Abstract
Background
The striated muscle costamere, a multi-protein complex at the boundary between the sarcomere and the sarcolemma, plays an integral role in maintaining striated muscle structure and function. Multiple costamere-associated proteins, such as integrins and integrin-interacting proteins, have been identified and shown to play an increasingly important role in the pathogenesis of human cardiomyopathy. Kindlin-2 is an adaptor protein that binds to the integrin β cytoplasmic tail to promote integrin activation. Genetic deficiency of Kindlin-2 results in embryonic lethality, and knockdown of the Kindlin-2 homolog in Caenorhabditis elegans and Danio rerio suggests it has an essential role in integrin function and normal muscle structure and function. The precise role of Kindlin-2 in the mammalian cardiac myocyte remains to be determined.
Methods and Results
The current studies were designed to investigate the role of Kindlin-2 in the mammalian heart. We generated a series of cardiac myocyte-specific Kindlin-2 knockout mice with excision of the Kindlin-2 gene in either developing or adult cardiac myocytes. We found that mice lacking Kindlin-2 in early developing heart are embryonic lethal. We demonstrate that deletion of Kindlin-2 at late gestation or in adult cardiac myocytes resulted in heart failure and premature death, which was associated with enlargement of the heart, and extensive fibrosis. In addition, integrin β1D protein expression was significantly downregulated in adult heart.
Conclusions
Kindlin-2 is required to maintain integrin β1D protein stability. Postnatal loss of Kindlin-2 from cardiomyocytes leads to progressive heart failure showing the importance of costameric proteins like Kindlin-2 for homeostasis of normal heart function.
Keywords: cardiac dysfunction, Kindlin-2, Integrin β1D
In general, as cells contact with the extracellular matrix (ECM), a multi-protein complex is organized that is commonly known as the focal adhesion (FA)1. In striated muscle, an equivalent structure to the FA is the costamere, a structural and functional component that bridges, anchors, and strengthens the connection of the muscle Z-disc to the sarcolemmal membrane1, 2. In the heart, it is a major site of localization for integrins3, 4. Integrins are heterodimeric transmembrane receptors that link the ECM with the cytoskeleton, and modulate bidirectional signaling across the cell membrane5. Integrins, together with integrin-associated proteins, form FA complexes, and in the heart are key components of the costamere where they perform critical roles in normal cardiac development, structure, and function6–8. Importantly, integrins are mechanotransducers1, 4. Dysfunction of cell-matrix adhesion in both animal models and human diseases is implicated in the pathogenesis of cardiomyopathy and heart failure7, 8. In mice, global deletion of the integrin β1 results in lethality shortly after implantation9, 10, while loss of β1 function in the cardiac myocyte (CM) leads to dilated cardiomyopathy7. Similar to integrin β1, global ablation of integrin-linked kinase (ILK), a critical component of the integrin-associated complex, results in murine lethality around the time of implantation11. In addition, CM-specific ablation of ILK results in disruption of the FA complex, leading to dilated cardiomyopathy and heart failure8. Mutations in ILK have also been found to be associated with human dilated cardiomyopathy12. Taken together, these findings suggest that integrins and their associated partners are critical for normal cardiac muscle function.
Kindlins are a family of adaptor proteins that are essential components of the integrin adhesion complex13–17. The Kindlin family consists of three isoforms (Kindlin-1, -2, and -3), which show tissue-specific expression patterns14, 16, 18. Only Kindlin-2, also known as Mig-2, is reported to be expressed in cardiac tissue3, 16. The structural hallmark of Kindlins is the evolutionary conserved FERM (four point one ezrin radixin moesin) domain, which has been shown to associate with the integrin β cytoplasmic tail, and in some cell types to promote integrin activation14, 19–23. Kindlin-2 is also an interacting partner of ILK24. In the absence of Kindlins, the conformational shift of integrins from the low- to high-affinity state does not occur, providing compelling evidence that Kindlins are essential regulators of integrin function13, 25, 26. Furthermore, global deficiency of Kindlin-2 in mice is peri-implantation lethal, likely because of defective integrin function and failed endodermal and epiblast attachment3, 13. Further, loss of the Caenorhabditis elegans (C. elegans) Kindlin-2 homolog, UNC-112, also results in embryonic lethality caused by integrin dysfunction leading to defects in muscle attachments15, 27. Interestingly, Kindlin-2 knockdown in Danio rerio leads to abnormalities in cardiac structure and function3. Together, these reports suggest an essential requirement for mammalian Kindlin-2 in integrin function and normal myocardium structure and function.
To begin to investigate the precise role of Kindlin-2 in the mammalian heart, we generated CM-specific knockout mice by conditionally deleting Kindlin-2 in developing, late gestational, or adult myocardium. Analysis of these mouse models revealed that loss of Kindlin-2 at late gestation or in adult myocardium leads to heart failure and premature death. Lethality was associated with enlargement of the heart and extensive fibrosis, with significant downregulation of integrin β1D protein expression. These results suggest that Kindlin-2 is essential for adult cardiac function, and is a key mediator of integrin β1D protein stability in the myocardium.
Methods
Animal models
The generation of kindlin-2 floxed (f) mice has been described previously28. To generate CM-specific Kindlin-2 KO mice, floxed mice were crossed to alpha (α)-myosin heavy chain (MHC)-Cre29 mice, beta (β)-MHC-Cre mice30, or α-MHC-MerCreMer transgenic mice31 to create Kindlin-2f/f; α-MHC-Cre KO mice, Kindlin-2f/f; β-MHC-Cre KO (cKO) mice, and Kindlin-2f/f; α-MHC-MerCreMer (IcKO) mice, respectively. All mice were of a mixed 129/SvJ and Black Swiss background. The control (Ctrl or KN2f/f) and KO (cKO or IcKO) mice used in the study were age and as analyzed, gender matched. Genotyping of mice was confirmed by PCR analysis using mouse tail DNA and kindlin-2 primers (forward: 5′-TGTGTTTCAAAGGTACTGGTCA-3′; reverse: 5′-ACAATGGTGCTTTGCCTACA-3′), and Cre primers (forward: 5′-TGTGTTTCAAAGGTACTGGTCA-3′; reverse, 5′-ACAATGGTGCTTTGCCTACA-3′). All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of California, San Diego.
Tamoxifen induction
4-hydroxytamoxifen (4OH-TAM, Sigma) was dissolved in sesame oil (Sigma) at a concentration of 10 mg/mL. Adult (2 month old) Kindlin-2f/f and Kindlin-2f/f; α-MHC-MerCreMer mice were treated with 4OH-TAM by intraperitoneal injection once daily for 5 days at a dose of 30 mg/kg body weight. As a control, sesame oil alone was injected in the same way.
Protein isolation and western blot analysis
Protein isolation and western blot analysis were performed as described in our previous publication32. Briefly, total protein extracts were prepared by suspending ground heart tissue or isolated CMs in RIPA/Triple Detergent Lysis Buffer (50 mM Tris, 10 mM EDTA, 150 mM NaCl, 0.25% Deoxycholic acid, 0.1% SDS, 2% NP-40 substitute, 0.01% Sodium azide). Concentration of the protein samples was determined by Bradford dye-binding protein assay (BioRad). Protein lysates were separated on 4–12% SDS-PAGE gels (Life Technologies) and transferred on to Nitrocellulose membrane (Biorad) at 4°C at a constant voltage of 55V in transfer buffer (25 mM Tris, 190 mM Glycine, 20% Methanol, pH 8.3). After blocking for 1–2 hours in Tris Buffered Saline containing 0.1% Tween 20 and 5% non-fat dry milk, membranes were incubated overnight at 4°C with the indicated primary antibody in blocking buffer. Blots were washed and incubated with horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature. Immunoreactive protein bands were visualized using enhanced chemiluminescence reagent (Thermo), analyzed by densitometry, and expressed as pixel density normalized to GAPDH. Primary antibodies: Kindlin-2 (1:1000, Sigma), Integrin β1D33 (1:5000), ILK (1:2000, Sigma), Talin 134, 35 (1:1000), Talin 234, 35 (1:100), Vinculin (1:2000, Sigma), Pan-Cadherin (1:2000, Sigma), N-Cadherin (1:1000, Sigma), α-Catenin (1:1000, Sigma), β-Catenin (1:1000, Sigma), Plakoglobin (1:1000, Santa Cruz), Connexin 43 (1:5000, Sigma), Desmoglein (1:1000, Fitzgerald), GAPDH (1:4000, Santa Cruz). Secondary antibodies: Anti-Mouse (1:4000, Dako), Anti-Rabbit (1:4000, Dako), Anti-Goat (1:4000, Dako).
Histology
Following isolation, mouse hearts were quickly rinsed with phosphate buffered saline (PBS), and fixed in formalin for 24 hours. Hearts were subsequently dehydrated in 70% ethanol, embedded in paraffin, and coronally sectioned (10 µm). Sections were stained with Masson’s Trichrome or Hematoxylin and Eosin as previously described36–38, mounted and imaged using a NanoZoomer 2.0HT Slide Scanning System (Hamamatsu).
Echocardiography
Echocardiography was performed as previously described32. Briefly, mice were anesthetized with 1% isoflurane via a nose cone mask and underwent echocardiography using VisualSonics, SonoSite FUJIFILM, Vevo 2100 ultrasound system with a linear transducer 32–55 MHz. Percentage fractional shortening (%FS) was used as an indicator of systolic cardiac function. Measurements of end-diastolic left ventricular internal diameter (LVIDd), end-systolic left ventricular internal diameter (LVIDs), interventricular septal thickness (IVSd), and LV posterior wall thickness (LVPWd) were determined from the LV M-mode tracing.
Quantitative RT-PCR
Total RNA was extracted from whole heart tissue using Trizol reagent (Life Technologies) according to the manufacturer’s instructions. cDNA was synthesized using MMLV Reverse Transcriptase (Bio-Rad). Primer sequences for qRT-PCR are listed in Supplementary Table 1. RT-PCR reactions were performed using Sso-Fast EvaGreen Real Time PCR (Bio-Rad) master mix in 96-well low profile PCR plates in the CFX96 Biorad Thermocycler.
Immunocytochemistry
Isolated CMs were fixed in acetone at −20°C for 5 minutes. After permeabilization with wash buffer (PBS with 0.2% TX-100), CMs were blocked in blocking solution (PBS with 5% normal donkey serum, 1% BSA, 0.2% TX-100) for 1 hour at room temperature, then incubated overnight with the indicated primary antibody in blocking buffer in a humidified chamber at 4°C. Cells were rinsed in wash buffer and incubated at room temperature with fluorescently-conjugated secondary antibodies and DAPI, diluted in blocking buffer for 1 hour. Cells were rinsed again in wash buffer and mounted in mounting buffer (Dako). Confocal microscopy was performed using an Olympus FluoView™ FV1000 confocal microscope. Primary antibodies: Kindlin-2 (1:150, Sigma), α-Actinin (1:200, Sigma), Vinculin (1:200, Sigma).
Statistical analysis
Numerical data are expressed as mean ± standard deviation (SD) or standard error of the mean (SEM). Statistical analysis was performed using Prism 6.0 software (GraphPad Software, La Jolla, CA). Differences between groups were analyzed by two-tailed Student’s t-test or two-way analysis of variance (ANOVA), and P-values <0.05 were considered significant. Where appropriate, post-hoc analysis utilizing the Bonferroni or Tukey correction method was used to define statistical significance when analyzing multiple time points and groups.
Results
Loss of Kindlin-2 in the heart leads to cardiomyopathy and premature death
Global genetic deficiency of Kindlin-2 results in embryonic lethality3, 13. Although ubiquitously expressed18, Kindlin-2 is most abundantly expressed in cardiac and skeletal muscle3, 16. To investigate the potential role of Kindlin-2 in the mammalian heart, we generated CM-specific knockout (KO) mice by conditionally deleting Kindlin-2 in developing CMs at around embryonic day (E) 8.0, by crossing Kindlin-2f/f mice28 with the α-MHC-Cre mouse line29. No viable Kindlin-2f/f-Cre positive mice were obtained at birth, indicating that KO of Kindlin-2 in early embryogenesis as a result of α-MHC-driven Cre expression, results in embryonic lethality (data not shown).
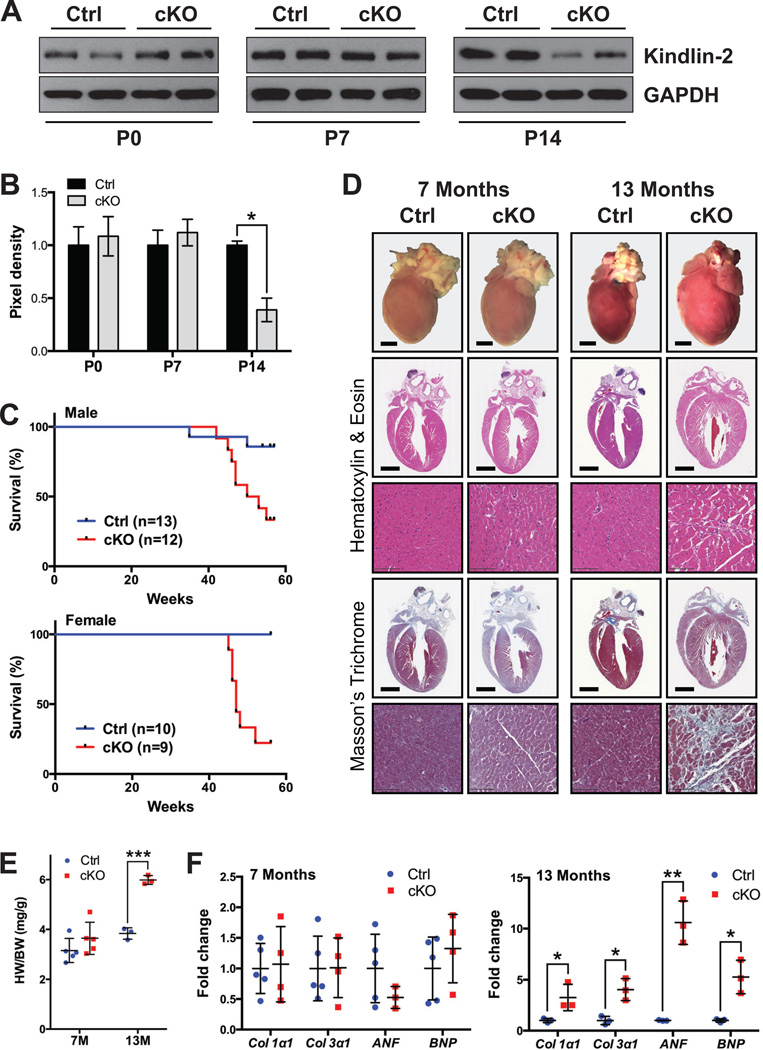
Given this result, we next crossed the Kindlin-2f/f mice28 with transgenic β-MHC-Cre mice30, since Cre-mediated DNA recombination effected by this promoter does not begin until E12.5 in CMs39. At this time point, gene excision only occurs with low efficiency, while high efficiency starts at E17.539. Kindlin-2f/f; β-MHC-Cre mice (referred to as cKO mice hereafter) were born at expected Mendelian ratios and appeared morphologically normal at birth. Kindlin-2 protein expression was not significantly different at postnatal day (P) 0 or 7 in cKO hearts, compared with Cre-negative Kindlin-2f/f (control) hearts, yet by P14, a significant reduction was evident in cKO hearts (Figure 1A–B). Over time, cKO mice exhibited progressive signs of heart failure with some gender variance (Figure 1C). Males began to die at 42 weeks (W) of age, while the majority of female cKO mice (78%) had died by 52W compared to 100% survival of control littermates. Only 29% of cKO mice survived past (13 months (M)) compared to 91% of control littermates (Figure 1C), indicating the essential role of Kindlin-2 in the CM.
Figure 1. Loss of Kindlin-2 in the heart results in postnatal lethality.
(A) Western blot and (B) corresponding quantitative densitometric analysis of Kindlin-2 in control (Ctrl) and Kindlin-2f/f; β-MHC Cre knockout (cKO) mouse hearts at postnatal day (P) 0, 7 and 14. GAPDH served as a loading control. Kindlin-2 pixel density is normalized to the level of GAPDH (*P<0.05, two-tailed Student’s t-test). A representative example of at least two independent experiments is shown (n=2 per genotype/experiment). (C) Kaplan-Meier survival curves of male (top) and female (bottom) Ctrl and cKO mice. Male and female cKO mice die between 42–55 and 45–52 weeks, respectively. (D) Macroscopic cross-sectional views of Hematoxylin and Eosin- and Masson’s Trichrome-stained hearts isolated from Ctrl and cKO mice, at 7 and 13 months (scale bar: 2 mm). Representative high magnification views are shown below each corresponding whole heart section (scale bar: 100 µm) (n=2–3 per genotype). (E) Heart weight to body weight ratio (HW/BW) of Ctrl versus cKO mice at 7 and 13 months (M) (***P<0.001, two-tailed Student’s t-test). (F) qRT-PCR analysis of indicated genes in Ctrl and cKO mouse hearts, at 7 (left) and 13 (right) months. Data is normalized to corresponding 18S levels, and cKO is expressed as fold change versus Ctrl (*P<0.05; **P<0.01, two-tailed Student’s t-test). Col1α1, collagen alpha 1 type I; Col3α1, collagen alpha1 type III; ANF, atrial natriuretic factor; BNP, B-type natriuretic peptide.
When we evaluated mice at 7M, there were no morphological, histological, or heart weight: body weight ratio (HW/BW) differences evident between cKO and control littermates (Figure 1D–E). Yet consistent with the survival curves, when live cKO were evaluated at 13M, morphological analysis revealed a marked enlargement of the heart (Figure 1D). HW/BW was significantly increased from 3.84±0.23 in control to 5.99±0.18 in cKO mice at 13M (Figure 1E), with no difference observed in body weight (data not shown). Histological analysis at this time point revealed enlargement of the left ventricular chamber with extensive fibrosis (Figure 1D). Accordingly, the fibrotic genes, collagen α1 types I and III, were significantly increased in 13M cKO mice (Figure 1F), as were the cardiac fetal gene markers, atrial natriuretic factor (ANF) and B-type natriuretic peptide (BNP) (Figure 1F), consistent with molecular evidence of cardiac remodeling40.
Loss of Kindlin-2 leads to progressive heart failure
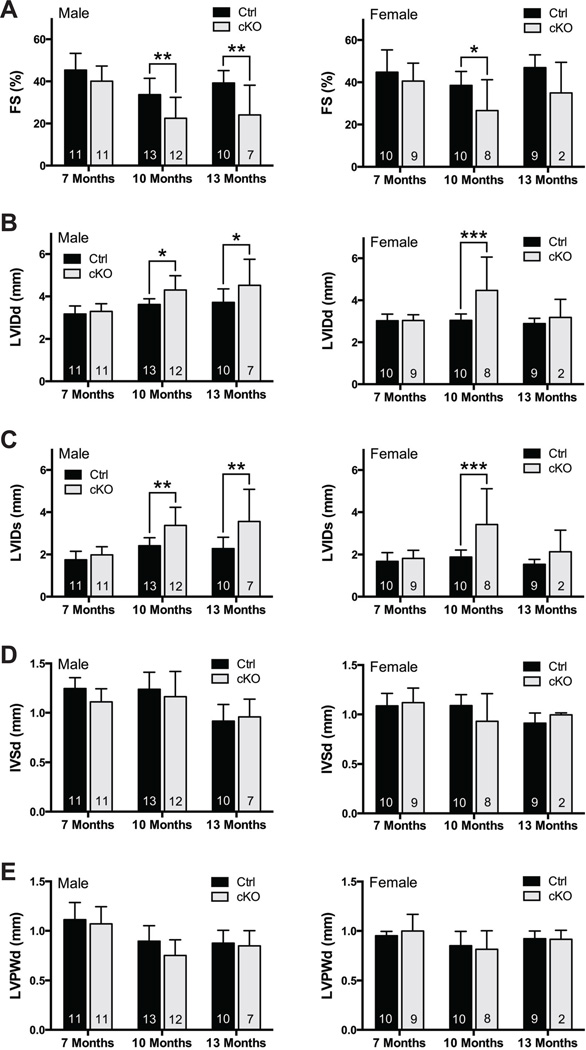
Given these morphological findings, we next evaluated the cardiac function of cKO and control mice from 7 to 13 months of age by echocardiography (Figure 2). Loss of Kindlin-2 resulted in a significant decrease in LV systolic function (fractional shortening (%FS)) beginning at 10M and progressing at 13M in those male survivors (Figure 2A). Interestingly, though trends were also noted for decreased function in female cKO mice at 10M and 13M vs. control, only dysfunction at 10M reached statistical significance. Accompanying the functional changes was evidence of LV chamber dilation in male mice, with dilation also seen in females at 10M, but no significant change at 13M, likely because of the small number of surviving female cKO mice (Figure 2B–C). Consistent with our histological observations (Figure 1D), end-diastolic interventricular septal thickness (IVSd) and LV posterior wall thickness (LVPWd) remained unchanged in both male and female cKO hearts (Figure 2D–E).
Figure 2. Loss of Kindlin-2 leads to progressive heart failure.
Echocardiographic analysis of (A) fractional shortening (FS); (B) left ventricle internal diameter, diastole (LVIDd); (C) left ventricle internal diameter, systole (LVIDs); (D) interventricular septum diameter (IVSd); and (E) LV posterior wall, diastole (LVPWd), in male (left) and female (right) control (Ctrl) and Kindlin-2f/f; β-MHC Cre knockout (cKO) mice at 7, 10, and 13 months (*P<0.05, **P<0.01, ***P<0.001, two-way ANOVA (Bonferroni post-hoc analysis); n=noted in each bar).
Loss of Kindlin-2 in the heart results in decreased protein level of integrin β1D
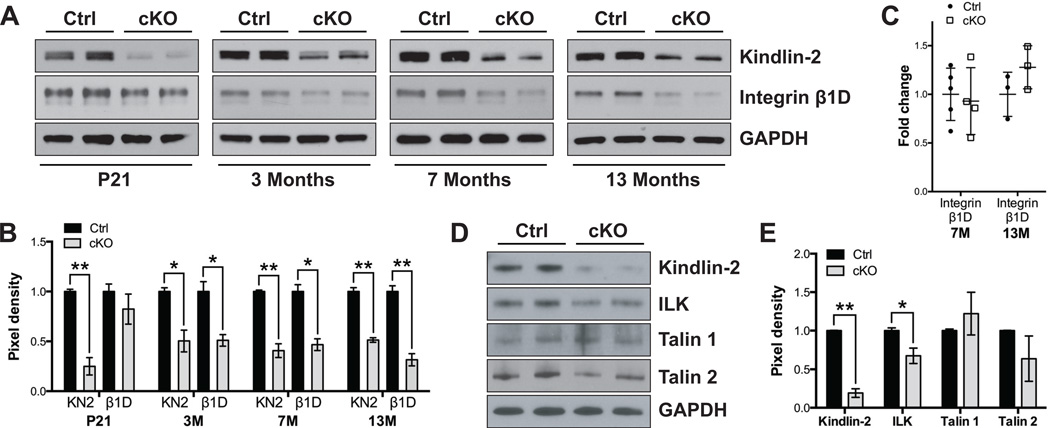
Since Kindlin-2 is an integrin-interacting protein that binds to the integrin β cytoplasmic tail to promote integrin activation19–21, 23, 41, and since expression of UNC-112, the C. elegans homolog of mammalian Kindlin-2, is necessary for organization of β integrin homologs in the muscle cell membrane and for proper muscle attachment15, 27, we next investigated how loss of Kindlin-2 in CMs might affect integrin expression or function. First we determined the localization of Kindlin-2, by performing immunomicroscopy of CMs isolated from control mouse hearts (Supplementary Figure 1A), owing to lack of antibodies specific to Kindlin-2 for immunofluorescence studies on cardiac sections. Our results revealed that, Kindlin-2, like Vinculin, is localized at costameres. Unlike Vinculin however, Kindlin-2 could not be detected at intercalated discs, nor did it co-localize with sarcomeric α-Actinin in isolated adult CMs. This result is in contrast with previous studies, which report that Kindlin-2 also localizes at intercalated discs3, and the Z-line41. We then proceeded to perform Western blot analyses of an array of proteins in heart tissue, concentrating on components of the integrin complex, adherens and gap junction, as well as the desmosome (Figure 3A and Supplementary Figure 1B–C). Interestingly, the protein expression level of integrin β1D, the β1 integrin isoform dominantly expressed in striated muscle, was significantly reduced in Kindlin-2 cKO hearts beginning at age 3M (Figure 3A–B). Integrin β1D mRNA levels remained unchanged even when evaluated up to 13M of age (Figure 3C).
Figure 3. Loss of Kindlin-2 in the heart results in decreased protein levels of integrin β1D.
(A) Western blot and (B) corresponding quantitative densitometric analysis of Kindlin-2 (KN2) and integrin β1D (β1D) in control (Ctrl) and Kindlin-2f/f; β-MHC Cre knockout (cKO) mouse hearts at postnatal day (P) 21, and 3, 7, and 13 months. GAPDH served as a loading control. KN2 and β1D pixel density is normalized to the level of GAPDH (*P<0.05; **P<0.01, two-tailed Student’s t-test). A representative example of at least two independent experiments is shown (n=2 per genotype/experiment). (C) qRT-PCR analysis of integrin β1D in Ctrl and cKO mouse hearts, at 7 and 13 months. Data is normalized to corresponding 18S levels, and cKO is expressed as fold change versus Ctrl (n=noted in each bar). (D) Western blot and (E) corresponding quantitative densitometric analysis of Kindlin-2, integrin-linked kinase (ILK), Talin 1, and Talin 2 in isolated cardiomyocytes from 5 month old Ctrl and cKO mice. GAPDH served as a loading control. Pixel density is normalized to the level of GAPDH (*P<0.05; **P<0.01, two-tailed Student’s t-test). A representative example of at least two independent experiments is shown (n=2 per genotype/experiment).
In addition, we specifically evaluated CM protein samples isolated from 5M cKO hearts. This result showed a significant decrease in integrin-linked kinase (ILK) (Figure 3D–E), another Kindlin-2 interacting partner24 and critical regulator of cardiac function42. These data suggest that Kindlin-2 is essential to maintain the protein stability of integrin β1D and ILK, which is required for adult cardiac function. Of interest, expression of other integrin-binding proteins, Talin 1 and 2, did not significantly differ between cKO and control hearts/CMs (Figure 3D–E and Supplementary Figure 1B–C). β-Catenin and the gap junction protein Connexin 43 were significantly decreased due to loss of Kindlin-2 at 13M. However, all other proteins analyzed showed no difference between control and cKO hearts (Supplementary Figure 1B–C).
Deletion of cardiac Kindlin-2 in adulthood leads to cardiomyopathy and premature death similarly to early loss of Kindlin-2 in the heart
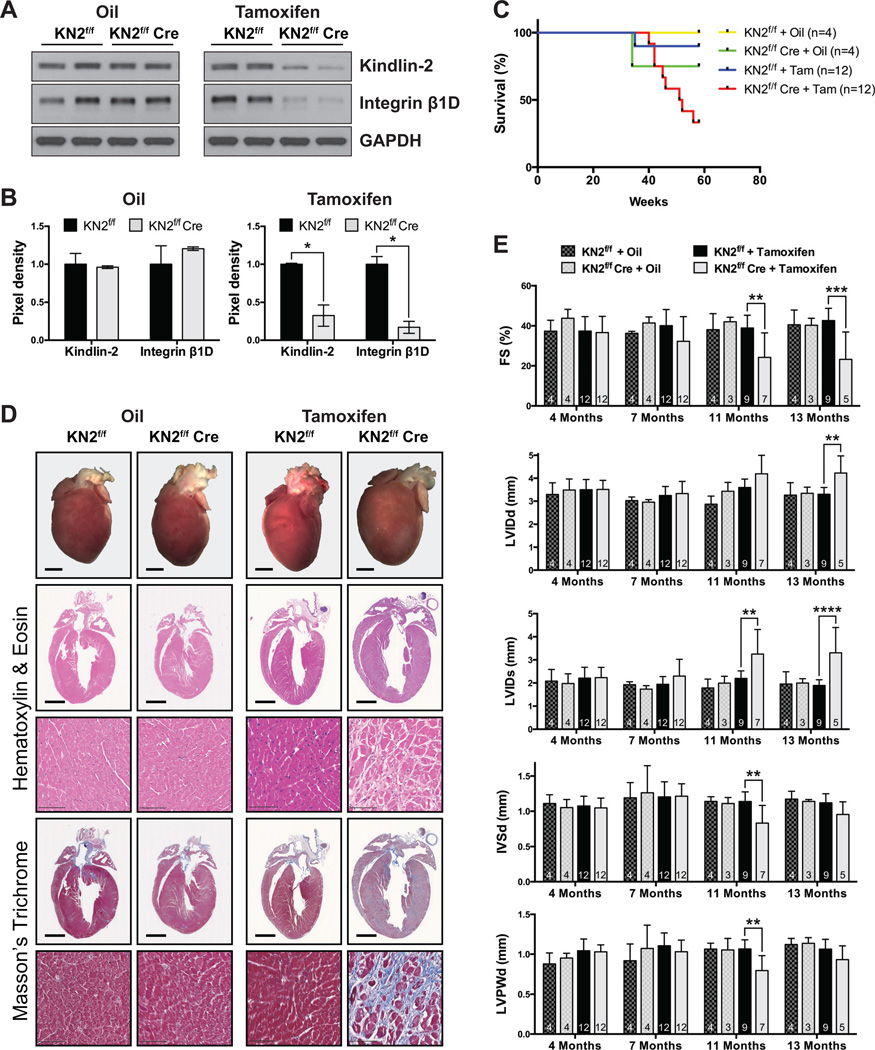
Our study to this point focused on using the β-MHC Cre30 transgene to excise the Kindlin-2 floxed allele. Cre expression from β-MHC Cre starts at E12.539. Therefore to exclude the possibility that adult cardiac dysfunction in cKO mice was due to the effect of decreased Kindlin-2 in the developing myocardium, we further generated an inducible CM-specific KO mouse strain so that Kindlin-2 protein reduction would not occur until adult CMs had fully matured. For this, Kindlin-2f/f mice28 were crossed with α-MHC-MerCreMer transgenic mice31 and tamoxifen treated at 2 months of age. These inducible Kindlin-2 KO mice are hereafter referred to as IcKO mice. Three months after tamoxifen injection for 5 consecutive days, expression of Kindlin-2 protein in IcKO mouse hearts was almost totally abolished when compared with control mice in which either 1) the Kindlin-2f/f line (without Cre) was also similarly injected with tamoxifen, or 2) Kindlin-2f/f and Kindlin-2f/f; α-MHC-MerCreMer mice were injected with the diluent sesame oil (Figure 4A–B). As we saw loss of Kindlin-2 in the IcKO mice, reduction in integrin β1D protein was likewise found (Figure 4A–B), confirming that Kindlin-2 is required to maintain stability of integrin β1D in the mature CM.
Figure 4. Inducible cardiac-specific deletion of Kindlin-2 in adult cardiomyocytes mimics early loss of Kindlin-2 in the heart.
(A) Western blot and (B) corresponding quantitative densitometric analysis of Kindlin-2 (KN2) and integrin β1D in the heart of KN2f/f αMHC-MerCreMer mice 3 months after injection with tamoxifen (KN2f/f Cre). KN2f/f mice injected with tamoxifen, and KN2f/f and KN2f/f Cre mice injected with oil served as littermate controls. GAPDH served as a loading control. Kindlin-2 and integrin β1D pixel density is normalized to the level of GAPDH (*P<0.05, two-tailed Student’s t-test). A representative example of at least two independent experiments is shown (n=2 per genotype/experiment). (C) Kaplan-Meier survival curves of KN2f/f and KN2f/f Cre mice injected with oil or tamoxifen (Tam) show that Tam-treated KN2f/f Cre mice die between 40–56 weeks. (D) Macroscopic cross-sectional views of Hematoxylin and Eosin- and Masson’s Trichrome-stained hearts isolated from KN2f/f and KN2f/f Cre mice, 13 months following injection with oil or tamoxifen (scale bar: 2 mm). Representative high magnification views are shown below each corresponding whole heart section (scale bar: 100 µm) (n=2–3 per genotype). (E) Echocardiographic analysis of fractional shortening (FS); left ventricle internal diameter, diastole (LVIDd); LVID, systole (LVIDs); interventricular septum diameter (IVSd); and LV posterior wall, diastole (LVPWd), in KN2f/f and KN2f/f Cre mice, 4, 7, 11, and 13 months following injection with oil or tamoxifen (**P<0.01, ***P<0.001, ****P<0.0001, two-way ANOVA (Tukey post-hoc analysis); n=noted in each bar).
IcKO mice began to die at 40 weeks of age (Figure 4C), with only 33% surviving past 13M. Consistent with the Kindlin-2 cKO mouse, histology (Figure 4D) and echocardiography (Figure 4E) confirmed significant enlargement of the 13M IcKO mouse hearts, compared with all control groups. Histological observations of surviving 13M IcKO mice showed enlargement of the LV chamber size, which was also evident by echocardiography (LVIDd and LVIDs) (Figure 4D–E). Extensive fibrosis was also evident (Figure 4D). More importantly, the changes in cardiac dimensions in IcKO mice were accompanied by a significant decrease in fractional shortening when compared with controls (Figure 4E). Collectively, the data obtained from the IcKO mice, show that whether Kindlin-2 gene excision is initiated in late embryogenesis or in the mature adult CM, Kindlin-2 is found to be essential for the preservation of normal adult cardiac structure and function.
Discussion
Kindlin-2 is an integrin-interacting protein14, 19–23, and is the Kindlin isoform abundantly expressed in cardiac and skeletal muscle3, 16. Previous studies, in a number of model organisms, have demonstrated that global ablation of Kindlin-2 results in embryonic lethality caused by abrogated integrin function and associated attachment defects3, 13, 15, 27. To investigate the role of Kindlin-2 in the mammalian heart, we generated CM-specific KO mice by conditionally deleting Kindlin-2 in either developing or adult CMs. Kindlin-2f/f; β-MHC-Cre (cKO) mice developed cardiomyopathy and died prematurely with cardiac dysfunction associated with enlargement of the heart and extensive myocardial fibrosis. Similar observations were made in an inducible CM-specific KO mouse strain, Kindlin-2f/f; α-MHC-MerCreMer (IcKO). Thus, ablation of Kindlin-2, either in perinatal or adult CMs, results in cardiac dysfunction and premature death, indicating an essential role for Kindlin-2 in cardiac function.
Previous studies showed that Kindlin-2 is localized at the Z-line where it interacts with α-Actinin41, and at the intercalated disc where it has been proposed to be critical for the establishment of myofibrillar attachments in cardiac and skeletal muscle3. In contrast to these observations, we only detected Kindlin-2 in the costamere of adult isolated CMs. Positive detection of Vinculin at the intercalated disc ruled out the possibility that isolation and staining of single CMs disrupted the structure and therefore localization of Kindlin-2.
Localization of Kindlin-2 at the costamere supports its role as an integrin-interacting protein. Aberrations in Kindlin-2 expression or activity may therefore potentially contribute to the pathogenesis of cardiomyopathy via Kindlin-2 interaction with integrins, or modulation of their signaling and mechanotransductive properties7, 43, 44. Kindlin-2 has previously been shown to bind to the integrin β cytoplasmic tail and promote integrin activation13, 19–21, 23, 41, 45. In this study, we provide further evidence that Kindlin-2 is essential for integrin function by regulating integrin β1D protein expression levels in adult CMs. Loss of Kindlin-2 from CMs resulted in a dramatic decrease in the level of integrin β1D protein, but not mRNA. In support of our findings, ablation of β1D in CMs results in cardiomyopathy and premature death7 similar to the phenotypes observed in our cKO and IcKO Kindlin-2 mutant mice. While there are reports showing that both Talin and Kindlin are required for integrin activation13, 45, 46, in myoblasts and mammary epithelial cells, activation of β1 integrins can proceed in the absence of Talin47, 48. In this study, we found that despite the continued normal expression of both Talin 1 and 2, loss of Kindlin-2 protein still resulted in cardiac dysfunction. In addition to inside-out signaling, characterized by the conformational shift of integrins from the inactive (low affinity) to active (high affinity) state14, 45, Kindlin-2 therefore could also function as an essential adaptor protein in outside-in signaling in the CM. Kindlin-2 recruits ILK24, which binds to the cytoplasmic tail of β integrins, thus linking cell-ECM interactions to signals regulating cytoskeletal remodeling and cellular processes including growth, proliferation, survival, and differentiation49, 50. Here, we found that loss of Kindlin-2 from CMs resulted in a dramatic decrease in the level of ILK. CM ablation of ILK in the murine heart causes early onset of dilated cardiomyopathy, as well as spontaneous heart failure8, consistent with the results we present here.
In summary, our data demonstrate that in the mammalian heart, Kindlin-2 is localized to the costamere, and is essential for adult myocardial function. Loss of Kindlin-2 from the CM leads to downregulation of integrin β1D, and results in premature death from cardiac dysfunction. Thus, future strategies for manipulation of Kindlin-2 activity may be useful in promoting beneficial cardiac remodeling and repair.
Supplementary Material
Clinical Perspective.
Interaction of cardiac myocytes (CMs) with the extracellular matrix (ECM) is of fundamental importance to normal heart development and postnatal function of the myocardium. Integrins are transmembrane receptors that link the ECM with the cytoskeleton and together with a complex of integrin-associated proteins, form the focal adhesion (FA) complex. In the cardiac myocyte, an equivalent structure to the FA is the costamere, which plays essential roles in preservation of myocyte structure and function. Multiple costameric proteins play important roles in the pathogenesis of human cardiomyopathy. Kindlins are a family of three adaptor proteins that are essential components of the integrin adhesion complex. Kindlin-2 is the only reported isoform to be expressed in cardiac tissue. It binds to the β integrin cytoplasmic tails to promote integrin activation. Global deficiency of Kindlin-2 in mice causes peri-implantation lethality, likely because of defective integrin function, with resultant failure of endodermal and epiblast attachment. Kindlin-2 knockdown in zebrafish leads to abnormalities in cardiac structure and function. Together, these reports suggest that Kindlin-2 plays an essential requirement for mammalian integrin function, along with normal myocardial structure and function. In this study we demonstrate that Kindlin-2 in the mammalian heart is localized to the costamere and is essential for adult myocardial function. Loss of Kindlin-2 specifically from the adult CM leads to reduced expression of the muscle-dominant integrin β1D protein, and results in premature death from cardiac dysfunction. Thus, we would propose that defects in Kindlin-2 protein function could be considered as potentially causal of human heart failure, and future strategies for manipulation of Kindlin-2 expression or activity may be useful for promoting beneficial cardiac remodeling and repair.
Acknowledgments
Sources of Funding
J. Chen holds the American Heart Association Endowed Chair, and is funded by grants from NIH/NHLBI. R.S. Ross is funded by grants from NIH/NHLBI and the Veterans Administration.
Footnotes
Disclosures
None.
References
- 1.Israeli-Rosenberg S, Manso AM, Okada H, Ross RS. Integrins and integrin-associated proteins in the cardiac myocyte. Circulation research. 2014;114:572–586. doi: 10.1161/CIRCRESAHA.114.301275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peter AK, Cheng H, Ross RS, Knowlton KU, Chen J. The costamere bridges sarcomeres to the sarcolemma in striated muscle. Progress in pediatric cardiology. 2011;31:83–88. doi: 10.1016/j.ppedcard.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowling JJ, Gibbs E, Russell M, Goldman D, Minarcik J, Golden JA, Feldman EL. Kindlin-2 is an essential component of intercalated discs and is required for vertebrate cardiac structure and function. Circulation research. 2008;102:423–431. doi: 10.1161/CIRCRESAHA.107.161489. [DOI] [PubMed] [Google Scholar]
- 4.Ross RS, Borg TK. Integrins and the myocardium. Circulation research. 2001;88:1112–1119. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- 5.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 6.Danen EH, Sonnenberg A. Integrins in regulation of tissue development and function. The Journal of pathology. 2003;201:632–641. doi: 10.1002/path.1472. [DOI] [PubMed] [Google Scholar]
- 7.Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, Omura M, Leil TA, Becker KD, Jiang M, Smith DJ, Cherry SR, Loftus JC, Ross RS. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circulation research. 2002;90:458–464. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- 8.White DE, Coutu P, Shi YF, Tardif JC, Nattel S, St Arnaud R, Dedhar S, Muller WJ. Targeted ablation of ILK from the murine heart results in dilated cardiomyopathy and spontaneous heart failure. Genes & development. 2006;20:2355–2360. doi: 10.1101/gad.1458906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes & development. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 10.Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes & development. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 11.Sakai T, Li S, Docheva D, Grashoff C, Sakai K, Kostka G, Braun A, Pfeifer A, Yurchenco PD, Fassler R. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes & development. 2003;17:926–940. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knoll R, Postel R, Wang J, Kratzner R, Hennecke G, Vacaru AM, Vakeel P, Schubert C, Murthy K, Rana BK, Kube D, Knoll G, Schafer K, Hayashi T, Holm T, Kimura A, Schork N, Toliat MR, Nurnberg P, Schultheiss HP, Schaper W, Schaper J, Bos E, Den Hertog J, van Eeden FJ, Peters PJ, Hasenfuss G, Chien KR, Bakkers J. Laminin-alpha4 and integrin-linked kinase mutations cause human cardiomyopathy via simultaneous defects in cardiomyocytes and endothelial cells. Circulation. 2007;116:515–525. doi: 10.1161/CIRCULATIONAHA.107.689984. [DOI] [PubMed] [Google Scholar]
- 13.Montanez E, Ussar S, Schifferer M, Bosl M, Zent R, Moser M, Fassler R. Kindlin-2 controls bidirectional signaling of integrins. Genes & development. 2008;22:1325–1330. doi: 10.1101/gad.469408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moser M, Legate KR, Zent R, Fassler R. The tail of integrins, talin, and kindlins. Science. 2009;324:895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- 15.Rogalski TM, Mullen GP, Gilbert MM, Williams BD, Moerman DG. The UNC-112 gene in Caenorhabditis elegans encodes a novel component of cell-matrix adhesion structures required for integrin localization in the muscle cell membrane. The Journal of cell biology. 2000;150:253–264. doi: 10.1083/jcb.150.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ussar S, Wang HV, Linder S, Fassler R, Moser M. The Kindlins: subcellular localization and expression during murine development. Experimental cell research. 2006;312:3142–3151. doi: 10.1016/j.yexcr.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Calderwood DA, Campbell ID, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nature reviews Molecular cell biology. 2013;14:503–517. doi: 10.1038/nrm3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhan J, Yang M, Chi X, Zhang J, Pei X, Ren C, Guo Y, Liu W, Zhang H. Kindlin-2 expression in adult tissues correlates with their embryonic origins. Science China Life sciences. 2014;57:690–697. doi: 10.1007/s11427-014-4676-4. [DOI] [PubMed] [Google Scholar]
- 19.Bledzka K, Bialkowska K, Nie H, Qin J, Byzova T, Wu C, Plow EF, Ma YQ. Tyrosine phosphorylation of integrin beta3 regulates kindlin-2 binding and integrin activation. The Journal of biological chemistry. 2010;285:30370–30374. doi: 10.1074/jbc.C110.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harburger DS, Bouaouina M, Calderwood DA. Kindlin-1 and -2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects. The Journal of biological chemistry. 2009;284:11485–11497. doi: 10.1074/jbc.M809233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perera HD, Ma YQ, Yang J, Hirbawi J, Plow EF, Qin J. Membrane binding of the N-terminal ubiquitin-like domain of kindlin-2 is crucial for its regulation of integrin activation. Structure. 2011;19:1664–1671. doi: 10.1016/j.str.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi X, Ma YQ, Tu Y, Chen K, Wu S, Fukuda K, Qin J, Plow EF, Wu C. The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. The Journal of biological chemistry. 2007;282:20455–20466. doi: 10.1074/jbc.M611680200. [DOI] [PubMed] [Google Scholar]
- 23.Xu Z, Gao J, Hong J, Ma YQ. Integrity of kindlin-2 FERM subdomains is required for supporting integrin activation. Biochemical and biophysical research communications. 2013;434:382–387. doi: 10.1016/j.bbrc.2013.03.086. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda K, Bledzka K, Yang J, Perera HD, Plow EF, Qin J. Molecular basis of kindlin-2 binding to integrin-linked kinase pseudokinase for regulating cell adhesion. The Journal of biological chemistry. 2014;289:28363–28375. doi: 10.1074/jbc.M114.596692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma YQ, Qin J, Wu C, Plow EF. Kindlin-2 (Mig-2): a co-activator of beta3 integrins. The Journal of cell biology. 2008;181:439–446. doi: 10.1083/jcb.200710196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma YQ, Yang J, Pesho MM, Vinogradova O, Qin J, Plow EF. Regulation of integrin alphaIIbbeta3 activation by distinct regions of its cytoplasmic tails. Biochemistry. 2006;45:6656–6662. doi: 10.1021/bi060279h. [DOI] [PubMed] [Google Scholar]
- 27.Williams BD, Waterston RH. Genes critical for muscle development and function in Caenorhabditis elegans identified through lethal mutations. The Journal of cell biology. 1994;124:475–490. doi: 10.1083/jcb.124.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C, Jiao H, Lai Y, Zheng W, Chen K, Qu H, Deng W, Song P, Zhu K, Cao H, Galson DL, Fan J, Im HJ, Liu Y, Chen J, Chen D, Xiao G. Kindlin-2 controls TGF-beta signalling and Sox9 expression to regulate chondrogenesis. Nature communications. 2015;6:7531. doi: 10.1038/ncomms8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, Minnemann T, Boers ME, Hadro E, Oberste-Berghaus C, Quist W, Lowell BB, Ingwall JS, Kahn BB. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. The Journal of clinical investigation. 1999;104:1703–1714. doi: 10.1172/JCI7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsons SA, Millay DP, Wilkins BJ, Bueno OF, Tsika GL, Neilson JR, Liberatore CM, Yutzey KE, Crabtree GR, Tsika RW, Molkentin JD. Genetic loss of calcineurin blocks mechanical overload-induced skeletal muscle fiber type switching but not hypertrophy. The Journal of biological chemistry. 2004;279:26192–26200. doi: 10.1074/jbc.M313800200. [DOI] [PubMed] [Google Scholar]
- 31.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circulation research. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Stroud MJ, Zhang J, Fang X, Ouyang K, Kimura K, Mu Y, Dalton ND, Gu Y, Bradford WH, Peterson KL, Cheng H, Zhou X, Chen J. Normalization of Naxos plakoglobin levels restores cardiac function in mice. The Journal of clinical investigation. 2015;125:1708–1712. doi: 10.1172/JCI80335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pham CG, Harpf AE, Keller RS, Vu HT, Shai SY, Loftus JC, Ross RS. Striated muscle-specific beta(1D)-integrin and FAK are involved in cardiac myocyte hypertrophic response pathway. Am J Physiol Heart Circ Physiol. 2000;279:H2916–H2926. doi: 10.1152/ajpheart.2000.279.6.H2916. [DOI] [PubMed] [Google Scholar]
- 34.Manso AM, Li R, Monkley SJ, Cruz NM, Ong S, Lao DH, Koshman YE, Gu Y, Peterson KL, Chen J, Abel ED, Samarel AM, Critchley DR, Ross RS. Talin1 has unique expression versus talin 2 in the heart and modifies the hypertrophic response to pressure overload. The Journal of biological chemistry. 2013;288:4252–4264. doi: 10.1074/jbc.M112.427484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Praekelt U, Kopp PM, Rehm K, Linder S, Bate N, Patel B, Debrand E, Manso AM, Ross RS, Conti F, Zhang MZ, Harris RC, Zent R, Critchley DR, Monkley SJ. New isoform-specific monoclonal antibodies reveal different sub-cellular localisations for talin1 and talin2. Eur J Cell Biol. 2012;91:180–191. doi: 10.1016/j.ejcb.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng M, Cheng H, Li X, Zhang J, Cui L, Ouyang K, Han L, Zhao T, Gu Y, Dalton ND, Bang ML, Peterson KL, Chen J. Cardiac-specific ablation of Cypher leads to a severe form of dilated cardiomyopathy with premature death. Human molecular genetics. 2009;18:701–713. doi: 10.1093/hmg/ddn400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang X, Sun Y, Schneider J, Ding JH, Cheng H, Ye M, Bhattacharya S, Rearden A, Evans S, Chen J. Pinch1 is required for normal development of cranial and cardiac neural crest-derived structures. Circulation research. 2007;100:527–535. doi: 10.1161/01.RES.0000259041.37059.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang X, Zhou Q, Li X, Sun Y, Lu M, Dalton N, Ross J, Jr, Chen J. PINCH1 plays an essential role in early murine embryonic development but is dispensable in ventricular cardiomyocytes. Mol Cell Biol. 2005;25:3056–3062. doi: 10.1128/MCB.25.8.3056-3062.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakraborty S, Yutzey KE. Tbx20 regulation of cardiac cell proliferation and lineage specialization during embryonic and fetal development in vivo. Developmental biology. 2012;363:234–246. doi: 10.1016/j.ydbio.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nature reviews Molecular cell biology. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 41.Qi L, Yu Y, Chi X, Xu W, Lu D, Song Y, Zhang Y, Zhang H. Kindlin-2 interacts with alpha-actinin-2 and beta1 integrin to maintain the integrity of the Z-disc in cardiac muscles. FEBS letters. 2015;589:2155–2162. doi: 10.1016/j.febslet.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 42.Hannigan GE, Coles JG, Dedhar S. Integrin-linked kinase at the heart of cardiac contractility, repair, and disease. Circulation research. 2007;100:1408–1414. doi: 10.1161/01.RES.0000265233.40455.62. [DOI] [PubMed] [Google Scholar]
- 43.Hatcher CJ, Basson CT. Disrupted intercalated discs. Is kindlin-2 required? Circulation research. 2008;102:392–394. doi: 10.1161/CIRCRESAHA.108.172171. [DOI] [PubMed] [Google Scholar]
- 44.Li R, Wu Y, Manso AM, Gu Y, Liao P, Israeli S, Yajima T, Nguyen U, Huang MS, Dalton ND, Peterson KL, Ross RS. beta1 integrin gene excision in the adult murine cardiac myocyte causes defective mechanical and signaling responses. Am J Pathol. 2012;180:952–962. doi: 10.1016/j.ajpath.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Theodosiou M, Widmaier M, Bottcher RT, Rognoni E, Veelders M, Bharadwaj M, Lambacher A, Austen K, Muller DJ, Zent R, Fassler R. Kindlin-2 cooperates with talin to activate integrins and induces cell spreading by directly binding paxillin. eLife. 2016;5:e10130. doi: 10.7554/eLife.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Margadant C, Kreft M, de Groot DJ, Norman JC, Sonnenberg A. Distinct roles of talin and kindlin in regulating integrin alpha5beta1 function and trafficking. Current biology : CB. 2012;22:1554–1563. doi: 10.1016/j.cub.2012.06.060. [DOI] [PubMed] [Google Scholar]
- 47.Conti FJ, Monkley SJ, Wood MR, Critchley DR, Muller U. Talin 1 and 2 are required for myoblast fusion, sarcomere assembly and the maintenance of myotendinous junctions. Development. 2009;136:3597–3606. doi: 10.1242/dev.035857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang P, Ballestrem C, Streuli CH. The C terminus of talin links integrins to cell cycle progression. The Journal of cell biology. 2011;195:499–513. doi: 10.1083/jcb.201104128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nature reviews Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- 50.Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.