Abstract
Background
Prostaglandin E2 (PGE2) EP receptors EP3 and EP4 signal via decreased and increased cAMP production, respectively. Previously we reported that cardiomyocyte-specific EP4 KO mice develop dilated cardiomyopathy with reduced ejection fraction. We thus hypothesized that PGE2 increases contractility via EP4 but decreases contractility via EP3.
Methods and Results
The effects of PGE2 and the EP1/EP3 agonist sulprostone on contractility were examined in the mouse langendorff preparation and in adult mouse cardiomyocytes (AVM). Isolated hearts of adult male C57Bl/6 mice were perfused with PGE2 (10−6M) or sulp (10−6M) and compared to vehicle. Both PGE2 and sulprostone decreased +dp/dt (p<0.01) and left ventricular developed pressure, LVDP, (p<0.001) with reversal by an EP3 antagonist. In contrast, the EP4 agonist had the opposite effect. AVM contractility was also reduced after treatment with either PGE2 or sulprostone for 10 min. We then examined the acute effects of PGE2, sulprostone and the EP4 agonist on expression of phosphorylated phospholamban (PLN) and SERCA2a in AVM using Western blot. Treatment with either PGE2 or sulprostone decreased expression of phosphorylated PLN corrected to total PLN whereas treatment with the EP4 agonist had the opposite effect. SERCA2a expression was unaffected. Finally, we examined the effect of these compounds in vivo using pressure volume loops. Both PGE2 and sulprostone decreased +dp/dt while the EP4 agonist increased +dp/dt.
Conclusions
Contractility is reduced via the EP3 receptor but increased via EP4. These effects may be mediated through changes in PLN phosphorylation and has relevance to detrimental effects of inflammation.
Keywords: phospholamban, prostaglandin, contractility
Prostaglandin E2 (PGE2) elicits biological effects through four distinct receptor sub-types termed EP1, EP2, EP3 and EP4 that couple to different second messenger systems. Whereas activation of EP2 and EP4 increases cAMP, activation of EP1 increases intracellular calcium and activation of EP3 decreases cAMP. Thus, the effect of PGE2 is dependent on the profile of EP receptors expressed in different tissues and cell types.
Our laboratory recently reported that male mice lacking the EP4 receptor sub-type only in cardiac myocytes (EP4 KO) have reduced cardiac function with age and develop a phenotype of dilated cardiomyopathy 1. However, these in vivo studies could not discern whether the EP4 KO mice have intrinsic defects in myocyte contractility or whether decreased cardiac function results from whole system abnormalities in the sympathetic nervous system that regulates both the speed and force of contraction or whether these mice have conduction defects. Moreover, although we reported that EP3 mRNA is not increased in the heart of EP4 KO mice to compensate for lack of EP4 2; we now suggest that the absence of EP4 allows the effects of EP3 stimulation to proceed unopposed. There are very few studies that have reported on the influence of PGE2 on isolated myocyte contractility and the EP receptor(s) involved. Wang et al 3 showed that cardiomyocyte specific deletion of cyclooxygenase-2 increased ventricular tachycardia in response to electrical stimulation of the heart and infusion of PGE2 was reported to depress premature ventricular beats in humans, consistent with its ability to reduce ischemia-induced arrhythmias in animal models. There is only one published paper describing the effect of PGE2 on isolated rat myocytes in vitro and it found that PGE2 increased contractility without effects on intracellular calcium 4. However, these few reports do not allude to potential mechanisms by which PGE2 increases the rate or force of contractions, including which PGE2 receptor sub-type is involved.
Phospholamban (PLN) is a crucial component of excitation-contraction coupling. The phosphorylation of PLN physically separates it from SERCA2a and therefore removes its inhibitory effect; making for increased SERCA activity and triggering the release of large amounts of Ca2+ from the sarcoplasmic reticulum to increase contractility. We therefore tested the ability of PGE2 and various EP agonists to alter contraction and examined the hypothesis that PGE2 acting through its EP4 receptor can increase contraction via increased PLN phosphorylation whereas PGE2 acting through its EP3 receptor has the opposite effect. This hypothesis was tested in isolated mouse ventricular myocytes, in the ex vivo mouse working heart Langendorff preparation and acutely in vivo using pressure volume loops.
METHODS
Animal Use
The wild type and EP4 KO mice used in this study were bred and genotyped at Henry Ford Hospital and have been previously described 1. C57Bl/6 mice used for the myocardial infarction (MI) studies and contractility studies were from Jackson labs. The mouse model of MI using permanent ligation of the left anterior descending coronary artery was previously described by us 2 as was the isolation of adult mouse ventricular cardiomyocytes (AVM) 5. All studies involving the use of animals were approved by the institutional review committee at Henry Ford Hospital, in accordance with federal guidelines.
Chemicals
The EP1/EP3 agonist, sulprostone; EP4 agonist, CAY 10598; and the EP3 antagonist, L798-106 were obtained from Cayman Chemical (Ann Arbor, MI). All drugs were dissolved in 100% ethanol. All other chemicals were obtained from Sigma Aldrich.
Mouse Langendorff studies
Mice were anesthetized with isoflurane and injected with 250 U heparin. Hearts were rapidly excised and placed in ice-cold Krebs solution (mmol/L: NaCl 118.5, KCl 4.7, MgSO4 2.46, KH2PO4 1.21, glucose 12, CaCl2 1.7, Na pyruvate 2, NaHCO3 25, pH 7.4, bubbled in 95% O2/5% CO2). The aortas were cannulated and retrogradely perfused constantly at 3.5 ml/min at 37°C. Hearts were allowed to beat spontaneously. Coronary perfusion pressure (CPP) was constantly measured using an in-line pressure transducer, MLT0380/D, ADInstruments, Australia). After 10 min equilibration, chemicals or vehicle were added to the buffer and perfusion was continued for 30 min. Left ventricular end diastolic pressure (LVEDP), left ventricular end systolic pressure (LVESP) and heart rate were monitored and recorded continuously using PowerLab system (ADInstruments). Left ventricular developed pressure (LVDP) was calculated by subtracting LVEDP from LVESP. Hearts were excluded from further study if they exhibited one or more of the following exclusion criteria: LVEDP higher than 20 mmHg; LVDP less than 60 mmHg, heart rate less than 300 bpm or arrhythmias.
Isolation of adult cardiomyocytes for contractility studies
Isolation of cardiomyocytes from 16–21 week-old C57Bl/6 male mice was performed using modifications of the method described by O’Connell et al 6 and has previously been described by us 1. 10 mmol/l 2,3-butanedione monoxime was omitted as it is a known inhibitor of contractility. Freshly isolated AVM prepared in Tyrode’s solution were loaded with1 μmol/L Fura-2 AM (Molecular Probes, Eugene, OR) for 5 min at room temperature, washed and rested for 15 minutes. After cells were loaded and rested, cardiomyocytes were divided into aliquots and treated with either vehicle or PGE2 (10−6 M). In another set of experiments, cells were divided and treated with either vehicle or 10−6 M sulprostone for 10 minutes and washed. An aliquot of cells was added to the chamber and cells were allowed to attach for 2 min, then superfused with Tyrode’s solution at 37°C and electrically stimulated at 3 Hz using a biphasic pulse. Contraction amplitude and intracellular calcium transients were recorded online using a dual excitation spectrofluorometer and video edge detection system (IonOptix) and a minimum of 50 transients were analyzed for each cell. As indicators of contractility; peak shortening, and the speed of contraction and relaxation were measured. To ensure that the response to the various agonists was not due to reduced cell viability, in some experiments the cells treated with test agents were assessed first and then the cells treated with vehicle were assessed.
Cell culture and Western Blot
Phospholamban (PLN) and phosphorylated phospholamban protein expression was measured by Western blot in AVM treated with PGE2, CAY10598 and sulprostone for various times. For the cell culture studies to investigate the effect of PGE2 and EP agonists on PLN and phospho-PLN expression, we used primary cultures of AVM from 18–20 week old C57Bl/6 male mice. Cells were plated and after 1 hr the media was changed to serum-minus media. After an additional hour, the cells were treated and cell lysates were harvested at appropriate times.
Western blot analysis was performed under reducing conditions using 20 μg of total protein. After electrophoresis, proteins were transferred overnight to a PVDF membrane. Membranes were blocked for 1 hr in 5% milk (w/v in TBS-Tween) and incubated overnight (4°C) with a 1:1000 dilution of PLN or a phospho-PLN antibody that recognizes phosphorylation at Ser16/Thr17(Cell Signaling, Danvers, MA). After washing with TBS-tween, membranes were incubated with a HRP-conjugated donkey anti-rabbit secondary antibody for 1 hr at room temperature at a dilution of 1:2000. After further washing they were developed using a Super Signal West Pico chemiluminescent substrate (Pierce, Rockford, IL).
Pressure-Volume Loops
Pressure volume loops were performed in male 18–20 week old C57Bl/6 mice using a closed chest method with Nembutal (sodium pentobarbital) anesthesia. Briefly, mice were surgically ventilated and the PV catheter (Transonic Scisense Inc. London, Ontario, Canada) was inserted into the left ventricle via the right carotid artery using the surgical procedure described by Pacher et al 7. The left jugular vein was also cannulated for drug administration. After stabilization of the signal for 10 min, baseline PV loops were obtained at steady state. PGE2, EP4 agonist or sulprostone was then infused at a rate of 30 μg/min/kg body weight and data was collected for over a period of 30 min. In other experiments, a vehicle (0.1%) ethanol was infused to ensure that the vehicle had no effect over the experimental period. All data was collected and analyzed using iWOX 408 Labscribe v3 software.
Statistical Analysis
All statistics were performed by a statistician in the Department of Public Health Sciences of Henry Ford Hospital using the statistical package SAS Version 9.4. For the contractility data, statistics are reported as means +/− standard error of the mean (SEM) with ‘n’ representing the number of cells. For all other data, ‘n’ represents the number of experiments or the number of mice used. Groups were compared with Student’s t-test except where normality was not present and a two-sample Wilcoxon test was used. For the pressure volume loop data, a paired t-test was used to compare parameters before and after administration of experimental drugs. A p-value <0.05 was considered as evidence of a statistically significant difference for experimental data with the p values being two-sided.
RESULTS
Effect of PGE2 and Sulprostone on AVM Contractility
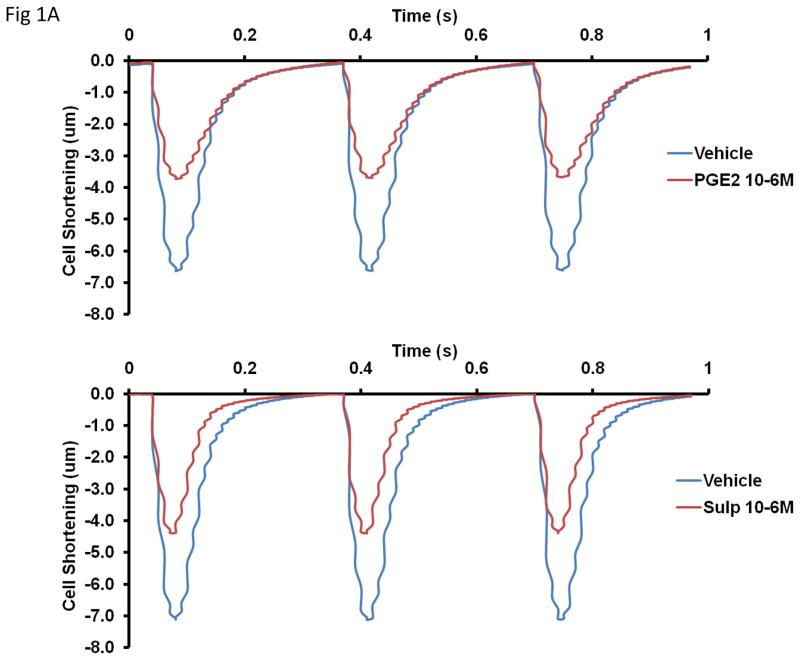
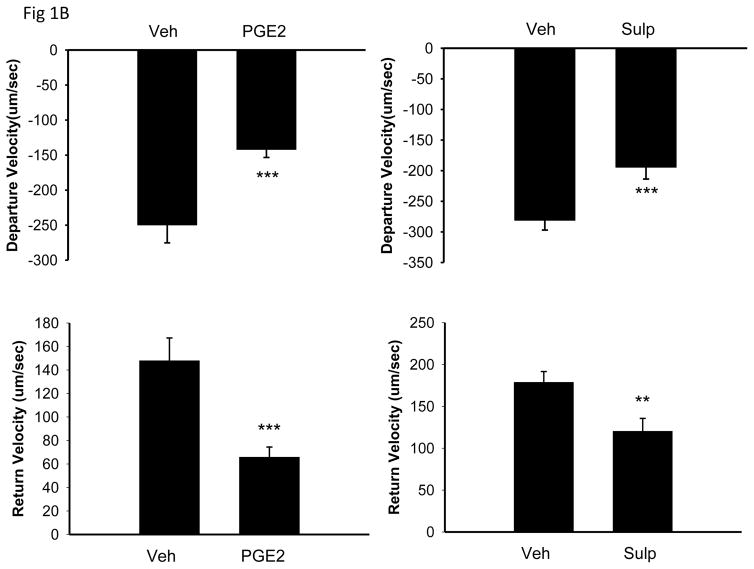
The top panel of Figure 1A shows mean data of transients from cells treated with vehicle and cells treated with PGE2. The bottom panel of the same figure shows mean transients from cells treated with vehicle and cells treated with sulprostone. Treatment with PGE2 (10−6M) for 10 min reduced contractility as measured by peak height (6.84 ± 0.7 for vehicle vs 3.85 ± 0.3 for PGE2, p < 0.001), departure velocity (−250.0 ± 25.2 um/sec for vehicle vs −142.2 ± 11.2 um/sec for PGE2, p < 0.001) and return velocity (147.9 ± 19.3 um/sec for vehicle vs 65.8 ± 8.6 um/sec for PGE2, p < 0.001).
Figure 1.
Effect of 10 min treatment with PGE2 (10−6M) or sulprostone 10−6M on isolated myocyte contractility. Panel A shows traces of mean cell shortening data when cells were treated with either vehicle or PGE2 (top panel) or vehicle or sulprostone (bottom panel). Cells were paced at 3 Hz. Panel B is mean quantitative data of contraction measured as the speed of contraction (departure velocity) and the speed of relaxation (return velocity). n = 49–73 cells from 5–8 mice. Statistical significance: **p<0.01 and ***p<0.001 versus vehicle control.
Under basal conditions, treatment of AVM for 10 min with 10−6M sulprostone also reduced contractility as measured by peak height (7.41 ± 0.4 for vehicle vs 4.44 ± 0.43 for sulprostone, p < 0.001), departure velocity (−281.3 ± 15.7 um/sec for vehicle vs −194.6± 19.0 um/sec for sulprostone, p < 0.001) and return velocity (178.8 ± 12.8 um/sec for vehicle vs 120.4 ± 15.3 um/sec for sulprostone, p = 0.008). The mean data for the effects of PGE2 and sulprostone are presented in Figure 1B.
With regard to changes in intracellular calcium, treatment with 10−6M PGE2 increased sin exp tau (the exponential decay time constant for calcium) from a value of 0.082 ± 0.003 in vehicle-treated cells to 0.094 ± 0.004 in PGE2-treated cells, p =0.022. Treatment with sulprostone did not significantly increase this parameter. These results indicate a slower return to baseline calcium levels in cells treated with PGE2.
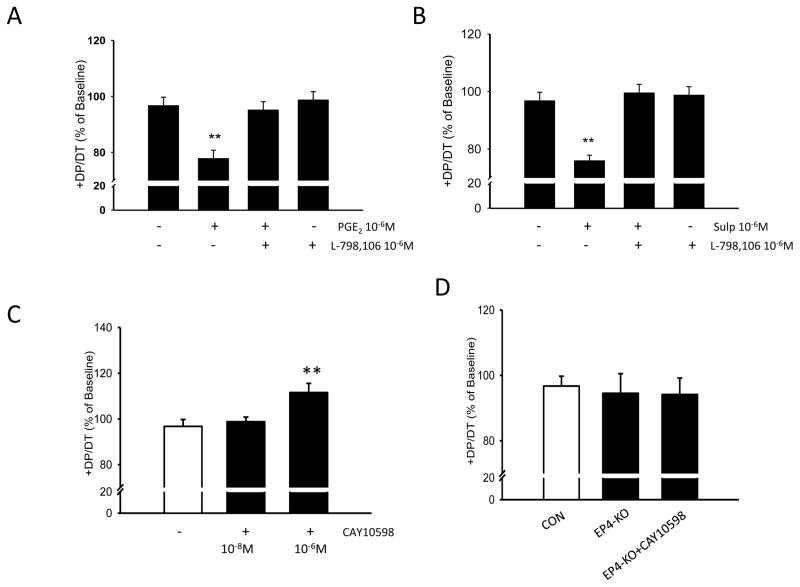
Effect of PGE2, Sulprostone and EP4 agonist (CAY10598) on contractility of the isolated heart (Figure 2)
Figure 2.
Cardiac function was determined using the Langendorff preparation. L798,106 is an EP3 inhibitor. A–B: PGE2 and sulprostone reduced percent LV +DP/DT via EP3, C: EP4 agonist (CAY10598) increased percent LV +dP/dt, D: EP4 agonist (CAY10598) had no effect on cardiac function in EP4-KO hearts. n=4–6/group. Statistical significance: *p<0.05,**p<0.01,***p<0.001
Isolated hearts of 18–20 wk old male C57Bl/6 mice were mounted on the Langendorff apparatus, equilibrated for 10 min and then perfused with PGE2 (10−6mol/l) or sulprostone (10−6mol/l) for 30 min. Values at the end of equilibration were set to 100%. Compared to vehicle, PGE2 decreased +dp/dt (77.8±3% for PGE2 vs 96.7±3% for vehicle, p=0.004) and left ventricular developed pressure, LVDP (77.2±2% vs 96.8±3%, p<0.001). Sulprostone decreased +dp/dt (75.9±2% vs 96.7±3%, p<0.001), −dp/dt (72.2±1% vs 85.7±1%, p = 0.01) and LVDP (70.9±1% vs 96.8±3%, p<0.001). The effects of both PGE2 and sulprostone were reversed by the EP3 antagonist, L789,106 (10−6mol/l). In contrast to the effect of sulprostone and PGE2, perfusion of the EP4 agonist into isolated working hearts increased their contractility. Compared to vehicle, the EP4 agonist, CAY10598 (10−6M) increased +dp/dt (117 ± 9 % vs 97 ± 7 %, p = 0.006), −dp/dt (105 ± 8 % vs 86 ± 3 %, p = 0.006) and left ventricular developed pressure, LVDP (112 ± 7% vs 97 ± 8%, p = 0.007, n = 5 mice). To confirm specificity of the EP4 agonist, we then performed experiments to determine the effect of the EP4 agonist in working hearts from EP4 KO mice in which the EP4 receptor is deleted only in cardiac myocytes. As anticipated, the EP4 agonist had no effect on contractility of hearts obtained from these knockout animals.
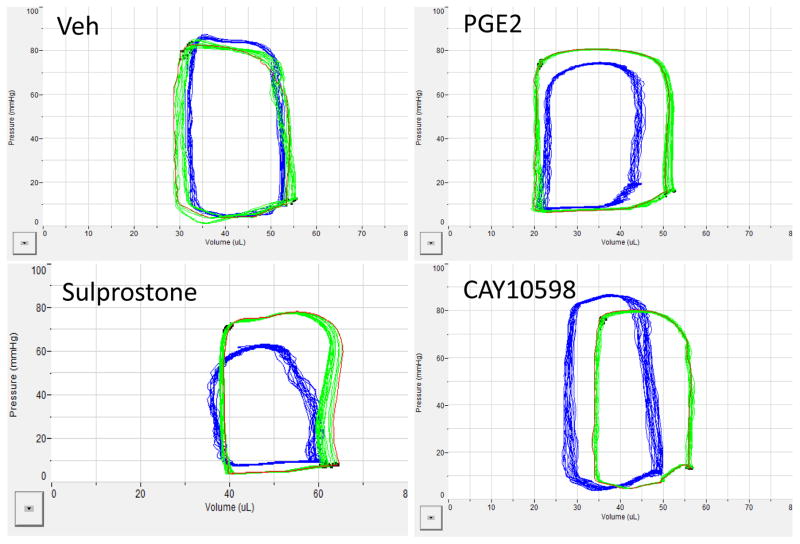
Effect of PGE2, Sulprostone and EP4 agonist in Vivo (Figure 3)
Figure 3.
Representative left ventricular pressure-volume loops obtained from closed chest preparations in C57Bl/6 mice. Green loop is baseline and blue loops are loops obtained after 30 min treatment with compounds (30ug/kg/min).
The acute in vivo effects of the above compounds were determined using PV loops in a closed chest approach. Similar to the results observed in the isolated adult myocytes and langendorff preparation, both PGE2 and sulprostone decreased contractility when compared to baseline measurements. In contrast, acute treatment with the EP4 agonist significantly improved contractility as measured by increased +dp/dt and heart rate. These results are presented in the Table.
Table.
Effect of PGE2, Sulprostone (EP1/EP3 agonist) and CAY10598 (EP4 agonist) on Pressure-Volume Relationship in C57BL/6 Mice
| Control
|
PGE2
|
Sulprostone
|
CAY10598
|
|||||
|---|---|---|---|---|---|---|---|---|
| Base | 30 min | Base | 30min | Base | 30min | Base | 30min | |
| HR (bpm) | 326.7±14.2 | 313.7±8.9 | 419.8±39.1 | 391.2±39.0* | 393±37.1 | 353±35.1 | 356.6±17.4 | 383.67±25.0* |
| SBPend (mmHg) | 85±2.5 | 85.3±1.9 | 87.2±11.0 | 80±6.5 | 86.4±4.4 | 79.4±4.6 | 79.16±2.9 | 85.5±3.0* |
| DBPend (mmHg) | 5.8±0.8 | 5.7±1.0 | 2.8±1.4 | 3.4±1.3 | 5.4±0.4 | 6.4±1.0 | 4±1.2 | 4.5±0.8 |
| ESV (μl) | 36.5±3.3 | 39±4.3 | 17±2.2 | 20.4±2.6 | 30.6±2.8 | 32.4±2.8* | 29.17±4.3 | 26.83±3.8 |
| EDV (μl) | 61.3±6.5 | 62.5±6.8 | 36±4.1 | 30.6±5.1 | 52.2±4.0 | 54.8±3.8 | 51.07±4.6 | 52.83±5.9 |
| SV (μl) | 23.3±2.2 | 21.5±2.8 | 17±3.6 | 10.8±2.7** | 22.6±1.8 | 24±3.5 | 21.5±1.3 | 26±3.5 |
| CO (μl/min) | 8065±1239 | 7823±1806 | 7588±971 | 4060 ±692* | 7823±1506 | 6840±1471 | 7958 ±563 | 10481±1992 |
| EF (%) | 39±2.2 | 36.2±1.3 | 52.8±5.5 | 31.8±5.0** | 41.4±3.2 | 40.04±3.7 | 43.16±4.4 | 49.17±4.5 |
| +DP/DT (mmHg/sec) | 5109±316 | 5095±291 | 5385±769 | 4634±576* | 5524±615 | 4271±326* | 4352±235 | 5550±328*** |
| −DP/DT (mmHg/sec) | 3721±279 | 3654±149 | 5063±732 | 4375±642* | 4305±515 | 3283±347* | 3705±140 | 4388±196*** |
Data represents mean ± SEM of 4–6 male 18–20 week old C57BL/6 mice. Pressure volume loop parameters obtained in anesthetized mice using a closed chest approach. Data was obtained at baseline and after 30 minute infusion with compounds. Abbreviations: HR, heart rate; SBPend, end systolic pressure; DBPend, end diastolic pressure; ESV, end systolic volume; EDV, end diastolic volume; SV, stroke volume; CO, cardiac output; EF, ejection fraction; + DP/DT and − DP/DT, maximum and minimum change in pressure with time respectively. Statistical significance: * p < 0.05, ** p < 0.01 and *** p < 0.005 by paired t-test versus baseline values.
Effect of PGE2, Sulprostone and EP4 Agonist (CAY 10598) on Phosphorylation of Phospholamban and SERCA2a Expression (Figure 4)
Figure 4.
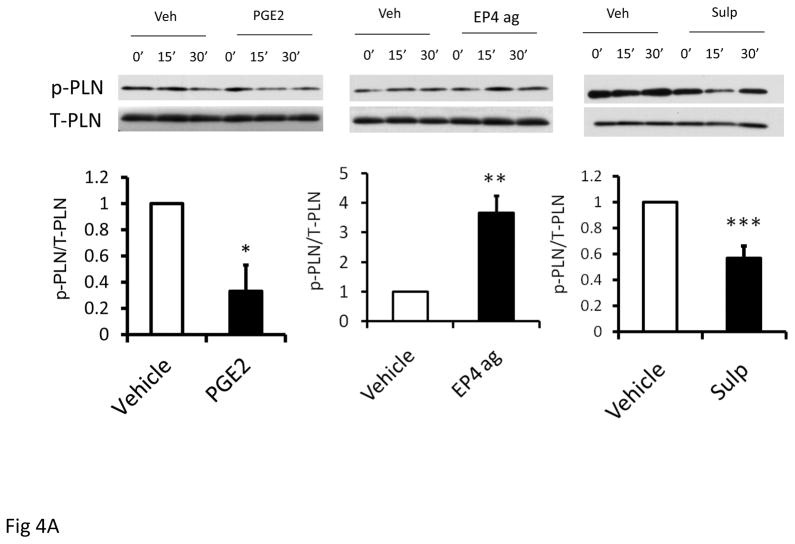
Figure 4A. Effect of PGE2(10−6M), sulprostone(10−6M) and EP4 agonist, CAY 10598 (10−6M) on p-PLN expression in isolated myocytes obtained from C57Bl/6 mice. Upper panels show representative western blots of phosphorylated and total PLN (p-PLN, T-PLN) and bottom panel is bar graph showing mean data after 15 min treatment. Statistical significance: *p<0.05, **p<0.01,***p<0.005. n=3 per group.
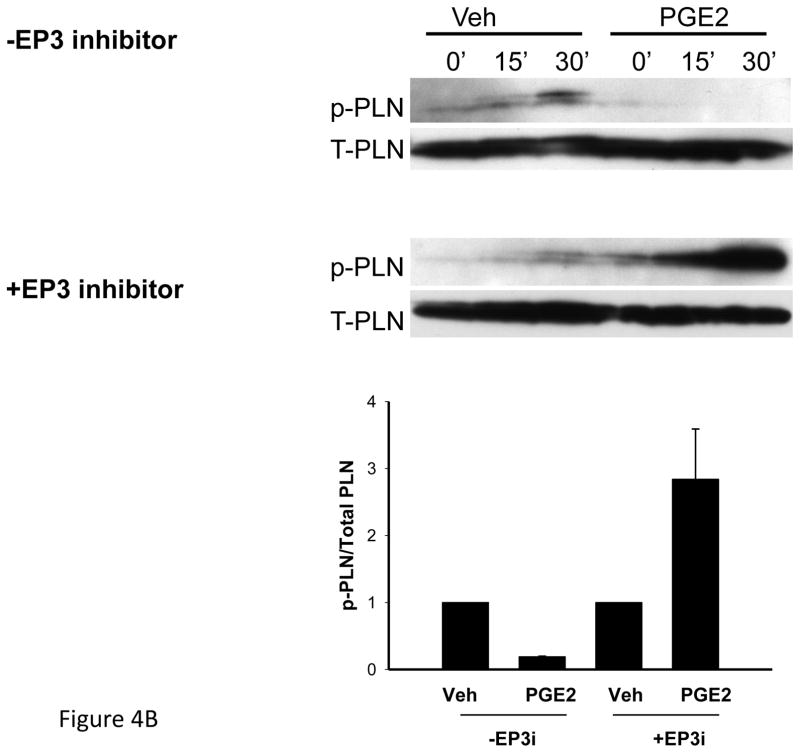
Figure 4B. Effect of PGE2 (10−6M) on p-PLN expression in isolated myocytes obtained from 13–15 week old EP4 KO mice. Experiments were performed in the absence or presence of the EP3 inhibitor (EP3i), L798, 106 (10−6M). Upper panels show representative western blots of phosphorylated and total PLN (p-PLN, T-PLN) and bottom panel is bar graph showing mean data after 15 min treatment.
Treatment of AVM for 15 min with either PGE2 or sulprostone decreased expression of phosphorylated PLN corrected to total PLN, by 67% and 43%. SERCA2a expression was unaffected (data not shown); which was anticipated in this short experimental time frame. In contrast, treatment with the EP4 agonist increased phosphorylated PLN corrected to total PLN by 3.7 ± 0.6-fold, p = 0.005, n = 3 separate preparations. To further explore the role for the EP receptors in PLN phosphorylation, we examined AVM from 13–15 week old male EP4 KO mice. In these mice, treatment with PGE2 for 15 min reduced p-PLN/total PLN by an average of 81%; a value that appeared greater than the 67% reduction observed in C57Bl/6 mice. Pre-treatment with the EP3 antagonist in AVM from EP4 KO mice prevented the ability of PGE2 to reduce p-PLN/total PLN (Figure 4B).
EP3 and EP4 are Increased in the MI Heart
To determine whether expression of EP3 and EP4 is altered in the failing heart, we performed real time RT-PCR on left ventricle samples from hearts obtained from C57Bl/6 mice that were subjected to permanent ligation of the LAD for 2 weeks. All samples were obtained from the border-remote zone and not the infarcted section. Our data shows that EP3 is increased 3.37 ± 0.8 fold in the MI heart compared to sham-operated controls (p = 0.016) and EP4 mRNA is also increased in those same hearts although to a lesser extent (2.12 ± 0.35 fold, p = 0.007).
DISCUSSION
This study provides direct evidence that exogenous PGE2 reduces contractility of the in vivo heart, the isolated working heart and single adult ventricular myocytes via its EP3 receptor subtype while stimulation of the EP4 receptor has opposite results. These effects may be mediated by alterations in the phosphorylation state of phospholamban, a protein that negatively regulates SERCA activity.
There have been a number of studies reporting the effect of PGE2 on contractility but the results have been conflicting and do not elucidate the contribution of the various receptor subtypes. Using the PGE2-perfused mouse isolated heart, Liu et al reported that PGE2 attenuates the adrenergic-induced cardiac contractile response in animal hearts 8 whereas studies by Pecha et al 9 did not support a role for PGE2 in regulating catecholamine–induced inotropy. Whether these differences relate to the different preparations used (isolated heart versus atrial and ventricular trabeculae) is not known. Moreover, Klein and Wold 4 used a system similar to the one used in our studies to observe that PGE2 augmented peak shortening in adult rat cardiomyocytes independent of changes in calcium whereas concentrations greater than 10−5M reduced intracellular calcium. Church et al 10 also reported that PGE2 (10−5M) increases the contraction frequency of neonatal rat cardiomyocytes but only spontaneous beating was measured. Although our recent data conflict with those described above, our results across a spectrum of preparations ranging from isolated cardiomyocytes through the Langendorff preparation and in vivo using pressure volume loops are consistent and suggest that the main effect of PGE2 via EP3 is to reduce contractility whereas it augments contractility via EP4. Our data are thus consistent with the reduced contractility noted in our previous studies using the cardiomyocyte-specific EP4 KO mouse.
Clinical reports have also implicated the EP1/EP3 receptor agonist, sulprostone, in heart failure or cardiomyopathy. Vital and colleagues 11 reported a case of a peripartum heart failure while another case showed that sulprostone caused coronary spasm, bradycardia, and subsequent asystole 12. In EP3 over-expressing mice, LV ejection fraction was severely decreased in transgenic hearts while the relative LV mass was significantly increased 13. Using isolated rat atria, Wolkowicz et al 14 also reported that an EP1 agonist increased contractile force that was sensitive to Rho kinase inhibitors. In a Langendorff preparation, both endogenous PGE2 and an exogenous EP4 agonist were shown to protect the heart from I/R injury via EP4 15. These latter results are consistent with our findings that the EP4 agonist improves cardiac function in the same working heart preparation.
It is well established that PGE2 signals through four receptors (EP1, EP2, EP3 and EP4) that signal via different mechanisms. In the mouse left ventricle, expression of EP3 and EP4 mRNAs are higher than EP1 and EP2. Indeed Xiao et al 15 were unable to detect EP1 in the mouse heart using quantitative PCR whereas other literature supports its presence 16, 17. The results in our present study provide direct evidence that exogenous PGE2 can reduce contractility of both the isolated heart and isolated myocytes acutely via its EP3 receptor. We were rather surprised to note that the effect of PGE2 mirrored that of the EP1/EP3 selective agonist sulprostone, despite the fact that both the isolated heart and the isolated cardiac myocyte express EP4 receptors abundantly as measured by real time RT-PCR. Although the reason for this finding is not clear, one could speculate that either EP4 but not EP3 is rapidly internalized after agonist stimulation 18–20 or that the receptors are compartmentalized differently 21. However, we have data (not shown) indicating that within a 1 hr time frame, AVM are able to increase cAMP production in response to repeated doses of either PGE2 or the EP4 agonist; suggesting that the former may not be correct in cardiac myocytes. Further experiments are needed to examine these possibilities.
Since both cyclooxygenase-2 and microsomal PGE synthase-1 are upregulated in the infarcted heart to increase production of PGE2, we were interested to examine whether the expression of EP3 and/or EP4 were also affected. Although our experiments were limited to studies of mRNA levels due to difficulties with the commercially available EP3 and-4 antibodies, our data show that both EP3 and EP4 mRNAs are up-regulated after MI although this is seemingly greater for EP3. We thus speculate that the altered balance between EP3 and EP4 in heart failure contributes to the diminished contractility observed in pathologic conditions characterized by increased PGE2.
Almost a decade ago, Schutte and co-workers 22 reported that the administration of PGE2 to healthy sheep improved cardiac contractility and relaxation while decreasing heart rate. In contrast, however, similar administration to sheep in congestive heart failure reduced cardiac function with increased pre-load. These results lead the authors to speculate that PGE2 might not be a suitable agent for treatment of congestive heart failure because of the worsening effect it had on the cardiodynamics of the failing heart. Our results suggest that administration of a selective EP4 agonist might be a more promising option. Indeed, the first clinical report was very recently published showing that an EP4 agonist had a lucistropic and vasodilator effect in healthy volunteers 23. This was followed with a concurrent publication showing that acute infusion of an EP4 agonist to normal anesthetized dogs increased ejection fraction and +dp/dt but decreased end systolic pressure 24. Our results add to evidence for a protective effect of EP4 but provide additional mechanistic insight.
Ca2+ is known to be very important in myocyte contraction and relaxation. Selective stimulation of EP2 or EP4 receptors attenuates histamine-evoked Ca(2+) signals 9 potentially demonstrating that PGE2 via these receptors can regulate heart contractility by alterations in intracellular calcium. Our data with isolated myocytes shows that treatment with PGE2 slows calcium reuptake. Surprisingly, we could not detect such an effect using sulprostone but whether this relates to our inability to detect smaller changes with this drug is unknown. Phospholamban (PLN) is a phosphoprotein in cardiac sarcoplasmic reticulum (SR) that is a reversible regulator of the Ca(2) (+)-ATPase (SERCA2a) activity and cardiac contractility. Dephosphorylated PLN inhibits SERCA2a and PLN phosphorylation, at either Ser16 by PKA or Thr17 by Ca(2) (+)-calmodulin-dependent protein kinase (CaMKII), reverses this inhibition. Through this mechanism, PLN is a key modulator of SR Ca(2) (+) uptake, Ca(2) (+) load, contractility, and relaxation 2. Previously, our gene array data on left ventricles from EP4 KO mice showed reduced PLN in KO mice with the reduction correlating with ejection fraction (Data submitted to the GEO database-NCBI). However, this data was obtained from older mice in various stages of heart failure and could not discern phosphorylation status. In support of our data, Liu S et al 8 reported that PGE2 inhibits adrenergic-induced phosphorylation of PLN and the contractile response in animal hearts. However, they did not observe any effect under basal conditions and they implicated the EP4 receptor whereas our results showing that PGE2 reduces phosphorylated PLN in isolated myocytes suggests that this is an EP3-mediated event, consistent with the Langendorff data.
In conclusion, our data clearly shows that PGE2 has an acute and direct effect on cardiac contractility; a positive inotropic effect mediated by its EP4 receptor and a negative inotropic effect mediated by its EP3 receptor. These effects were consistent in experiments ranging from isolated myocyte contractility studies through those in the intact animal. Our results have importance in situations where PGE2 is elevated such as various inflammatory conditions and thus suggest a new deleterious relationship between inflammation and cardiac function that is mediated via the PGE2 EP3 receptor sub-type.
Clinical Perspective.
Our laboratory previously reported that cardiomyocyte-specific EP4 KO mice develop dilated cardiomyopathy with reduced ejection fraction. We thus hypothesized that PGE2 increases contractility via EP4 but decreases contractility via EP3. Our present study examined the influence of prostaglandin E2 (PGE2) and its receptor subtypes (EP1-4) on cardiac contractility using isolated mouse cardiomyocytes, the Langendorff heart preparation and in vivo using pressure volume loops in the anesthetized mouse. Our studies clearly show that PGE2 has opposing effects on contractility dependent upon which receptor sub-type is activated. In general, contractility was reduced via the EP3 receptor but increased via EP4. In the working heart, both PGE2 and the EP1/EP3 agonist sulprostone decreased left ventricular developed pressure via EP3 whereas the EP4 agonist had the opposite effect. Single myocyte contractility was also reduced after treatment with either PGE2 or sulprostone. The negative inotropic effects of PGE2 and the EP3 agonist appeared to be mediated by decreased phosphorylation of phospholamban without effects on SERCA2a expression. The in vitro hemodynamic effects of PGE2 and sulprostone were mimicked acutely in vivo using pressure volume loops. Both PGE2 and sulprostone decreased +dp/dt while the EP4 agonist increased +dp/dt. Our results may have potential clinical significance as we also observed increased EP3 expression in mice subject to myocardial infarction. If these results translate to the patient population, they suggest that blockade of the EP3 receptor could ameliorate worsening cardiac function observed in inflammatory conditions characterized by increased PGE2. Furthermore they suggest a potentially protective role for EP4 agonists.
Acknowledgments
The authors would like to thank David Taube for excellent technical assistance. Thanks also to Edward Shesely for the genotyping and maintenance of the EP4 KO mouse colony.
Sources of Funding
These studies were funded by a National Institutes of Health grant [5P01HL028982] (subproject 2) to PH.
Footnotes
Disclosures
None.
Reference List
- 1.Harding P, Yang XP, Yang J, Shesely E, He Q, LaPointe MC. Gene expression profiling of dilated cardiomyopathy in older male ep4 knockout mice. Am J Physiol Heart Circ Physiol. 2010;298:H623–H632. doi: 10.1152/ajpheart.00746.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian JY, Harding P, Liu Y, Shesely E, Yang XP, LaPointe MC. Reduced cardiac remodeling and function in cardiac-specific ep4 receptor knockout mice with myocardial infarction. Hypertension. 2008;51:560–566. doi: 10.1161/HYPERTENSIONAHA.107.102590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Patel VV, Ricciotti E, Zhou R, Levin MD, Gao E, Yu Z, Ferrari VA, Lu MM, Xu J, Zhang H, Hui Y, Cheng Y, Petrenko N, Yu Y, FitzGerald GA. Cardiomyocyte cyclooxygenase-2 influences cardiac rhythm and function. Proc Natl Acad Sci U S A. 2009;106:7548–7552. doi: 10.1073/pnas.0805806106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein AL, Wold LE, Ren J. The cyclooxygenase-2 product prostaglandin e2 modulates cardiac contractile function in adult rat ventricular cardiomyocytes. Pharmacol Res. 2004;49:99–103. doi: 10.1016/j.phrs.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Taube D, Xu J, Yang XP, Undrovinas A, Peterson E, Harding P. Fractalkine depresses cardiomyocyte contractility. PLoS One. 2013;8:e69832. doi: 10.1371/journal.pone.0069832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 7.Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3:1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S, Li Y, Kim S, Fu Q, Parikh D, Sridhar B, Shi Q, Zhang X, Guan Y, Chen X, Xiang YK. Phosphodiesterases coordinate camp propagation induced by two stimulatory g protein-coupled receptors in hearts. Proc Natl Acad Sci U S A. 2012;109:6578–6583. doi: 10.1073/pnas.1117862109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pecha S, Mudersbach E, Sohren KD, Hakmi S, Reichenspurner H, Eschenhagen T, Christ T. Prostaglandin e2 does not attenuate adrenergic-induced cardiac contractile response. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:963–968. doi: 10.1007/s00210-014-1012-2. [DOI] [PubMed] [Google Scholar]
- 10.Church DJ, Rebsamen MC, Morabito D, van DBV, Vallotton MB, Lang U. Role of cell contractions in camp-induced cardiomyocyte atrial natriuretic peptide release. Am J Physiol Heart Circ Physiol. 2000;278:H117–H125. doi: 10.1152/ajpheart.2000.278.1.H117. [DOI] [PubMed] [Google Scholar]
- 11.Vital M, Lebert C, Reignier J, Ducarme G. Peripartum cardiomyopathy vs. Sulprostone-associated heart failure? A case report and analysis of the literature. Arch Gynecol Obstet. 2013;288:1423–1424. doi: 10.1007/s00404-013-2905-x. [DOI] [PubMed] [Google Scholar]
- 12.Lampati L, Colantonio LB, Calderini E. Cardiac arrest during sulprostone administration--a case report. Acta Anaesthesiol Scand. 2013;57:395–397. doi: 10.1111/aas.12022. [DOI] [PubMed] [Google Scholar]
- 13.Meyer-Kirchrath J, Martin M, Schooss C, Jacoby C, Flogel U, Marzoll A, Fischer JW, Schrader J, Schror K, Hohlfeld T. Overexpression of prostaglandin ep3 receptors activates calcineurin and promotes hypertrophy in the murine heart. Cardiovasc Res. 2009;81:310–318. doi: 10.1093/cvr/cvn312. [DOI] [PubMed] [Google Scholar]
- 14.Wolkowicz PE, Ku DD, Grenett HE, Urthaler F. Occupation of the prostaglandin e2-type 1 receptor increases rat atrial contractility via a y-27632-sensitive pathway. Prostaglandins Other Lipid Mediat. 2002;70:91–105. doi: 10.1016/s0090-6980(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 15.Xiao CY, Yuhki K, Hara A, Fujino T, Kuriyama S, Yamada T, Takayama K, Takahata O, Karibe H, Taniguchi T, Narumiya S, Ushikubi F. Prostaglandin e2 protects the heart from ischemia-reperfusion injury via its receptor subtype ep4. Circulation. 2004;109:2462–2468. doi: 10.1161/01.CIR.0000128046.54681.97. [DOI] [PubMed] [Google Scholar]
- 16.Harding P, LaPointe MC. Prostaglandin e2 increases cardiac fibroblast proliferation and increases cyclin d expression via ep1 receptor. Prostaglandins Leukot Essent Fatty Acids. 2011 doi: 10.1016/j.plefa.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyatake S, Manabe-Kawaguchi H, Watanabe K, Hori S, Aikawa N, Fukuda K. Prostaglandin e2 induces hypertrophic changes and suppresses alpha-skeletal actin gene expression in rat cardiomyocytes. J Cardiovasc Pharmacol. 2007;50:548–554. doi: 10.1097/FJC.0b013e318145ae2e. [DOI] [PubMed] [Google Scholar]
- 18.Desai S, April H, Nwaneshiudu C, Ashby B. Comparison of agonist-induced internalization of the human ep2 and ep4 prostaglandin receptors: Role of the carboxyl terminus in ep4 receptor sequestration. Mol Pharmacol. 2000;58:1279–1286. doi: 10.1124/mol.58.6.1279. [DOI] [PubMed] [Google Scholar]
- 19.Desai S, Ashby B. Agonist-induced internalization and mitogen-activated protein kinase activation of the human prostaglandin ep4 receptor. FEBS Lett. 2001;501:156–160. doi: 10.1016/s0014-5793(01)02640-0. [DOI] [PubMed] [Google Scholar]
- 20.Negishi M, Sugimoto Y, Irie A, Narumiya S, Ichikawa A. Two isoforms of prostaglandin e receptor ep3 subtype. Different cooh-terminal domains determine sensitivity to agonist-induced desensitization. J Biol Chem. 1993;268:9517–9521. [PubMed] [Google Scholar]
- 21.Bhattacharya M, Peri K, Ribeiro-da-Silva A, Almazan G, Shichi H, Hou X, Varma DR, Chemtob S. Localization of functional prostaglandin e2 receptors ep3 and ep4 in the nuclear envelope. J Biol Chem. 1999;274:15719–15724. doi: 10.1074/jbc.274.22.15719. [DOI] [PubMed] [Google Scholar]
- 22.Schutte PJ, du Plooy WJ, Hay L. Some adverse cardiodynamic effects of prostaglandin e2 in congestive heart failure. Prostaglandins Leukot Essent Fatty Acids. 1996;54:207–210. doi: 10.1016/s0952-3278(96)90018-6. [DOI] [PubMed] [Google Scholar]
- 23.Ward CL, Jamieson V, Nabata T, Sharpe J, Dozono K, Suto F, Hashimoto Y, Gussak I. First clinical experience with ono-4232: A randomized, double-blind, placebo-controlled healthy volunteer study of a novel lusitropic agent for acutely decompensated heart failure. Clinical therapeutics. 2016 doi: 10.1016/j.clinthera.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 24.Honda A, Nakamura Y, Ohara H, Cao X, Nomura H, Katagi J, Wada T, Izumi-Nakaseko H, Ando K, Sugiyama A. Effects of a prostagrandin ep4-receptor agonist ono-ae1-329 on the left ventricular pressure-volume relationship in the halothane-anesthetized dogs. Eur J Pharmacol. 2016;775:130–137. doi: 10.1016/j.ejphar.2016.02.029. [DOI] [PubMed] [Google Scholar]