Abstract
Objective
To compare the outcome of immediate versus delayed radical prostatectomy (RP) in men with low-grade prostate cancer (PCa).
Materials and Methods
Nationwide population-based cohort in the National Prostate Cancer Register (NPCR) of Sweden, of 7608 men with clinically localized, biopsy Gleason score 6 PCa who underwent immediate or delayed RP in 1997-2007. Multivariable models compared RP pathology, use of salvage radiotherapy and prostate cancer mortality based on timing of RP (<1, 1-2, >2 years after diagnosis). Median follow-up was 8.1 years.
Results
Men undergoing RP more than 2 years after diagnosis had a higher risk of Gleason upgrading (OR 2.93, 95% CI 2.34-3.68) and an increased risk of salvage radiotherapy (HR 1.90, 95%CI 1.41-2.55), but no significant increase in PCa-specific mortality (HR 1.85, 95%CI 0.57-5.99). In competing risk analysis, 7-year PCa-specific cumulative mortality was similar at <1% for immediate RP and AS regardless of later intervention. Limitations of our study include lack of data on follow-up biopsies and a limited follow-up time.
Conclusion
Men undergoing RP more than 2 years after diagnosis had more adverse pathology features and second-line therapy, highlighting the trade-off by deferring immediate curative therapy. However, men with delayed RP constitute a minority with higher-risk cancer among the much larger group of low-risk men initially surveilled and the overall risk of prostate cancer mortality at 7 years was similarly low with immediate RP or AS.
Keywords: prostate cancer, active surveillance, radical prostatectomy, surgical delay, prognosis, outcomes
Introduction
Treatment of favorable-risk prostate cancer is curative in the vast majority of men,[1] but can have a significant negative impact on quality of life.[2] Active surveillance is an important alternative to delay or avoid curative treatment. Historically, only 10% of low risk prostate cancers in the United States were managed conservatively[3], although by 2010-2013 this had increased to approximately 40% among a large group of US practices.[4] In other countries such as Sweden, the adoption of active surveillance has also increased with 59% of very low risk and 41% of low risk prostate cancers diagnosed from 2007 to 2011 managed with active surveillance.[5]
Although many studies have shown favorable short-term outcomes with active surveillance, there is very little long-term data to confirm that this strategy does not miss the window for cure in men who might have been treated upon diagnosis. In a recent review, the median follow-up across active surveillance programs was only 3 years, and the longest median follow-up of any individual cohort was 7 years.[6] More recently, Klotz et al. reported updated follow-up of the Sunnybrook cohort (median follow-up of 6 years from first biopsy) in which 1.5% had died from prostate cancer and an additional 1.3% developed metastatic disease.[7] Updated data from the Johns Hopkins active surveillance program (median follow-up of 5 years) reported 2 prostate cancer deaths (0.15%) and 0.4% with metastatic disease.[8]
Overall, there remains limited data in the literature on the cancer specific outcomes of men with low-grade tumors undergoing surgery 1 or more years after diagnosis. The objective of our study was to examine the impact of longer treatment delays on outcomes in the National Prostate Cancer Register (NPCR) of Sweden.
Materials and Methods
The NPCR of Sweden includes 98% of all prostate cancer cases compared to the Swedish Cancer Register to which reporting is mandated by law. As previously described, data on tumor characteristics and primary treatment are recorded, and in the Prostate Cancer Data Base (PCBaSe), the NPCR has been cross-linked to other health care registries and demographic databases [9].
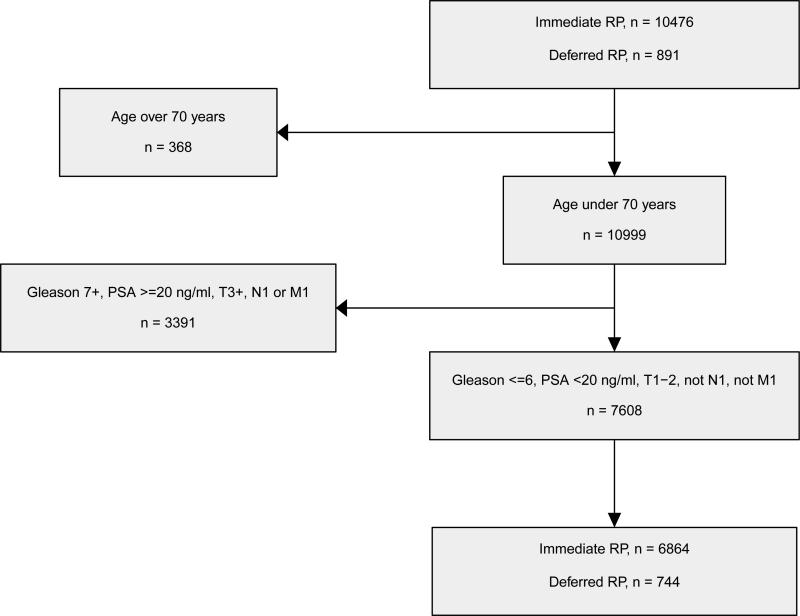
In this study, data in PCBaSe for men diagnosed with prostate cancer from 1997 to 2007 were analysed (Figure 1). The inclusion criteria were age ≤70 at diagnosis, clinical stage T1/T2, PSA <20 ng/ml, biopsy Gleason score ≤6 and receipt of an immediate or delayed radical prostatectomy, resulting in a final study population of 7608 men. Subset analysis was also performed in the 5987 men with PSA <10 ng/ml (D'Amico low risk), including 5347 treated at <1 year, 335 treated at 1-2 years, and 305 treated at >2 years after diagnosis.
Figure 1.
Selection of the main study population comparing immediate versus deferred prostatectomy
The exposure in our analysis was time from date of prostate cancer diagnosis to date of surgery. We calculated this both as a continuous variable and as a categorical variable (<1, 1-2, and >2 years). We examined the following endpoints: adverse prostatectomy pathology (upgrading to a Gleason >6, extraprostatic extension or positive surgical margins), the receipt of salvage radiation therapy, and prostate cancer mortality.
The following covariates were included in the analysis: age (continuous), PSA (continuous, in 1 ng/ml increments), clinical local stage (≤T1c versus T2), comorbidity, educational level (low, middle or high), marital status (married, divorced/separated/widow, or single), and type of hospital (non-university versus university hospital). Comorbidity was assessed by use of the Charlson Comorbidity Index (CCI) which is a score based on International Classification of Diseases (ICD) codes that we applied to the discharge diagnoses in the In-Patient Register during ten years prior to the date of diagnosis of the index case, as previously described.[10, 11] The term “healthy men” is used for men with no registered comorbidity i.e. CCI 0. Information on the level of education was obtained from the Longitudinal Integration Database for Health Insurance and Labour Market Studies (LISA), a socioeconomic database, and was categorized as low (< 10 years, mandatory school), intermediate (10-12 years, high school) or high (> 12 years, university or equivalent).
Statistical Analysis
All statistical analysis was performed using R, version 3.0.3 (R Foundation for Statistical Computing, Vienna, Austria) and statistical significance was set at α=0.05. Logistic regression was used to examine predictors of upgrading from diagnosis to RP (prostatectomy Gleason ≥7), extraprostatic extension and positive surgical margins. Cumulative incidence of salvage radiation therapy and prostate cancer specific mortality in the presence of competing risks were estimated, treating other-cause mortality as a competing risk. This was also used to assess the need to adjust for such competing risks in the multivariable models by employing more sophisticated methods. Cox proportional hazards models were ultimately used to examine the endpoints of salvage radiation therapy and prostate cancer specific mortality after delayed and immediate prostatectomy.
Finally, to compare PCa-specific mortality between immediate prostatectomy and active surveillance in an unselected setting, 7-year cumulative incidences were estimated in a competing risk analysis of 6981 men who underwent a primary RP within 6 months from diagnosis versus 1729 men who were prospectively registered as choosing active surveillance (Supplemental Figure 1),[12] regardless of later treatment (i.e. date of diagnosis was used as t=0 and time to treatment was disregarded). The Research Ethics Board at Umeå University Hospital approved of the study.
Results
The median age at diagnosis was 62 years (IQR 58.3-65.5), median PSA level was 6.5 ng/ml (IQR 4.8-9.2), and the majority of men (67%) had clinical stage T1c disease (Table 1). Radical prostatectomy was performed within 1 year after diagnosis in 6864 men, 1-2 years in 397 men, and >2 years in 347 men. Gleason scores ≥7 were present in 27%, 37%, and 49% of the prostatectomy specimens, extraprostatic extension was present in 13%, 13% and 21%, and positive surgical margins were present in 23%, 17%, and 24% of men with delays <1, 1-2 and >2 years, respectively.
Table 1.
Demographics and tumor characteristics for men in the Prostate Cancer Database of Sweden (PCBaSe) 3.0 who underwent immediate or delayed radical prostatectomy with follow-up data
| Time to RP | <1 yr | 1-2 yrs | 2-7 yrs | Total |
|---|---|---|---|---|
| Men | 6864 | 397 | 347 | 7608 |
| Year of diagnosis | ||||
| 1997-1998 | 243 (4%) | 15 (4%) | 10 (3%) | 268 (4%) |
| 1999 | 328 (5%) | 22 (6%) | 11 (3%) | 361 (5%) |
| 2000 | 460 (7%) | 27 (7%) | 22 (6%) | 509 (7%) |
| 2001 | 512 (8%) | 33 (9%) | 21 (6%) | 566 (8%) |
| 2002 | 623 (9%) | 32 (8%) | 20 (6%) | 675 (9%) |
| 2003 | 956 (14%) | 60 (16%) | 42 (12%) | 1058 (14%) |
| 2004 | 1204 (18%) | 62 (16%) | 41 (12%) | 1307 (18%) |
| 2005 | 1125 (17%) | 48 (12%) | 52 (15%) | 1225 (16%) |
| 2006-2007 | 1261 (19%) | 88 (23%) | 121 (36%) | 1470 (20%) |
| Age at diagnosis (yrs) | ||||
| Median (IQR) | 62.0 (58.1-65.5) | 62.7 (59.7-65.3) | 62.4 (59.1-65.7) | 62.0 (58.3-65.5) |
| Charlson Comorbidity Index | ||||
| CCI 0 | 6098 (89%) | 361 (91% | 301 (87%) | 6760 (89%) |
| CCI 1 | 466 (7%) | 21 (5%) | 25 (7%) | 512 (7%) |
| CCI 2+ | 300 (4%) | 15 (4%) | 21 (6%) | 336 (4%) |
| Marital status | ||||
| Married | 5211 (76%) | 288 (73%) | 266 (77%) | 5765 (76%) |
| Separated/Divorced/Widower | 1086 (16%) | 75 (19%) | 53 (15%) | 1214 (16%) |
| Single | 565 (8%) | 34 (9%) | 28 (8%) | 627 (8%) |
| Missing data | 2 (0%) | 0 (0%) | 0 (0%) | 2 (0%) |
| Educational level | ||||
| High | 1933 (28%) | 112 (28%) | 99 (29%) | 2144 (28%) |
| Middle | 2849 (42%) | 179 (45%) | 148 (43%) | 3176 (42%) |
| Low | 2061 (30%) | 106 (27%) | 98 (28%) | 2265 (30%) |
| Type of hospital | ||||
| Non-university hospital | 3936 (57%) | 197 (50%) | 183 (53%) | 4316 (57%) |
| University hospital | 2928 (43%) | 200 (50%) | 164 (47%) | 3292 (43%) |
| Serum PSA (ng/mL) | ||||
| Median (IQR) | 6.7 (4.8-9.3) | 6.1 (4.5-8.6) | 5.4 (4.2-7.6) | 6.5 (4.8-9.2) |
| <4 | 860 (13%) | 64 (16%) | 61 (18%) | 985 (13%) |
| 4-10 | 4487 (65%) | 271 (68%) | 244 (70%) | 5002 (66%) |
| 10-20 | 1517 (22%) | 62 (16%) | 42 (12%) | 1621 (21%) |
| Clinical stage | ||||
| T1a | 51 (1%) | 10 (3%) | 8 (2%) | 69 (1%) |
| T1b | 34 (0%) | 3 (1%) | 3 (1%) | 40 (1%) |
| T1c | 4528 (66%) | 301 (76%) | 285 (82%) | 5114 (67%) |
| T2 | 2251 (33%) | 83 (21%) | 51 (15%) | 2385 (31%) |
| Number of biopsy cores | ||||
| Median (IQR) | 6 (6-8) | 6 (6-8) | 8 (6-10) | 6 (6-8) |
| <10 | 3313 (48) | 189 (48) | 167 (48) | 3669 (48) |
| 10+ | 740 (11) | 52 (13) | 61 (18) | 853 (11) |
| Ratio of positive cores | ||||
| <33% | 1926 (28) | 178 (45) | 168 (48) | 2272 (30) |
| 33-66% | 1696 (25) | 54 (14) | 51 (15) | 1801 (24) |
| >66% | 424 (6) | 7 (2) | 9 (3) | 440 (6) |
| Missing data | 2818 (41%) | 158 (40%) | 119 (34%) | 3095 (41%) |
| Prostatectomy Gleason score | ||||
| <=6 | 4805 (70%) | 236 (59%) | 163 (47%) | 5204 (68%) |
| 7 | 1757 (26%) | 136 (34%) | 150 (43%) | 2043 (27%) |
| 8-10 | 108 (2%) | 11 (3%) | 19 (5%) | 138 (2%) |
| Missing data | 194 (3%) | 14 (4%) | 15 (4%) | 223 (3%) |
| Pathological features | ||||
| OC | 4940 (72%) | 289 (73%) | 237 (68%) | 5466 (72%) |
| EPE | 875 (13%) | 51 (13%) | 73 (21%) | 999 (13%) |
| Missing data | 1049 (15%) | 57 (14%) | 37 (11%) | 1143 (15%) |
| Positive margin | ||||
| No | 4514 (66%) | 290 (73%) | 221 (64%) | 5025 (66%) |
| Yes | 1572 (23%) | 66 (17%) | 84 (24%) | 1722 (23%) |
| Missing data | 778 (11%) | 41 (10%) | 42 (12%) | 861 (11%) |
| Follow-up after RP (yrs) | ||||
| Median (IQR) | 8.3 (6.8-10.2) | 7.6 (5.7-9.7) | 4.7 (3.0-7.3) | 8.1 (6-6-10.1) |
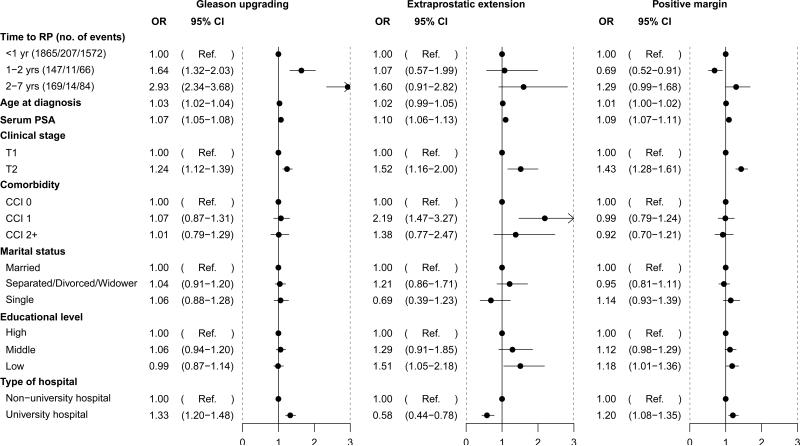
Multivariable models to predict adverse prostatectomy pathology are shown in Figure 2. Compared to men who had radical prostatectomy within the first year, delays of 1-2 years and 2-7 years were associated increased risk of Gleason upgrading, OR 1.64 (95% CI 1.32-2.03) and OR 2.93 (95% CI 2.34-3.68), respectively. A separate model showed that for each additional year of delay, the odds ratio for upgrading was 1.45 (95% CI, 1.35-1.55) (Supplemental Table 1). However, there was no significant difference in risk of extraprostatic extension or positive surgical margins between immediate and delayed prostatectomy.
Figure 2.
Multivariable logistic regression for adverse surgical pathology
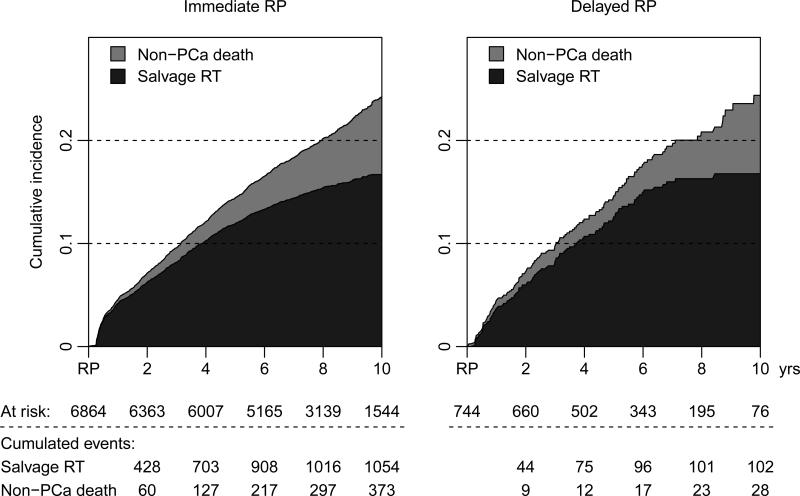
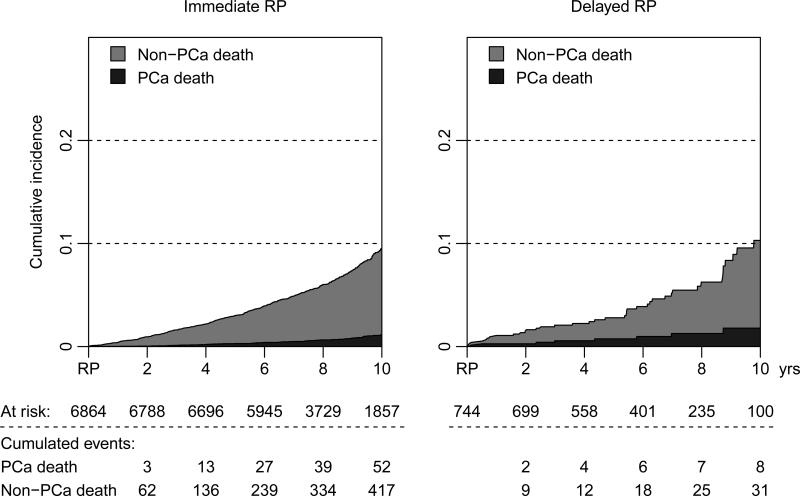
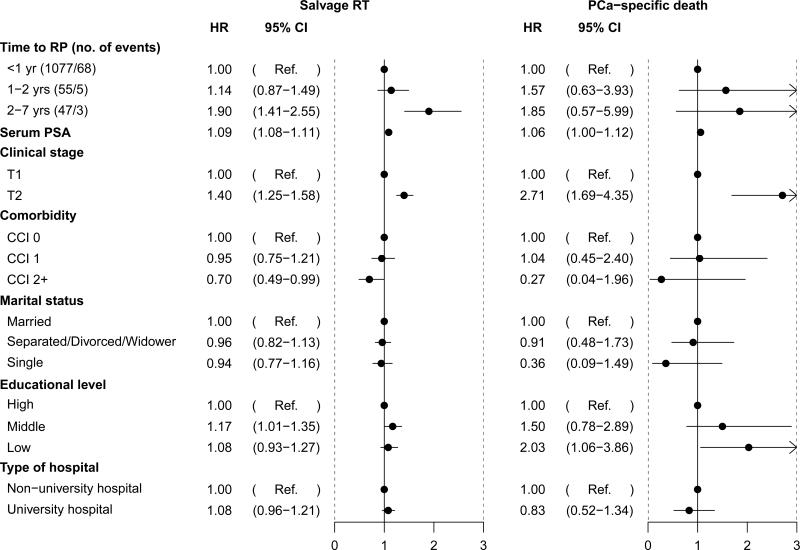
During a median follow-up of 8.1 years (IQR 6.6-10.1), 1179 (15%) men received salvage radiation therapy, and there were 76 prostate cancer deaths among a total of 608 deaths in the study population during the follow-up period (Figures 3 and 4). Figure 5 and Supplemental Table 2 show the results of multivariable analyses to predict salvage radiation therapy and prostate cancer death. Although there was no difference in use of secondary radiation therapy between men treated immediately and within the first two years after date of diagnosis, longer delays of 2-7 years were associated with a significantly higher risk of radiation therapy (OR 1.90, 95% CI 1.41-2.55). The hazard ratio for prostate cancer death among men who received deferred radical prostatectomy after 2-7 years of surveillance was 1.85 (95% CI, 0.57-5.99). Clinical stage T2 (HR 2.71, 95% CI 1.69-4.35) and low education (HR 2.03, 95% CI 1.06-3.86) were also significantly associated with prostate cancer death.
Figure 3.
Cumulative incidence of salvage radiation therapy after immediate versus delayed radical prostatectomy (RP), treating non-PCa death as a competing risk
Figure 4.
Cumulative incidence of prostate cancer death after immediate versus delayed radical prostatectomy (RP), treating non-PCa death as a competing risk
Figure 5.
Multivariable Cox regression for salvage radiotherapy and prostate cancer-specific death after radical prostatectomy
Subset analysis of men with PSA <10 ng/ml is shown in Supplemental figures 2 and 3. In this subgroup of 5987 men, 1610 men (27%) had prostatectomy Gleason scores of 7-10, 733 (12%) had extraprostatic extension, and 1224 (20%) had positive margins at prostatectomy. Similar to the overall results, the risk of upgrading was significantly higher with delays of 1-2 years (OR 1.82, 95% CI 1.43-2.30) and >2 years (OR 2.96, 95% CI 2.32-3.78). There was also a significantly higher risk of salvage therapy with delays >2 years (HR 1.91, 95% CI 1.37-2.68) but the difference in prostate cancer death did not reach statistical significance (HR 2.29, 95% CI 0.70-7.56).
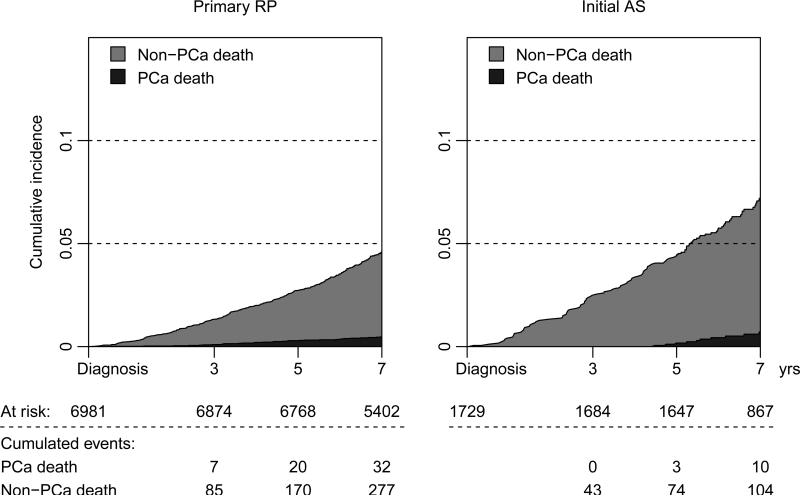
Finally, in competing risk analysis, the 7-year PCa-specific cumulative mortality from date of diagnosis to last follow-up, i.e within six months after date of diagnosis, was similar at <1% for 6981 men with primary RP and 1729 men managed with active surveillance regardless of later intervention (Figure 6).
Figure 6.
Competing risk analysis comparing all men undergoing primary prostatectomy versus active surveillance irrespective of subsequent treatment.
Discussion
In this large population based study, we compared outcome in men with biopsy Gleason ≤6 prostate cancer undergoing immediate versus delayed prostatectomy after a period of surveillance. Delays in surgery longer than 2 years were associated with a higher risk of adverse pathology features, salvage radiation therapy, and non-significant increase in the risk of prostate-cancer death at 8 years of median follow-up. Importantly, men undergoing delayed prostatectomy represent a selected subset of men with higher risk features out of all men on active surveillance. Overall, we observed a <1% prostate cancer mortality at 7 years, with no difference between men undergoing primary prostatectomy compared to men on active surveillance irrespective of later treatment.
This is the first population-based study to examine the impact of treatment delays of >2 years on prostate cancer death, and such data are important as the use of active surveillance is rapidly increasing for Gleason 6 prostate cancer worldwide. Several previous studies have reported the outcomes of immediate prostatectomy for men who would have met criteria for active surveillance[13, 14], or have examined the impact of very short treatment delays. At a European referral center, Graefen et al reported that short delays (median of 54 days) did not increase the risk of biochemical progression, but only 12 and 4 patients in this study had delays longer than 6 and 12 months, respectively.[15] In the US SEARCH database, Abern et al. compared pathology results and biochemical progression rates at a median follow-up of 53 months between men having surgery at <3, 3-6, 6-9, and >9 months.[16] In the low-risk group, there were no differences in adverse pathology or biochemical recurrence based on timing of surgery; whereas, for intermediate risk patients, delays >9 months were associated with significantly higher rates of positive surgical margins and biochemical progression.
Using a similar study design, Andrews et al. examined time to radiation therapy (<3, 3-6, 6-9 and >9 months) and treatment outcomes in 1322 patients from a single institution.[17] During a median follow-up of approximately 5 years, there were no differences in biochemical progression-free, metastasis-free or cancer-specific survival between the groups. By contrast, O'Brien et al. reported significantly higher rates of biochemical progression on multivariable analysis for low-risk patients with surgical delays ≥6 months.[18] Salvage therapy and cancer-specific survival were not evaluated in that study.
Other groups have performed matched comparisons between men undergoing immediate versus delayed prostatectomy. For example, van den Bergh compared 158 immediate versus 69 delayed prostatectomy for men with low risk disease (T1/T2, PSA <10, PSAD <0.2, Gleason 6 and 1-2 positive cores) from the Swedish section of the ERSPC.[19] They found no significant differences in tumor volume, upgrading, capsular penetration, positive surgical margins or biochemical recurrence between the groups. The median follow-up in this study was 6 years after diagnosis.
An important limitation of the study design in all observational studies on immediate versus deferred RP is the selection process that occurs at several steps. First, men on surveillance in this study have lower risk features at the time of diagnosis compared to men who undergo immediate prostatectomy, eg. in our study significantly lower PSA (p<0.001), less clinical stage T2 disease (p<0.001), and a lower ratio of positive biopsy cores (p<0.001). Second, men who ultimately undergo radical prostatectomy after a period of surveillance constitute a subgroup with higher-risk cancer within the group of low-risk cancer of whom the majority remain on surveillance without any trigger for conversion.
One explanation for a higher rate of upgrading in the delayed RP group is that increases in grade during surveillance are a common reason for conversion to active treatment. Indeed, biopsy progression was the reason for discontinuing surveillance in 24% of men in our cohort, with the remainder discontinuing due to PSA progression (52%), patient preference (20%) or other reasons (3%).[12] Thus, men on surveillance who receive delayed prostatectomy represent a selection of men with higher risk features within the group of men with low-risk prostate cancer on AS. Notably, Tosoian et al. recently compared 3788 men undergoing immediate prostatectomy at Johns Hopkins versus 89 men who had delayed prostatectomy after active surveillance (at a median of 2 years, IQR 1-4).[20] The delayed surgery group was selected to represent the entire cohort, including 36% with disease reclassification leading to treatment and the remaining 64% without a protocol-based trigger for intervention. The risk of adverse pathology was not significantly different between the groups (multivariable OR 1.33, 0.82-2.79, p=0.13), although the sample size was small.
Another potential source of selection bias in studies of delayed radical prostatectomy is that men who progress to incurable disease during surveillance in the absence of definitive treatment are not included. However, the 7-year PCa-specific cumulative mortality in our study was similarly low for men who received immediate prostatectomy or active surveillance regardless of later intervention, suggesting that this was not the case.
It is important to note that data on biochemical recurrence were not available and the use of secondary radiation therapy in our study was not per protocol, but was performed at the discretion of the individual provider and the patient. Due to the inherent subjectivity, we recommend caution when evaluating this endpoint. In addition, since this was a nationwide registry with data from all health care providers in Sweden, uniform criteria were not used to initiate active surveillance or curative treatment, nor was there a standardized follow-up protocol. Data on follow-up biopsies are not available in this dataset, precluding an analysis of the impact of more stringent followup on the outcomes of delayed treatment. Finally, in order to examine long-term treatment outcomes we included men treated between 1997-2007, and there has been a significant stage migration over time also within the low-risk group. Thus, our results for deferred prostatectomy are a worst-case scenario compared to per- protocol selection and follow-up of surveillance, as is currently implemented in trials such as the Study on Active Monitoring in Sweden (SAMS) and PRIAS.[21, 22] Finally, we did not compare quality of life between the early and delayed groups, since our dataset did not include information on this topic.
Strengths of the study include our nationwide population-based dataset, resulting in a large group of men who underwent delayed RP and greater generalizability than many previous studies reporting on single institutions. Also, we examined treatment delays of >2 years, which is longer than any of the previous papers on this topic and the median overall follow-up was 8 years. Although this is substantially longer than previous studies on surgical delay and on active surveillance,[23] at least 15 years of follow-up are needed for an optimal assessment of prostate cancer mortality in men with low risk disease so these results should be interpreted with caution. In addition, PCBaSe contains extensive and complete data on important confounders such as comorbidity and educational level which were not considered in most other studies.[23] Indeed, we did find a significant association between low education with adverse pathology and prostate cancer death after radical prostatectomy. Since the majority of men on active surveillance remain free from intervention at 5 years,[12, 24, 25] the impact of longer delays on prostatectomy outcomes is an important but understudied issue. Our study also offers methodological advantages over previous studies from our group and others comparing immediate versus delayed treatment between men matched on a limited number of features.[26, 27] Finally, we examined multiple endpoints ranging from pathology features to cancer-specific survival which was not included in previous studies, and performed an “intent-to-treat” comparison of men who received primary prostatectomy versus active surveillance to place these data into a larger context. Given the very low rate of disease-specific mortality among men with favorable-risk prostate cancer, very large datasets will be necessary to compare prostate cancer mortality based upon initial management strategy.
In conclusion, delayed RP after more than two years was associated with a significantly higher risk of adverse pathology and salvage radiation, and a non-significant increase in risk of death from prostate cancer highlighting the trade-offs by deferring initial curative therapy. However, men who receive delayed RP are a selection of higher-risk cancer among men with low-risk cancer on surveillance and since our population-based study was based on real-life data, these men represent a worst case scenario compared to men who are selected and followed per protocol. Importantly, there was no difference in 7-year prostate cancer mortality between men with localized Gleason 6 disease on surveillance compared to men who underwent immediate prostatectomy indicating that active surveillance is a viable management strategy for men with low-risk prostate cancer.
Supplementary Material
Acknowledgement
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden (NPCR) steering group: Pär Stattin (chairman), Anders Widmark, Camilla Thellenberg, Ove Andrén, Anna Bill Axelson, Ann-Sofi Fransson, Magnus Törnblom, Stefan Carlsson, Marie Hjälm-Eriksson, Bill Pettersson, David Robinson, Mats Andén, Jan-Erik Damber, Jonas Hugosson, Ingela Franck Lissbrant, Maria Nyberg, Göran Ahlgren, Ola Bratt, René Blom, Lars Egevad, Calle Walller, Olof Akre, Per Fransson, Eva Johansson, Fredrik Sandin, Hans Garmo, Mats Lambe, Karin Hellström.
Funding: This work was supported by The Swedish Research Council 825-2012-5047 and The Swedish Cancer Foundation 11 0471, Västerbotten County Council, the Lion's Cancer Research Foundation at Umeå University, the Swedish Cancer Foundation (2012/475) to OB, the Louis Feil Charitable Lead Trust to SL, the Laura and Isaac Perlmutter Cancer Center at NYU Langone Medical Center to SL, and the National Institutes of Health (Award Number K07CA178258) to SL. The content is solely the responsibility of the authors and does not represent the official views of the NIH.
Abbreviations
- AS
active surveillance
- PCa
Prostate cancer
- RP
Radical prostatectomy
- NPCR
National Prostate Cancer Register
Footnotes
Prior presentations: Abstract presented at the 2014 American Urological Association meeting
COI: PS reports honoraria from Ferring and Astra Zeneca.
Contributor Information
Stacy Loeb, New York University and Manhattan Veterans Affairs Medical Center, NY, NY, USA.
Yasin Folkvaljon, Regional Cancer Centre Uppsala Örebro, Uppsala University Hospital, Uppsala, Sweden.
David Robinson, Department of Surgery and Perioperative Sciences, Urology and Andrology, Umeå University Hospital, Umeå, Sweden; Department of Urology, Ryhov County Hospital, Jönköping, Sweden.
Danil V. Makarov, New York University and Manhattan Veterans Affairs Medical Center, NY, NY, USA
Ola Bratt, Department of Urology, CamPARI Clinic, Addenbrooke's Hospital, Cambridge, UK and Department of Translational Sciences, Lund University, Lund, Sweden.
Hans Garmo, Faculty of Life Sciences and Medicine, Division of Cancer Studies, King's College London, London, UK.
Pär Stattin, Department of Surgery and Perioperative Sciences, Urology and Andrology, Umea University Hospital, Umea, Sweden, and Department of Surgical Sciences, Uppsala University, Uppsala, Sweden.
References
- 1.Stephenson AJ, Kattan MW, Eastham JA, Bianco FJ, Jr., Yossepowitch O, Vickers AJ, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009;27:4300–5. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Resnick MJ, Koyama T, Fan KH, Albertsen PC, Goodman M, Hamilton AS, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368:436–45. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178:14–9. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990-2013. JAMA. 2015314:80–2. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 5.Loeb S, Berglund A, Stattin P. Population Based Study of Use and Determinants of Active Surveillance and Watchful Waiting for Low and Intermediate Risk Prostate Cancer. J Urol. 2013;1901742-9 doi: 10.1016/j.juro.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 6.Dall'era MA, Albertsen PC, Bangma C, Carroll PR, Carter HB, Cooperberg MR, et al. Active Surveillance for Prostate Cancer: A Systematic Review of the Literature. Eur Urol. 2012;62976-83 doi: 10.1016/j.eururo.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 7.Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–7. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 8.Tosoian JJ, Mamawala M, Epstein JI, Landis P, Wolf S, Trock BJ, et al. Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol. 2015;333379-85 doi: 10.1200/JCO.2015.62.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Hemelrijck M, Wigertz A, Sandin F, Garmo H, Hellstrom K, Fransson P, et al. Cohort Profile: The National Prostate Cancer Register of Sweden and Prostate Cancer data Base Sweden 2.0. Int J Epidemiol. 2013;42956-67 doi: 10.1093/ije/dys068. [DOI] [PubMed] [Google Scholar]
- 10.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;571288-94 doi: 10.1016/j.jclinepi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Sundararajan V, Quan H, Halfon P, Fushimi K, Luthi JC, Burnand B, et al. Cross-national comparative performance of three versions of the ICD-10 Charlson index. Med Care. 2007;45:1210–5. doi: 10.1097/MLR.0b013e3181484347. [DOI] [PubMed] [Google Scholar]
- 12.Loeb S, Folkvaljon Y, Makarov DV, Bratt O, Bill-Axelson A, Stattin P. Five-year Nationwide Follow-up Study of Active Surveillance for Prostate Cancer. Eur Urol. 2015;67233-8 doi: 10.1016/j.eururo.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suardi N, Briganti A, Gallina A, Salonia A, Karakiewicz PI, Capitanio U, et al. Testing the most stringent criteria for selection of candidates for active surveillance in patients with low-risk prostate cancer. BJU Int. 2010;105:1548–52. doi: 10.1111/j.1464-410X.2009.09057.x. [DOI] [PubMed] [Google Scholar]
- 14.Wong LM, Neal DE, Johnston RB, Shah N, Sharma N, Warren AY, et al. International multicentre study examining selection criteria for active surveillance in men undergoing radical prostatectomy. Br J Cancer. 2012;107:1467–73. doi: 10.1038/bjc.2012.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimopoulou C, Ceausu I, Depypere H, Lambrinoudaki I, Mueck A, Perez-Lopez FR, et al. EMAS position statement: Testosterone replacement therapy in the aging male. Maturitas. 2016;8494-9 doi: 10.1016/j.maturitas.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Abern MR, Aronson WJ, Terris MK, Kane CJ, Presti JC, Jr., Amling CL, et al. Delayed radical prostatectomy for intermediate-risk prostate cancer is associated with biochemical recurrence: possible implications for active surveillance from the SEARCH database. Prostate. 2013;73409-17 doi: 10.1002/pros.22582. [DOI] [PubMed] [Google Scholar]
- 17.Andrews SF, Horwitz EM, Feigenberg SJ, Eisenberg DF, Hanlon AL, Uzzo RG, et al. Does a delay in external beam radiation therapy after tissue diagnosis affect outcome for men with prostate carcinoma? Cancer. 2005;104:299–304. doi: 10.1002/cncr.21184. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien D, Loeb S, Carvalhal GF, McGuire BB, Kan D, Hofer MD, et al. Delay of surgery in men with low risk prostate cancer. J Urol. 2011;1852143-7 doi: 10.1016/j.juro.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 19.van den Bergh RC, Steyerberg EW, Khatami A, Aus G, Pihl CG, Wolters T, et al. Is delayed radical prostatectomy in men with low-risk screen-detected prostate cancer associated with a higher risk of unfavorable outcomes? Cancer. 2010;1161281-90 doi: 10.1002/cncr.24882. [DOI] [PubMed] [Google Scholar]
- 20.Cui Y, Zong H, Yan H, Zhang Y. The effect of testosterone replacement therapy on prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2014;17132-43 doi: 10.1038/pcan.2013.60. [DOI] [PubMed] [Google Scholar]
- 21.Bratt O, Carlsson S, Holmberg E, Holmberg L, Johansson E, Josefsson A, et al. The Study of Active Monitoring in Sweden (SAMS): a randomized study comparing two different follow-up schedules for active surveillance of low-risk prostate cancer. Scand J Urol. 2013;47347-55 doi: 10.3109/21681805.2013.813962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bul M, Zhu X, Valdagni R, Pickles T, Kakehi Y, Rannikko A, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol. 2013;63597-603 doi: 10.1016/j.eururo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 23.van den Bergh RC, Albertsen PC, Bangma CH, Freedland SJ, Graefen M, Vickers A, et al. Timing of curative treatment for prostate cancer: a systematic review. Eur Urol. 2013;64:204–15. doi: 10.1016/j.eururo.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tosoian JJ, Trock BJ, Landis P, Feng Z, Epstein JI, Partin AW, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;292185-90 doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 25.Godtman RA, Holmberg E, Khatami A, Stranne J, Hugosson J. Outcome following active surveillance of men with screen-detected prostate cancer. Results from the Goteborg randomised population-based prostate cancer screening trial. Eur Urol. 2013;63101-7 doi: 10.1016/j.eururo.2012.08.066. [DOI] [PubMed] [Google Scholar]
- 26.Holmstrom B, Holmberg E, Egevad L, Adolfsson J, Johansson JE, Hugosson J, et al. Outcome of primary versus deferred radical prostatectomy in the National Prostate Cancer Register of Sweden Follow-Up Study. J Urol. 2010;1841322-7 doi: 10.1016/j.juro.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Warlick C, Trock BJ, Landis P, Epstein JI, Carter HB. Delayed versus immediate surgical intervention and prostate cancer outcome. J Natl Cancer Inst. 2006;98355-7 doi: 10.1093/jnci/djj072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.