3). Some symptoms were rated as considerably more problematic, on average, than others. Moreover, the distribution of the severity ratings for all of the symptoms shifted towards being more severe over time (Tables 2 and 3). For non-bulbar symptoms, strength and ability to move followed by fatigue were the most problematic symptoms. Others were less problematic and were clustered together. For the bulbar symptoms, the most problematic was speaking, with others clustered lower.
Figure 1.
Mean Non-Bulbar Symptom Scores Over Time. The symptom scores are a subset of the first 10 items of the ALS Specific Quality of Life Instrument, and range from 0 (no problem) to 10 (tremendous problem).
Figure 2.
Mean Bulbar Symptoms Scores Over Time. . The symptom scores are a subset of the first 10 items of the ALS Specific Quality of Life Instrument, and range from 0 (no problem) to 10 (tremendous problem).
Table 2.
Non-Bulbar Symptoms
| Symptom | Week 0 | Week 96 | P-value* |
|---|---|---|---|
|
| |||
| N (%) or Mean ± SD | N (%) or Mean ± SD | ||
|
| |||
| Pain score (0–10) | 1.60 ± 2.27 | 2.50 ± 2.37 | 0.002 |
|
| |||
| Pain severity | |||
| None | 38 (46.3) | 18 (22.0) | 0.001 |
| Mild | 32 (39.0) | 44 (53.7) | |
| Moderate | 6 (7.3) | 12 (14.6) | |
| Severe | 6 (7.3) | 8 (9.7) | |
|
| |||
| Fatigue score (0–10) | 3.24 ± 2.24 | 4.38 ± 2.22 | <0.001 |
|
| |||
| Fatigue severity | |||
| None | 10 (12.2) | 2 (2.4) | 0.036 |
| Mild | 37 (45.1) | 26 (31.7) | |
| Moderate | 28 (34.2) | 41 (50.0) | |
| Severe | 7 (8.5) | 13 (15.9) | |
|
| |||
| Breathing score (0–10) | 0.67 ± 1.33 | 2.83 ± 2.77 | <0.001 |
|
| |||
| Breathing severity | |||
| None | 59 (72.0) | 23 (28.1) | <0.001 |
| Mild | 19 (23.2) | 29 (35.4) | |
| Moderate | 3 (3.7) | 18 (22.0) | |
| Severe | 1 (1.2) | 12 (14.6) | |
|
| |||
| Strength/ability to move score (0–10) | 3.96 ± 2.57 | 6.46 ± 2.36 | <0.001 |
|
| |||
| Strength/ability to move severity | |||
| None | 7 (8.6) | 1 (1.2) | <0.001 |
| Mild | 32 (39.5) | 8 (9.9) | |
| Moderate | 27 (33.3) | 29 (35.8) | |
| Severe | 15 (18.5) | 43 (53.1) | |
|
| |||
| Sleep score (0–10) | 1.44 ± 1.90 | 2.85 ± 2.74 | <0.001 |
|
| |||
| Sleep severity | |||
| None | 37 (45.1) | 15 (18.3) | 0.002 |
| Mild | 32 (39.0) | 43 (52.4) | |
| Moderate | 11 (13.4) | 13 (15.9) | |
| Severe | 2 (2.4) | 11 (13.4) | |
|
| |||
| Bowel and bladder score (0–10) | 0.94 ± 1.79 | 2.18 ± 2.54 | <0.001 |
|
| |||
| Bowel and bladder severity | |||
| None | 53 (64.6) | 28 (34.2) | 0.006 |
| Mild | 22 (26.8) | 37 (45.1) | |
| Moderate | 5 (6.1) | 10 (12.2) | |
| Severe | 2 (2.4) | 7 (8.5) | |
Wilcoxon signed rank test or Bowker’s test of symmetry
Table 3.
Bulbar Symptoms
| Measure | Week 0 | Week 96 | P-value* |
|---|---|---|---|
|
| |||
| N (%) or Mean ± SD | N (%) or Mean ± SD | ||
|
| |||
| Eating score (0–10) | 0.91 ± 1.54 | 2.54 ± 3.09 | <0.001 |
|
| |||
| Eating severity | |||
| None | 51 (62.2) | 30 (36.6) | 0.005 |
| Mild | 23 (28.1) | 34 (41.5) | |
| Moderate | 7 (8.5) | 7 (8.5) | |
| Severe | 1 (1.2) | 11 (13.4) | |
|
| |||
| Excessive saliva score (0–10) | 1.12 ± 1.92 | 2.54 ± 2.93 | <0.001 |
|
| |||
| Excessive saliva severity | |||
| None | 52 (63.4) | 27 (32.9) | <0.001 |
| Mild | 20 (24.4) | 28 (34.2) | |
| Moderate | 7 (8.5) | 17 (20.7) | |
| Severe | 3 (3.7) | 10 (12.2) | |
|
| |||
| Mucus score (0–10) | 1.24 ± 1.73 | 2.74 ± 2.84 | <0.001 |
|
| |||
| Mucus severity | |||
| None | 43 (52.4) | 24 (29.3) | 0.002 |
| Mild | 31 (37.8) | 32 (39.0) | |
| Moderate | 6 (7.3) | 18 (22.0) | |
| Severe | 2 (2.4) | 8 (9.8) | |
|
| |||
| Speaking score (0–10) | 1.85 ± 2.59 | 4.30 ± 3.77 | <0.001 |
|
| |||
| Speaking severity | |||
| None | 45 (54.9) | 24 (29.3) | <0.001 |
| Mild | 18 (22.0) | 13 (15.9) | |
| Moderate | 13 (15.9) | 17 (20.7) | |
| Severe | 6 (7.3) | 28 (34.2) | |
Wilcoxon signed rank test or Bowker’s test of symmetry
When comparing the severity ratings for each of the 10 symptoms at each time point (4, 16, 32, 48, 64, 80, and 96 weeks) to baseline, the time at which the difference from baseline first became statistically significant varied between symptoms, occurring as early as 4 weeks for eating, but as late as 96 weeks for bowel and bladder. For pain and for fatigue, differences from baseline were statistically significant at some data collection points but not others (at weeks 16, 32, 64, and 96, but not 48 or 80 for pain; at weeks 32, 64, and 96, but not 48 or 80 for fatigue). However, the perception that these symptoms were increasingly problematic gradually increased during the data collection period, reaching statistical significance in all by week 96 (Tables 2 and 3, Figures 1 and 2).
Discussion
An understanding of what are perceived by patients to be the most problematic symptoms should aid in focusing clinical care and in providing direction for future research in clinical management and symptom control in ALS. Although all the symptoms in the ALSSQOL were acknowledged by patients, on average, to be problematic, some clearly had a greater impact than others, and all became more problematic over time.
That strength and the ability to move should be perceived as the greatest problem by patients reinforces the overwhelmingly motor-predominant and progressive nature of ALS. It also serves to reinforce the importance of addressing these concerns by means of a multidisciplinary team, to maximize function, mobility, and independence while preserving safety. And, it complicates the concept of a “response shift,” in which patients with life-threatening illnesses alter their expectations and goals to match reality(17–20), and may shift their perception of those activities necessary to maintain QOL from the physical to those that rely more on relationships or satisfaction with their environment. Clearly the loss of the ability to move has great impact.
The prominent attention given by patients to fatigue as a problem supplements our understanding of this symptom. Previous studies have demonstrated a high prevalence of fatigue in patients with ALS, showing that it affects 44–86% of these patients (21–23). One longitudinal study showed that at baseline, 44% had clinically significant fatigue. Of patients seen 3 months later, 75% of those who were fatigued at baseline remained fatigued, and 22% reported new onset fatigue (21). Fatigue rates were similar at a third visit 3 months later. The combination of the high prevalence and the problematic nature of fatigue suggest that attention to this symptom is of importance in the management of patients with ALS. The etiology of fatigue is multifactorial and not only includes the physical factors such as pain and weakness, but also central and psychological factors, effect of pain medications, and respiratory impairment (24). Poor sleep may also contribute to daytime fatigue(22). Patients with ALS may have difficulty falling asleep due to anxiety or depression, or may experience an inability to sustain sleep because of factors such as muscle cramps, restless legs or nocturia. A multimodality approach targeting these possible etiologies, education regarding energy conservation strategies, and psychostimulant therapy such as modafinil, should be considered(25). Non-invasive ventilation (NIV) also plays a role, as it has been found to improve daytime fatigue and sleepiness and to improve QOL(26–31)
In the context of multiple problematic symptoms in ALS, pain, breathing and bowel and bladder, while increasing in perceived impact of QOL over time, were not perceived to be as problematic as the other physical symptoms. Pain is now understood to be a predominant symptom in ALS, occurring in 50% and perhaps up to 85% of patients with ALS(32–35). There are many possible sources of pain in ALS including muscle atrophy, cramping and spasticity, joint contractors and inability to move (33,36), for which a variety of approaches to symptomatic management are available(1,37,38). The relatively low perception of breathing as a problematic symptom likely relates to the benefits of NIV and to established guidelines for respiratory management in ALS(2,5,26–31). Bowel and bladders symptoms were perceived as the least problematic of the non-bulbar symptoms. Nonetheless, they were perceived as a severe problem by nearly 9% of our patients at week 96, and thus warrant at least an inquiry by the clinical team. This is consistent with literature indicating that urinary incontinence is relatively common in patients with ALS and is associated with a high burden(39).
Speaking was the most problematic of the bulbar symptoms. Dysarthria occurs in more than 80% of ALS patients(40). Loss of communication affects ALS patients’ ability to socialize and imposes a significant burden to the individuals QOL(41,42). Early intervention by speech therapists and use of communication devices has been shown to have a positive impact on QOL in ALS patients(41,43) and provides the patients opportunity to improve the skills for later stages of the disease.
Although perceived as less problematic than speaking, eating, excessive saliva and mucus all were reported to become more problematic symptoms over time. Malnutrition, a significant risk for those with ALS, negatively affects prognosis and QOL, making early and frequent nutrition assessment and intervention essential(44). Implementation of an adequate calorie diet, dietary texture modification, use of adaptive eating utensils, and placement of a feeding tube aid in preventing malnutrition(45). Predictive equations have been established as a basis for recommending placement of a feeding gastrostomy in ALS patients who fail to meet their energy requirements by oral intake(46). Patient concerns regarding saliva and mucus reflect self-reporting of sialorrhea in about half of those with ALS, including 20% who characterized it as moderate to severe (47). Attention to this is important because of the potential for intervention with anticholinergic drugs, and for refractory cases with botulinum toxin injections or radiation therapy to salivary glands(48–54). Thick mucus that patients are unable to clear because of muscle weakness and an ineffective cough may respond to beta blockers or to guaifenesin or nebulized saline or acetylcysteine, often in conjunction with an insufflation-exsufflation (cough-assist) device(37).
We attempted to determine whether specific points in the disease trajectory could be identified at which the perceived severity of individual problems was significant enough to warrant consideration of intervention. However, the variability of the time points at which each of the problems achieved a statistically significant difference from baseline, the inconsistency of some problems in remaining significantly different from baseline throughout the trajectory, and a lack of information as to whether any interventions for these problems were undertaken in these study patients, placed such an analysis beyond the scope of this retrospective review. Also of importance is the fact that mean differences in problem severity rating between data collection points and baseline was often very small (less than one point), raising concerns about whether such changes, while statistically significant, are clinically meaningful. By assessing severity of problems at week 96 and comparing to week 0, differences for all problems were statistically significant and likely to be clinically meaningful. Future studies, conducted prospectively, focusing on a smaller number of problems, and attempting to determine the size of a clinically meaningful difference in problem severity scores, could help guide interventions.
This study has limitations. Patients were part of a clinical trial, and in some respects differed from the general ALS patient population. Those with a forced vital capacity of 60% of predicted or less were excluded, as were those with a symptom duration of 3 years or more and those who completed fewer than 96 weeks in the trial. While the investigational drug was ineffective, the study sample used for this analysis may differ from those of the general ALS population with regard to factors that impact their QOL. Specifically, the decline in QOL over time in this study is in contrast to that of previous studies(55,56), and may reflect a greater emphasis on physical strength and function, with the desire to “get better” as a result of being in a clinical trial. The clinical trial involved the use and care of an intravenous catheter which could have been an added burden. Twice daily infusions combined with frequent visits to the study sites for evaluation may have impacted on patient’s ratings of their problematic symptoms. Patients receiving ceftriaxone experienced significantly more frequent gastrointestinal and hepatobiliary adverse events than did those receiving placebo (14), possibly affecting perception of problematic symptoms, particularly pain, fatigue, and bowel and bladder, in this study population compared to the general ALS patient population. We do not know what interventions were provided to alleviate symptoms, or the extent of compliance with recommended care. This study is a secondary analysis of the finished trial, and the sample size cannot be powered on the primary outcome of this study.
Despite these limitations, this study provides a relatively broad view of the impact of symptoms as perceived by patients with ALS over a long course with the disease. This should help direct the ALS community in prioritizing research initiatives for symptom management, and hopefully will assist individual clinicians in their approach to patient care.
Acknowledgments
Funding for the Ceftriaxone clinical trial: NIH 5U01NS049640-08
This work was supported by the Paul and Harriet Campbell Fund for ALS Research, the ALS Association Greater Philadelphia Chapter, and many other private donations to the Penn State Hershey ALS Center.
We gratefully acknowledge the work of the individual sites and personnel of the ceftriaxone study team in the collection of the clinical trial data.
Footnotes
Disclosures of Interest
Dr. Simmons has received reimbursement from Neuralstem, Inc., for serving on a Data Safety Monitoring Board for an ALS therapeutic trial. Other authors have no conflicts of interest to disclose.
Contributor Information
Divisha Raheja, Department of Neurology, The Pennsylvania State University College of Medicine, Hershey, PA.
Helen E Stephens, Department of Neurology, The Pennsylvania State University College of Medicine, Hershey PA.
Erik Lehman, Department of Public Health Sciences, The Pennsylvania State University College of Medicine, Hershey PA.
Susan Walsh, ALS Association Greater Philadelphia Chapter, Harrisburg PA.
Chengwu Yang, Department of Public Health Sciences & Office for Scholarship in Learning and Education Research, The Pennsylvania State University College of Medicine, Hershey PA.
Zachary Simmons, Departments of Neurology and Humanities, The Pennsylvania State University College of Medicine, Hershey, PA.
References
- 1.Simmons Z. Management Strategies for Patients With Amyotrophic Lateral Sclerosis From Diagnosis Through Death. Neurologist. 2005;11:257–270. doi: 10.1097/01.nrl.0000178758.30374.34. [DOI] [PubMed] [Google Scholar]
- 2.Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al. Practice Parameter update: The care of the patient with amyotrophic lateral sclerosis: Drug, nutritional, and respiratory therapies (an evidence-based review) Neurology American Academy of Neurology. 2009;73:1218–1226. doi: 10.1212/WNL.0b013e3181bc0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al. Practice parameter update: The care of the patient with amyotrophic lateral sclerosis: Multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review) Neurology. 2009;73:1227–1233. doi: 10.1212/WNL.0b013e3181bc01a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller RG, Brooks BR, Swain-Eng RJ, Basner RC, Carter GT, Casey P, et al. Quality improvement in neurology: amyotrophic lateral sclerosis quality measures: report of the quality measurement and reporting subcommittee of the American Academy of Neurology. Neurology. 2013;81:2136–2140. doi: 10.1212/01.wnl.0000437305.37850.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen PM, Abrahams S, Borasio GD, de Carvalho M, Chio A, Van Damme P, et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS) – revised report of an EFNS task force. Eur J Neurol. 2012;19:360–375. doi: 10.1111/j.1468-1331.2011.03501.x. [DOI] [PubMed] [Google Scholar]
- 6.Van den Berg JP, Kalmijn S, Lindeman E, Veldink JH, de Visser M, Van der Graaff MM, et al. Multidisciplinary ALS care improves quality of life in patients with ALS. Neurology. 2005;65:1264–1267. doi: 10.1212/01.wnl.0000180717.29273.12. [DOI] [PubMed] [Google Scholar]
- 7.Traynor BJ, Alexander M, Corr B, Frost E, Hardiman O. Effect of a multidisciplinary amyotrophic lateral sclerosis (ALS) clinic on ALS survival: a population based study, 1996–2000. J Neurol Neurosurg Psychiatry. 2003;74:1258–1261. doi: 10.1136/jnnp.74.9.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiò A, Bottacchi E, Buffa C, Mutani R, Mora G. Positive effects of tertiary centres for amyotrophic lateral sclerosis on outcome and use of hospital facilities. J Neurol Neurosurg Psychiatry. 2006;77:948–950. doi: 10.1136/jnnp.2005.083402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rooney J, Byrne S, Heverin M, Tobin K, Dick A, Donaghy C, et al. A multidisciplinary clinic approach improves survival in ALS: a comparative study of ALS in Ireland and Northern Ireland. J Neurol Neurosurg Psychiatry. 2015;86:496–501. doi: 10.1136/jnnp-2014-309601. [DOI] [PubMed] [Google Scholar]
- 10.Aridegbe T, Kandler R, Walters SJ, Walsh T, Shaw PJ, McDermott CJ. The natural history of motor neuron disease: assessing the impact of specialist care. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:13–19. doi: 10.3109/17482968.2012.690419. [DOI] [PubMed] [Google Scholar]
- 11.Zoccolella S, Beghi E, Palagano G, Fraddosio A, Guerra V, Lepore V, et al. ALS multidisciplinary clinic and survival. Results from a population-based study in Southern Italy J Neurol. 2007;254:1107–1112. doi: 10.1007/s00415-006-0401-y. [DOI] [PubMed] [Google Scholar]
- 12.Stephens H, Young J, Felgoise S, Simmons Z. Multidisciplinary ALS Clinics in the USA: A Comparison of Those Who Attend and Those Who Do Not. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:196–201. doi: 10.3109/21678421.2014.994530. [DOI] [PubMed] [Google Scholar]
- 13.Simmons Z, Felgoise SH, Bremer BA, Walsh SM, Hufford DJ, Bromberg MB, et al. The ALSSQOL: balancing physical and nonphysical factors in assessing quality of life in ALS. Neurology. 2006;67:1659–1664. doi: 10.1212/01.wnl.0000242887.79115.19. [DOI] [PubMed] [Google Scholar]
- 14.Cudkowicz ME, Titus S, Kearney M, Yu H, Sherman A, Schoenfeld D, et al. Safety and efficacy of ceftriaxone for amyotrophic lateral sclerosis: a multi-stage, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13:1083–1091. doi: 10.1016/S1474-4422(14)70222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 16.Cedarbaum JM, Stambler N, Fuller C, Hilt D, Thurmond B, Nakanishi A, et al. The ALSFRS-R : a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz CE, Sprangers MA. Methodological approaches for assessing response shift in longitudinal health-related quality-of-life research. Soc Sci Med. 1999;48:1531–1548. doi: 10.1016/s0277-9536(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 18.Carr AJ, Gibson B, Robinson PG. Measuring quality of life: Is quality of life determined by expectations or experience? BMJ. 2001;322:1240–1243. doi: 10.1136/bmj.322.7296.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yardley L, Dibb B. Assessing subjective change in chronic illness: An examination of response shift in health-related and goal-oriented subjective status. Psychol Health. 2007;22:813–828. [Google Scholar]
- 20.Barclay R, Tate RB. Response shift recalibration and reprioritization in health-related quality of life was identified prospectively in older men with and without stroke. J Clin Epidemiol. 2014;67:500–507. doi: 10.1016/j.jclinepi.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 21.McElhiney MC, Rabkin JG, Gordon PH, Goetz R, Mitsumoto H. Prevalence of fatigue and depression in ALS patients and change over time. J Neurol Neurosurg Psychiatry. 2009;80:1146–1149. doi: 10.1136/jnnp.2008.163246. [DOI] [PubMed] [Google Scholar]
- 22.Lo Coco D, La Bella V. Fatigue, sleep, and nocturnal complaints in patients with amyotrophic lateral sclerosis. Eur J Neurol. 2012;19:760–763. doi: 10.1111/j.1468-1331.2011.03637.x. [DOI] [PubMed] [Google Scholar]
- 23.Gibbons CJ, Thornton EW, Young CA. The patient experience of fatigue in motor neurone disease. Front Psychol. 2013;4:788. doi: 10.3389/fpsyg.2013.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abraham A, Drory VE. Fatigue in motor neuron diseases. Neuromuscul Disord. 2012;22(Suppl 3):S198–202. doi: 10.1016/j.nmd.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Rabkin JG, Gordon PH, McElhiney M, Rabkin R, Chew S, Mitsumoto H. Modafinil treatment of fatigue in patients with ALS: a placebo-controlled study. Muscle Nerve. 2009;39:297–303. doi: 10.1002/mus.21245. [DOI] [PubMed] [Google Scholar]
- 26.Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol. 2006;5:140–147. doi: 10.1016/S1474-4422(05)70326-4. [DOI] [PubMed] [Google Scholar]
- 27.Butz M, Wollinsky KH, Wiedemuth-Catrinescu U, Sperfeld A, Winter S, Mehrkens HH, et al. Longitudinal effects of noninvasive positive-pressure ventilation in patients with amyotrophic lateral sclerosis. Am J Phys Med Rehabil. 2003;82:597–604. doi: 10.1097/01.PHM.0000078239.83545.D0. [DOI] [PubMed] [Google Scholar]
- 28.Aboussouan LS, Khan SU, Banerjee M, Arroliga AC, Mitsumoto H. Objective measures of the efficacy of noninvasive positive-pressure ventilation in amyotrophic lateral sclerosis. Muscle Nerve. 2001;24:403–409. doi: 10.1002/1097-4598(200103)24:3<403::aid-mus1013>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Jackson CE, Rosenfeld J, Moore DH, Bryan WW, Barohn RJ, Wrench M, et al. A preliminary evaluation of a prospective study of pulmonary function studies and symptoms of hypoventilation in ALS/MND patients. J Neurol Sci. 2001;191:75–78. doi: 10.1016/s0022-510x(01)00617-7. [DOI] [PubMed] [Google Scholar]
- 30.Lyall RA, Donaldson N, Fleming T, Wood C, Newsom-Davis I, Polkey MI, et al. A prospective study of quality of life in ALS patients treated with noninvasive ventilation. Neurology. 2001;57:153–156. doi: 10.1212/wnl.57.1.153. [DOI] [PubMed] [Google Scholar]
- 31.Bourke SC, Bullock RE, Williams TL, Shaw PJ, Gibson GJ. Noninvasive ventilation in ALS: indications and effect on quality of life. Neurology. 2003;61:171–177. doi: 10.1212/01.wnl.0000076182.13137.38. [DOI] [PubMed] [Google Scholar]
- 32.Ganzini L, Johnston WS, Hoffman WF. Correlates of suffering in amyotrophic lateral sclerosis. Neurology. 1999;52:1434–1440. doi: 10.1212/wnl.52.7.1434. [DOI] [PubMed] [Google Scholar]
- 33.Chiò A, Canosa A, Gallo S, Moglia C, Ilardi A, Cammarosano S, et al. Pain in amyotrophic lateral sclerosis: a population-based controlled study. Eur J Neurol. 2012;19:551–555. doi: 10.1111/j.1468-1331.2011.03540.x. [DOI] [PubMed] [Google Scholar]
- 34.Wallace VCJ, Ellis CM, Burman R, Knights C, Shaw CE, Al-Chalabi A. The evaluation of pain in amyotrophic lateral sclerosis: A case controlled observational study. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:520–527. doi: 10.3109/21678421.2014.951944. [DOI] [PubMed] [Google Scholar]
- 35.Hanisch F, Skudlarek A, Berndt J, Kornhuber ME. Characteristics of pain in amyotrophic lateral sclerosis. Brain Behav. 2015 Mar;5(3):e00296. doi: 10.1002/brb3.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Handy CR, Krudy C, Boulis N, Federici T. Pain in amyotrophic lateral sclerosis: a neglected aspect ofdisease. Neurol Res Int. 2011;2011:403808. doi: 10.1155/2011/403808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmons Z. Rehabilitation of motor neuron disease. In: Barnes M, Good D, editors. Neurorehabilitation. Handbook of Clinical Neurology. Elsevier; Amsterdam: 2013. pp. 483–498. (Michael Aminoff series ed) [DOI] [PubMed] [Google Scholar]
- 38.Weiss M, Simmons Z, Atassi N, Graves M, Parziale N, Salameh J, et al. A Phase 2 Study of Mexiletine in Sporadic Amyotrophic Lateral Sclerosis. Neurology. 2015;84:S50.004. [Google Scholar]
- 39.Nübling GS, Mie E, Bauer RM, Hensler M, Lorenzl S, Hapfelmeier A, et al. Increased prevalence of bladder and intestinal dysfunction in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:174–179. doi: 10.3109/21678421.2013.868001. [DOI] [PubMed] [Google Scholar]
- 40.Tomik B, Guiloff RJ. Dysarthria in amyotrophic lateral sclerosis: a review. Amyotroph Lateral Scler. 2010;11:4–15. doi: 10.3109/17482960802379004. [DOI] [PubMed] [Google Scholar]
- 41.Körner S, Sieniawski M, Siniawski M, Kollewe K, Rath KJ, Krampfl K, et al. Speech therapy and communication device: impact on quality of life and mood in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:20–25. doi: 10.3109/17482968.2012.692382. [DOI] [PubMed] [Google Scholar]
- 42.da Costa Franceschini A, Mourão LF. Dysarthria and dysphagia in amyotrophic lateral sclerosis with spinal onset: a study of quality of life related to swallowing. Neuro Rehabilitation. 2015;36:127–134. doi: 10.3233/NRE-141200. [DOI] [PubMed] [Google Scholar]
- 43.Londral A, Pinto A, Pinto S, Azevedo L, de Carvalho M. Quality of life in ALS patients and caregivers: impact of assistive communication from early stages. Muscle Nerve. 2015 Mar 25; doi: 10.1002/mus.24659. [DOI] [PubMed] [Google Scholar]
- 44.Kasarskis EJ, Mendiondo MS, Wells S, Malguizo MS, Thompson M, Healey M, et al. The ALS Nutrition/NIPPV Study: design, feasibility, and initial results. Amyotroph Lateral Scler. 2011;12:17–25. doi: 10.3109/17482968.2010.515225. [DOI] [PubMed] [Google Scholar]
- 45.Greenwood DI. Nutrition management of amyotrophic lateral sclerosis. Nutr Clin Pract. 2013;28:392–399. doi: 10.1177/0884533613476554. [DOI] [PubMed] [Google Scholar]
- 46.Kasarskis EJ, Mendiondo MS, Matthews DE, Mitsumoto H, Tandan R, Simmons Z, et al. Estimating daily energy expenditure in individuals with amyotrophic lateral sclerosis. Am J Clin Nutr. 2014;99:792–803. doi: 10.3945/ajcn.113.069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradley WG, Anderson F, Bromberg M, Gutmann L, Harati Y, Ross M, et al. Current management of ALS: comparison of the ALS CARE Database and the AAN Practice Parameter. Neurology. 2001;57:500–504. doi: 10.1212/wnl.57.3.500. [DOI] [PubMed] [Google Scholar]
- 48.Young CA, Ellis C, Johnson J, Sathasivam S, Pih N. Treatment for sialorrhea (excessive saliva) in people with motor neuron disease/amyotrophic lateral sclerosis. Cochrane database Syst Rev. 2011:CD006981. doi: 10.1002/14651858.CD006981.pub2. doi:0.1002/14651858.CD006981.pub2. [DOI] [PubMed] [Google Scholar]
- 49.Stokholm MG, Bisgård C, Vilholm OJ. Safety and administration of treatment with botulinum neurotoxin for sialorrhoea in ALS patients: review of the literature and a proposal for tailored treatment. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:516–520. doi: 10.3109/21678421.2013.830312. [DOI] [PubMed] [Google Scholar]
- 50.Giess R, Naumann M, Werner E, Riemann R, Beck M, Puls I, et al. Injections of botulinum toxin A into the salivary glands improve sialorrhoea in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2000;69:121–123. doi: 10.1136/jnnp.69.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verma A, Steele J. Botulinum toxin improves sialorrhea and quality of living in bulbar amyotrophic lateral sclerosis. Muscle Nerve. 2006;34:235–237. doi: 10.1002/mus.20545. [DOI] [PubMed] [Google Scholar]
- 52.Scott KR, Kothari MJ, Venkatesh YS, Murphy T, Simmons Z. Parotid gland injections of botulinum toxin a are effective in treating sialorrhea in amyotrophic lateral sclerosis. J Clin Neuromuscul Dis. 2005;7:62–65. doi: 10.1097/01.cnd.0000188865.88167.62. [DOI] [PubMed] [Google Scholar]
- 53.Neppelberg E, Haugen DF, Thorsen L, Tysnes O-B. Radiotherapy reduces sialorrhea in amyotrophic lateral sclerosis. Eur J Neurol. 2007;14:1373–1377. doi: 10.1111/j.1468-1331.2007.01983.x. [DOI] [PubMed] [Google Scholar]
- 54.Kasarskis EJ, Hodskins J, St Clair WH. Unilateral parotid electron beam radiotherapy as palliative treatment for sialorrhea in amyotrophic lateral sclerosis. J Neurol Sci. 2011;308:155–157. doi: 10.1016/j.jns.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 55.Gauthier A, Vignola A, Calvo A, Cavallo E, Moglia C, Sellitti L, et al. A longitudinal study on quality of life and depression in ALS patient-caregiver couples. Neurology. 2007;68:923–926. doi: 10.1212/01.wnl.0000257093.53430.a8. [DOI] [PubMed] [Google Scholar]
- 56.Cupp J, Simmons Z, Berg A, Felgoise SH, Walsh SM, Stephens HE. Psychological health in patients with ALS is maintained as physical function declines. Amyotroph Lateral Scler. 2011;12:290–296. doi: 10.3109/17482968.2011.554555. [DOI] [PubMed] [Google Scholar]