Abstract:
ATP-sensitive potassium (KATP) channels link membrane excitability to metabolic state to regulate a series of biological activities including the vascular tone. However, their ability to influence hypertension is controversial. Here we aim to investigate possible alteration of KATP channel in vascular smooth muscles (VSMs) during hypertension development process. In this study, we used 16-week-old spontaneously hypertensive rats (SHRs), 49-week-old SHRs, and their age-matched Wistar-Kyoto rats to study the expression of VSM KATP subunits at the mRNA and protein level and the function of VSM KATP by observing the relaxation reactivity of isolated aorta rings to KATP modulators. We found that the expression of VSM KATP subunits Kir6.1 and sulfonylurea receptor (SUR2B) decreased during hypertension. Moreover, the expression of SUR2B and Kir6.1 in 49-week-old SHRs decreased much more than that in 16-week-old SHRs. Furthermore, the aorta rings of 49-week-old SHRs showed lower reactivity to diazoxide than 16-week-old SHRs. This study suggests that KATP channels in VSM subunits Kir6.1 and SUR2B contribute to modify the functionality of this channel in hypertension with age.
Key Words: KATP channel, hypertension, mitoKATP, diazoxide, in vitro
INTRODUCTION
Hypertension, a worldwide cardiovascular disease affecting more than 30% of the population, is defined as a complex, multifactorial, environmental, quantitative trait under a polygenic control.1 It is known that the contraction and relaxation of vascular smooth muscles (VSMs) are related to membrane potential, which is critically determined by the activity of potassium channel.2 ATP-sensitive potassium (KATP) channels exist in VSM beds and play an important role in regulating vascular tone.3,4 In addition, the channel openers result in the decrease of blood pressure.5,6 Moreover, the channel blockers abolish the pharmacological function of channel openers to lower blood pressure.7,8 The KATP channel is composed of a hetero-octameric complex constituting an inward rectifier potassium channel (Kir6.1 and Kir6.2) and a sulfonylurea receptor (SUR1, SUR2A, and SUR2B), which is characterized by the inhibition of ATP and the activation of MgADP.9 The Kir6.x pore-forming subunit responds to the ATP sensitivity, and the SURx confers to sulfonylurea sensitivity and determines the efficacy of potassium channel openers (KCOs).10 KATP in different tissues is composed of different Kir and SUR subunits that “mix and match.” Sarcolemmal KATP (sarcKATP) channel of VSM are mainly constituted of Kir6.1/SUR2B,11 which are activated by nucleoside diphosphates and are rather insensitive to ATP.
KATP channels act as important metabolic sensors and target the response of VSM to a number of pharmacological and endogenous vasodilators: adenosine, prostacyclin, α-adrenoceptor agonist, nitric oxide, neurotransmitters, calcitonin-gene–related peptide, vasoactive intestinal peptide, angiotensin II, vasopressin, noradrenaline, neuropeptide Y, endothelin, and serotonin.12–14 Therefore, they probably participate in the process of hypertension.
In this study, we used 16-week-old spontaneously hypertensive rats (SHRs), 49-week-old SHRs, and their age-matched Wistar-Kyoto rats (WKY) to study the expression of VSM KATP subunits at the mRNA and protein level and the function of VSM KATP by observing isolated aorta rings' relaxation reactivity to KATP modulators. We aim to clarify the alteration of the expression and the function of KATP channels in hypertension at different age.
MATERIALS AND METHODS
Animals
All experimental animals were purchased from Vital River Laboratories (Beijing, China). Male WKY and SHRs were randomly divided into 4 groups: 16-week-old WKY, 16-week-old SHRs, 49-week-old WKY, and 49-week-old SHRs. Conscious rats were instrumented with tail-cuff system (Kent Scientific) to record the blood pressure. Systolic blood pressure, diastolic blood pressure, and mean blood pressure were measured with the rat tails at 31°C as previously described.15 The investigation conforms to the Guidelines for the Care and Use of Laboratory Animals, and the procedures for care and use of animals were approved by the Ethics Committee of Chinese PLA General Hospital.
Vessel Contractility and Reactivity Measurement
Male rats were intraperitoneally anesthetized with 1% pelltobarbitalum natricum (Solarbo, China) 1 mL/100 g; the aorta was rapidly dissected out, placed in Krebs–Henseleit solution (KHS, in mmol/L: NaCl 115, CaCl2 2.5, KCl 4.6, KH2PO4 1.2, MgSO4·7H2O 1.2, NaHCO3 25, glucose 11.1, with pH of 7.4) at 4°C, and cleaned off the connective tissue and blood vessel endothelium. The isolated arteries were cut 3–4 mm in length and passed through the lumen of the vessel segment by 2 parallel steel triangles: one was fixed to the organ bath and the other connected to a tension transducer (TSD125B; Biopac), which was linked to AcqKnowledge software (MP150; Biopac). Vessel segments were bathed in 15 mL of KHS continuously saturated with a 95% O2–5% CO2 mixture at 37°C. The aortic rings were equilibrated for 90 minutes with 2.0 g of basal tension. Arterial viability was measured using KCl solution (final concentration of 60 mmol/L, the same as below), and arterial maximum contractility was determined using phenylephrine (PE, 1 μmol/L). Endothelial denudation was confirmed by acetylcholine (Ach, 10 μmol/L).16 Diazoxide (10−9 to 10−5 mol/L) or pinacidil (10−9 to 10−5 mol/L) was added to the aorta ring in the plateau phase of PE (1 μmol/L) to describe the concentration–response curves. To study the participation of the specific KATP channel on vasodilator response, the specific KATP channel blocker glibenclamide (Gli) or the mitoKATP channel blocker 5-hydroxydecanoate (5-HD) was added 30 minutes before the concentration–response curves were obtained. Gli (10 μmol/L) was used to block the vasodilator response of pinacidil, and 5-HD (10 μmol/L) was used to block the vasodilator response of diazoxide. All the changes in tension were recorded, and relaxation was expressed as a percent of the precontraction.
Quantitative Relative Real-Time Polymerase Chain Reaction
Total RNA was extracted from aorta arteries with TRIzol agent (Invitrogen, CA), and the concentration was determined by Nano Drop 2000 (Thermo Scientific). Reverse transcription–polymerase chain reaction (PCR) were performed using iScript cDNA Synthesis Kit (Bio-Rad), and quantitative relative real-time PCR was performed with the Power SYBR Green PCR Master Mix Kit (ABI) on ABI prism 7900 instrument, according to the manufacturer's instructions. Primers were based on published researches: (sequence 5′ to 3′) F: CAGAGGTGCTGGACATCACAGAG, R: GGCAACAGTCGGGTAGCCAATC for 36β4; F: GGAGTGCGATACTGGTCCAAACCT, R: CCCGATGCAGAGAACGAGACACT for SUR2A; F: CATAGCTCATCGGGTTCACACCATT, R: GCATCGAGACACAGGTGCTGTTGT for SUR2B; F: AGCTGGCTGCTCTTCGCTATCA, R: CCCTCCAAACCCAATGGTCACT for Kir6.1; and F: CAACGTCGCCCACAAGAACATC, R: CCAGCTGCACAGGAAGGACATG for Kir6.2. Quantitative relative real-time PCR consisted of 2 steps: hold at 95°C for 10 minutes; 40 cycles at 95°C for 15 seconds and at 60°C for 60 seconds. Relative expression was normalized to 36β4.
Western Blot
Total proteins were isolated from aorta tissues with RIPA buffer (Solarbo), and the concentration was measured by BCA protein assay kit (Solarbo). Protein samples (60 μg each well) were loaded on 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto nitrocellulose filter membranes. The membranes was blocked in 5% nonfat milk (BD) for 2 hours, incubated overnight at 4°C in primary antibodies, and then incubated with appropriate secondary antibodies at room temperature for 50 minutes. The primary antibody dilutions were 1:300 for Kir6.1 (ABcam, Britain), 1:500 for Kir6.2 (Alomone, Israel), 1:300 for SUR2B (Santa, Japan), and 1:30,000 for GAPDH (ABcam) antibodies. Signals were detected with the electrochemiluminescence Plus (Applygen, China). All experiments were measured in triplicate.
Statistical Analysis
Statistical analysis was performed with SPSS 17.0 and Graph Pad Prism 5.0 software. All data showed in figures were expressed as mean ± standard error of the mean. The 2 groups' unpaired data were analyzed with Student's t-tests. Differences in means among groups and treatments were compared using repeated-measure analysis of variance, when appropriate. Differences with 2-tailed P values <0.05 were considered to be statistically significant.
RESULTS
Body Weight and Blood Pressure
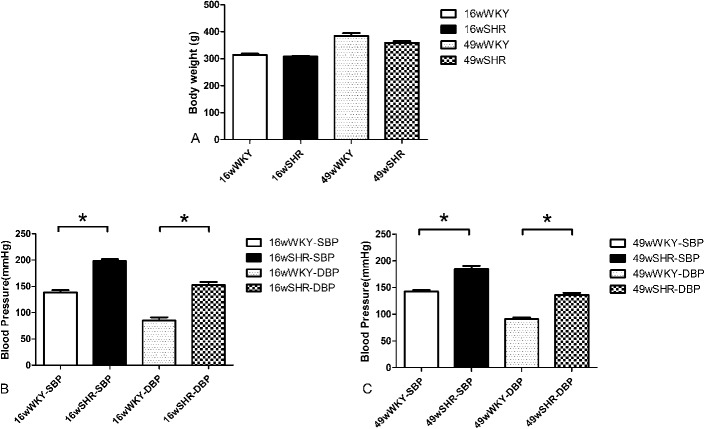
Figure 1 shows the mean body weight and blood pressure of all groups. There was no difference in body weight between the 16-week-old SHRs (314.2 ± 4.36 g) and 16-week-old WKY (307.6 ± 2.41 g) (P > 0.05) and also for the 49-week-old SHRs (384.3 ± 10.69 g) versus 49-week-old WKY (358.5 ± 6.74 g) (P > 0.05) (Fig. 1A).
FIGURE 1.
Body weight and blood pressure of rats. A, Body weight, (B) systolic blood pressure (SBP), and (C) diastolic blood pressure (DBP). Data shown are the means ± standard error of the mean of 6 separate experiments. *P < 0.05 for the SHR group compared with the respective WKY controls.
Blood pressure was increased in SHR groups when compared with the respective controls (P < 0.01). However, no difference in blood pressure level was observed between the 16-week-old (198.2 ± 3.86/152.3 ± 6.02 mm Hg) and 49-week-old SHRs (184.8 ± 5.89/136.0 ± 4.08 mm Hg) (Figs. 1B, C).
mRNA Expression of Kir6.1 and SUR2B Is Reduced in Aorta SM From SHRs
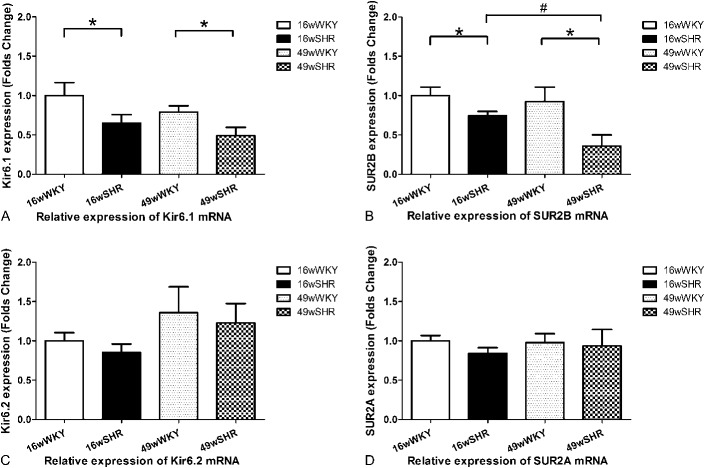
In aorta smooth muscle, the mRNA expression of Kir6.1 subunits was decreased by 35% in 16-week-old SHRs (0.65 ± 0.11) when compared with 16-week-old controls (1.00 ± 0.16, P < 0.05). Moreover, it was decreased to an even lower extent of 38% in 49-week-old SHRs (0.49 ± 0.11) compared with 49-week-old controls (0.79 ± 0.08, P < 0.05). Furthermore, compared with 16-week-old SHRs, there was a reduction of about 25% in 49-week-old SHRs. Similarly, VSM mRNA expression of KATP SUR2B subunits showed a 26% reduction in 16-week-old SHRs (0.74 ± 0.06) when compared with 16-week-old controls (1.00 ± 0.11, P < 0.05), and a further reduction of 61% in 49-week-old SHRs (0.36 ± 0.14) compared with 49-week-old controls (0.92 ± 0.18, P < 0.05). Moreover, SUR2B subunits were decreased by 51% in 49-week-old SHRs when compared with 16-week-old SHRs (P < 0.05). However, there were no differences in the Kir6.2 and SUR2A of the mRNA expression between 16-week-old and 49-week-old SHRs when compared with their respective controls (Figs. 2A–D).
FIGURE 2.
Relative expression of Kir6.1, Kir6.2, SUR2A, and SUR2B mRNA. A, Kir6.1, (B) SUR2B, (C) Kir6.2, and (D) SUR2A. Data shown are the means ± standard error of the mean of 6 separate experiments. *P < 0.05 for the SHR group compared with the respective WKY controls. #P < 0.05 for the 49-week-old SHR group compared with the 16-week-old SHR group.
Protein Expression of Kir6.1 and SUR2B Is Reduced in Aorta SM From SHRs
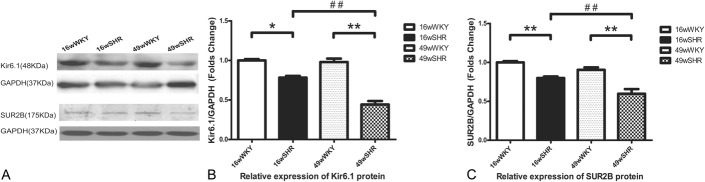
To examine whether the KATP expression is altered in the SHRs, we used Western blotting to measure the protein expression. Consistent with the mRNA data above, in both 16-week-old SHRs and 49-week-old SHRs, the protein expression of Kir6.1 and SUR2B was decreased than the respective controls. For Kir6.1, there was an almost 22% reduction in 16-week-old SHRs (0.78 ± 0.02, P < 0.05) and about 54% reduction in 49-week-old SHRs (0.45 ± 0.05, P < 0.01). For SUR2B, 20% reduction in 16-week-old SHRs (0.80 ± 0.02) and 34% reduction in 49-week-old SHRs (0.66 ± 0.06, P < 0.01) was observed. Moreover, there was a significant difference in Kir6.1 and SUR2B between 16-week-old and 49-week-old SHRs. Compared with 16-week-old SHRs, Kir6.1 decreased 44% and SUR2B reduced 25% in 49-week-old SHRs (P < 0.01) (Figs. 3A–C).
FIGURE 3.
Representative Western blots for Kir6.1 and SUR2B expression. A, Western blot of Kir6.1, SUR2B, and GAPDH. B, Analysis of relative gray intensity of Kir6.1. C, Analysis of relative gray intensity of SUR2B. Results are expressed as the ratio between the signal for the Kir6.1/SUR2B expression and the signal for GAPDH. Data shown are the means ± standard error of the mean of 3 separate experiments. **P < 0.01 for the SHR group compared with the respective WKY controls. ##P < 0.01 for the 49-week-old SHR group compared with the 16-week-old SHR group.
Vascular Reactivity to Diazoxide Is Reduced in Aorta SHRs
To evaluate KATP channel functional condition, tissue bath myography was determined in this study. Both diazoxide and pinacidil evoked a concentration-dependent relaxation response in PE-preconstricted aortic segments isolated from either WKY or SHR groups. The relaxation response of diazoxide was apparently lower than that of pinacidil. No difference was observed in 16-week-old SHR aorta response to diazoxide compared with 16-week-old controls. However, diazoxide caused a marked lower relaxation in 49-week-old SHRs compared with 49-week-old WKY at concentrations of 10−6 mol/L (12.06 ± 0.61 vs. 20.87 ± 2.92, P < 0.05) and 10−5 mol/L (16.82 ± 0.26 vs. 48.72 ± 4.31, P < 0.001). The area under curve (AUC) of diazoxide response of 49-week-old SHRs decreased when compared with 49-week-old WKY (P < 0.05). Furthermore, the AUC of diazoxide response of 49-week-old SHRs also showed a greater decrease than 16-week-old SHRs (P < 0.05). MitoKATP channel inhibitor 5-HD blocked diazoxide-induced relaxation to a similar extent in SHRs and normotensive rats. Thus, the delta area under curve (dAUC) of 5-HD ranged from 13.14% to 16.50%, and there was no statistical differences in both strains (Figs. 4E, G).
FIGURE 4.
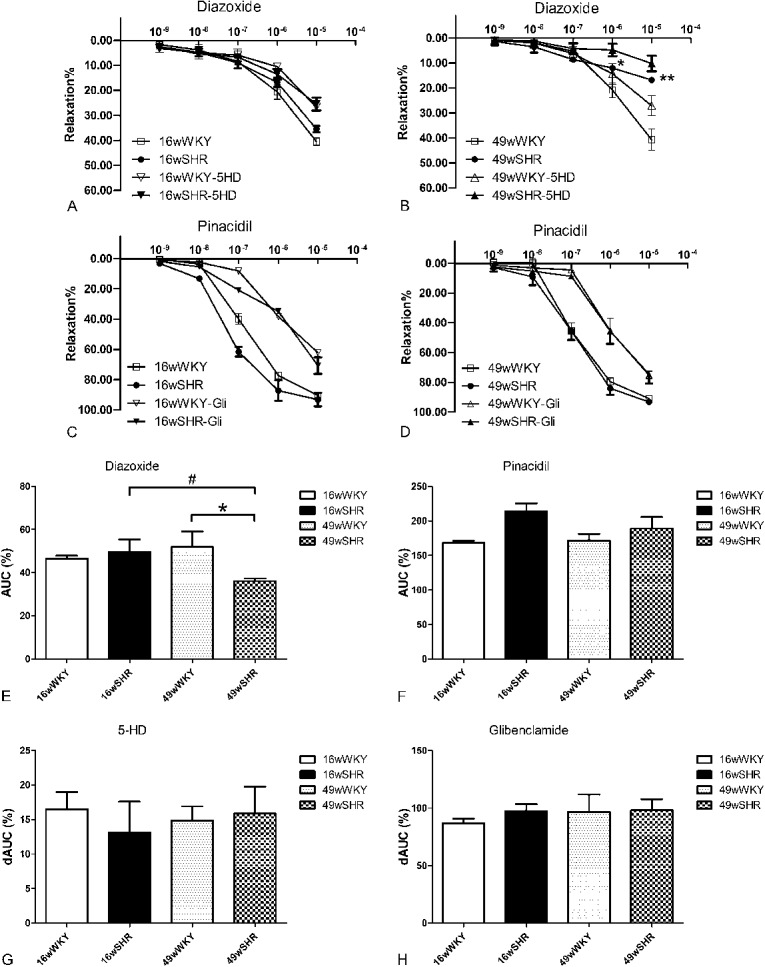
Vascular reactivity to KATP channel openers and blocker. Aorta rings were saturated in KHS and diazoxide or pinacidil were accumulatively added (10−9, 10−8, 10−7, 10−6, 10−5 mol/L). A, Concentration–relaxation curves of diazoxide in the presence or absence of 5-HD in the 16-week-old WKY and 16-week-old SHR group. B, Concentration–relaxation curves of diazoxide in the presence or absence of 5-HD in 49-week-old WKY and 49-week-old SHR group. C, Concentration–relaxation curves of pinacidil in the presence or absence of Gli in 16-week-old WKY and 16-week-old SHR group. D, Concentration–relaxation curves of pinacidil in the presence or absence of Gli in 49-week-old WKY and 49-week-old SHR group. E, AUC for diazoxide. F, AUC for pinacidil. G, dAUC is expressed as the difference between AUC for diazoxide and corresponding AUC for aortic segments in the presence of 5-HD; H, dAUC is expressed as the difference between AUC to pinacidil and the corresponding AUC for aortic segments in the presence of Gli. Results are expressed as percent of previous contraction with PE. Data shown are the means ± standard error of the mean of 4–8 separate experiments. *P < 0.05, **P < 0.01 for the SHR group compared with the respective WKY controls; #P < 0.05 for the 49-week-old SHR group compared with the 16-week-old SHR group.
Pinacidil elicited similar concentration-dependent relaxation curves in denuded aorta segments from SHRs and normotensive rats (both 16-week-old and 49-weekold SHRs). The AUC of pinacidil showed no difference in both strains. Furthermore, the pinacidil-induced relaxation was abolished by the KATP blocker Gli (10 μmol/L) to a similar extent. Thus, the dAUC of Gli kept unchanged in both strains about 86.65%–98.11% (Figs. 4F, H).
DISCUSSION
In this study, we present the following findings: (1) At the mRNA and protein level, expression of Kir6.1 and SUR2B is reduced in the aorta from 16-week-old and 49-week-old SHRs. (2) Moreover, the expression of Kir6.1 and SUR2B in 49-week-old SHRs decreases much more than that in 16-week-old SHRs. (3) The vasodilator response to diazoxide decreases in 49-week-old SHRs, although this trend of decline is not observed in 16-week-old SHRs. (4) The vasodilator response to pinacidil remains unchanged, and its inhibition by Gli is also similar in both strains of WKY and SHRs.
KATP channel is a tetrameric complex of Kir and SUR. In rat aorta, KATP channel has been demonstrated to be Kir6.1 and SUR2B.11,17 In this study, we found that at the mRNA and protein level, expression of Kir6.1 and SUR2B is reduced in the aorta for 16-week-old and 49-week-old SHRs, which is consistent with previous studies.18,19 However, in this study, we found that the protein expression of Kir6.1 and SUR2B in 49-week-old SHRs showed a much larger decrease than that in 16-week-old SHRs, although, the blood pressure remained unchanged between 49-week-old and 16-week-old SHRs. This is the first time we reported that the expression of Kir6.1 and SUR2B was reduced with age in SHRs. Although this different expression of VMS KATP channel subunits did not directly relate to the regulating of blood pressure, it probably affected other functions, such as the channel subunits affinity and the channel gating-dynamic, etc.
KATP channels act as targets of many vasoactive molecules and are widely distributed in various tissues and cells with different subunits and participate in both physiological and pathophysiological conditions including insulin resistance, hyperglycemia, hypoglycemia, hypoxia, and ischemia.20 Activation of KATP channel in VSM leads to a diminution of intracellular Ca2+ levels and an increase in vascular diameter, and thus lowers the vascular resistance.3 In this study, we evaluated the vasorelaxant response to the KCOs pinacidil and diazoxide in thoracic aorta from 16-week-old and 49-week-old SHRs. We found that pinacidil elicited similar concentration-dependent relaxation curve in both SHRs and WKY, and the relaxation was abolished by the KATP blocker Gli to a similar extent. However, the relaxant response to diazoxide was evidently declined in 49-week-old SHRs. 5-HD abolished the diazoxide-induced relaxation to a similar extent. These results indicate that the response to pinacidil, Gli, and 5-HD seems to be preserved, whereas the response to diazoxide diminishes in 49-week-old SHRs.
SUR2B subunit is mainly responsible for channel affinity with KATP channel openers and blockers.21,22 The SUR2B possesses 3 transmembrane domains (TMD0, TMD1, and TMD2), 2 cytoplasmic nucleotide binding domains (NBD1 and NBD2), and the Walker A, Walker B, and Linker L consensus sequences.23 It functions as a regulatory subunit to mediate gating of the Kir6.1 pore by sulfonylurea drugs, such as Gli. Uhde et al identified 2 regions, KCOI and KCOII, in rat SUR2B. Region KCOI was located from Thr1059 to Leu1087 and region KCOII was located from Arg1218 to Asn1320.24 The simultaneous presence of 2 regions was necessary for high-affinity binding of SUR2B, suggesting that the opener site could be made up of association of both regions. The downregulation of SUR2B expression in hypertension could directly affect the channel open state, gating regulation, and the affinity with modulators.
KCOs exhibit an extreme chemical diversity and comprise a number of different structural classes such as the benzopyrans (cromakalim), cyanoguanidines (pinacidil), benzothiadiazines (diazoxide), and nicotinamides (nicorandil).25,26 Diazoxide and pinacidil have been recognized as the first-generation KATP channel modulators, and both can act on Kir6.1/SUR2B. Basically, diazoxide activates mitoKATP channel, and pinacidil nonselectively activates both mitoKATP and sarcKATP channels. The potency of pinacidil is much stronger than diazoxide. Diazoxide activates the channel in a concentration-dependent manner with a half-maximal effective concentration of 30–60 μmol, but pinacidil has a half-maximal effective concentration of 2 μmol.27 Notably, diazoxide possesses a high affinity with SUR2B in the cardiovascular system, SUR2B (dissociation constant, Kd = 18 μmol) > SUR2A (Kd = 76 μmol).28 Researchers have also found that the transmembrane helix 16 and 17 in TMD2 and the section of the cytosolic loop linking helix 13 and 14 of SUR2B are required for the action of pinacidil, levocromakalin, and P1075, rather than diazoxide.24,29 Furthermore, the present regions such as TMD1, TMD2, and NBD1 cannot totally explain the binding site of diazoxide. Although the detailed binding mechanism remains to be studied, it is clear that diazoxide has a binding site distinct from all other openers.23,30
Taking the above discussion into account, the reason that the response to diazoxide diminished whereas the response to pinacidil was preserved may be explained as follows. First, diazoxide shows higher potency and efficacy to open mitoKATP than pinacidil.31–33 The expression of Kir6.1, which is also a subunit of mitoKATP in 49-week-old SHRs decreases much more than that in 16-week-old SHRs; hence, the response to diazoxide diminished in 49-week-old SHRs. Second, both diazoxide and pinacidil are able to bind to SUR2B and activate KATP channel. Pinacidil is reported to binding to TMD2 domains of SUR2B. Different from pinacidil, diazoxide is potent and has a more complex binding site in which SUR2B C-terminal domains are involved.34 Furthermore, diazoxide has a 4-fold higher affinity for SUR2B than SUR2A.35,36 Therefore, the much more decreased expression of SUR2B in 49-week-old SHRs may be another reason. Third, opening sarcKATP channel plays a much more important role in the vasodilation than mitoKATP.37,38 Compared with diazoxide, pinacidil activates sarcKATP channel in addition. The function of mitoKATP channel is mainly based on the modulation of mitochondrial function, including changes in mitochondrial matrix volume, mitochondrial potential, and oxygen consumption.27 In this study, we also found that pinacidil induced a stronger relaxation than diazoxide. Although the mitoKATP decreased in 49-week-old SHRs, the response of pinacidil to sarcKATP channel seemed to be preserved in 49 weeks.
In brief, this study found that (1) the expression of Kir6.1 and SUR2B reduced in SHRs, and the decrease in 49-week-old SHRs was much more than that in 16-week-old SHRs. (2) The response to diazoxide diminished, whereas the response to pinacidil, Gli, and 5-HD seems to be preserved in 49-week-old SHRs. Although the detailed mechanisms still need to be clarified, this study demonstrated that the decreased expression of the VSM KATP subunits Kir6.1 or SUR2B correlate with the functional changes of KATP channel in hypertension with age.
Footnotes
Supported by the grant from the National Natural Science Foundation of China (81070200, 81570349).
The authors report no conflicts of interest.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen LJ, Wu TP, Yang JJ, et al. Correction of vascular hypercontractility in spontaneously hypertensive rats using shRNAs-induced delta protein kinase C gene silencing. Biomed Res Int. 2013;718:401–407. [DOI] [PubMed] [Google Scholar]
- 3.Standen NB, Quayle JM, Davies NW, et al. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–180. [DOI] [PubMed] [Google Scholar]
- 4.Seino S, Miki T. Physiological and pathophysiological roles of atp-sensitive k channels. Prog Biophys Mol Biol. 2003;81:133–176. [DOI] [PubMed] [Google Scholar]
- 5.Coetzee WA. Multiplicity of effectors of the cardioprotective agent, diazoxide. Pharmacol Ther. 2013;140:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan Z, Huang J, Cui W, et al. Targeting hypertension with a new adenosine triphosphate-sensitive potassium channel opener iptakalim. J Cardiovasc Pharmacol. 2010;56:215–228. [DOI] [PubMed] [Google Scholar]
- 7.Kamata Y, Fujita T, Kato T, et al. An ATP-sensitive potassium channel blocker suppresses sodium-induced hypertension through increased secretion of urinary kallikrein. Hypertens Res. 2009;32:220–226. [DOI] [PubMed] [Google Scholar]
- 8.Dudek M, Razny K, Bilska-Wilkosz A, et al. Hypotensive effect of alpha-lipoic acid after a single administration in rats. Anatol J Cardiol. 2015; 10.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flagg TP, Kurata HT, Masia R, et al. Differential structure of atrial and ventricular KATP: atrial KATP channels require SUR1. Circ Res. 2008;103:1458–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Wet H, Proks P. Molecular action of sulphonylureas on KATP channels: a real partnership between drugs and nucleotides. Biochem Soc Trans. 2015;43:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inagaki N, Gonoi T, Clement JP, et al. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni-Chitnis M, Njie-Mbye YF, Mitchell L, et al. Inhibitory action of novel hydrogen sulfide donors on bovine isolated posterior ciliary arteries. Exp Eye Res. 2015;134:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Han W, Zhang Y, et al. The molecular pathway of ATP-sensitive potassium channel in endothelial cells for mediating arteriole relaxation. Life Sci. 2015;137:164–169. [DOI] [PubMed] [Google Scholar]
- 14.Brayden JE. Functional roles of KATP channels in vascular smooth muscle. Clin Exp Pharmacol Physiol. 2002;29:312–316. [DOI] [PubMed] [Google Scholar]
- 15.Li A, Knutsen RH, Zhang H, et al. Hypotension due to Kir6.1 gain-of-function in vascular smooth muscle. J Am Heart Assoc. 2013;2:e000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belin de Chantemele EJ, Muta K, Mintz J, et al. Protein tyrosine phosphatase 1B, a major regulator of leptin-mediated control of cardiovascular function. Circulation. 2009;120:753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flagg TP, Enkvetchakul D, Koster JC, et al. Muscle KATP channels: recent insights to energy sensing and myoprotection. Physiol Rev. 2010;90:799–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shih H, Lee B, Lee RJ, et al. The aging heart and post-infarction left ventricular remodeling. J Am Coll Cardiol. 2011;57:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xi Q, Cheranov SY, Jaggar JH. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Circ Res. 2005;97:354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minami K, Miki T, Kadowaki T, et al. Roles of ATP-sensitive K channels as metabolic sensors-studies of Kir6.x null mice. Diabetes. 2004;53(suppl 3):167–180. [DOI] [PubMed] [Google Scholar]
- 21.de Araujo ED, Alvarez CP, Lopez-Alonso JP, et al. Phosphorylation-dependent changes in nucleotide binding, conformation, and dynamics of the first nucleotide binding domain (NBD1) of the sulfonylurea receptor 2B (SUR2B). J Biol Chem. 2015;290:22699–22714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li CG, Cui WY, Wang H. Sensitivity of KATP channels to cellular metabolic disorders and the underlying structural basis. Acta Pharmacol Sin. 2016;37:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babenko AP, Gonzalez G, Bryan J. Pharmaco-topology of sulfonylurea receptors. Separate domains of the regulatory subunits of K(ATP) channel isoforms are required for selective interaction with K(+) channel openers. J Biol Chem. 2000;275:717–720. [DOI] [PubMed] [Google Scholar]
- 24.Uhde I, Toman A, Gross I, et al. Identification of the potassium channel opener site on sulfonylurea receptors. J Biol Chem. 1999;274:28079–28082. [DOI] [PubMed] [Google Scholar]
- 25.Mannhold R. KATP channel openers: structure-activity relationships and therapeutic potential. Med Res Rev. 2004;24:213–266. [DOI] [PubMed] [Google Scholar]
- 26.Bouider N, Fhayli W, Ghandour Z, et al. Design and synthesis of new potassium channel activators derived from the ring opening of diazoxide: study of their vasodilatory effect, stimulation of elastin synthesis and inhibitory effect on insulin release. Bioorg Med Chem. 2015;23:1735–1746. [DOI] [PubMed] [Google Scholar]
- 27.Foster MN, Coetzee WA. KATP channels in the cardiovascular system. Physiol Rev. 2016;96:177–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwanstecher M, Sieverding C, Dorschner H, et al. Potassium channel openers require ATP to bind to and act through sulfonylurea receptors. EMBO J. 1998;17:5529–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hambrock A, Loffler-Walz C, Russ U, et al. Characterization of a mutant sulfonylurea receptor SUR2B with high affinity for sulfonylureas and openers: differences in the coupling to Kir6.x subtypes. Mol Pharmacol. 2001;60:190–199. [DOI] [PubMed] [Google Scholar]
- 30.Moreau C, Jacquet H, Prost AL, et al. The molecular basis of the specificity of action of K(ATP) channel openers. EMBO J. 2000;19:6644–6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choma K, Bednarczyk P, Koszela-Piotrowska I, et al. Single channel studies of the ATP-regulated potassium channel in brain mitochondria. J Bioenerg Biomembr. 2009;41:323–334. [DOI] [PubMed] [Google Scholar]
- 32.Fancher IS, Dick GM, Hollander JM. Diabetes mellitus reduces the function and expression of ATP-dependent K(+) channels in cardiac mitochondria. Life Sci. 2013;92:664–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waza AA, Andrabi K, Hussain MU. Protein kinase C (PKC) mediated interaction between conexin43 (Cx43) and K(+)(ATP) channel subunit (Kir6.1) in cardiomyocyte mitochondria: implications in cytoprotection against hypoxia induced cell apoptosis. Cell Signal. 2014;26:1909–1917. [DOI] [PubMed] [Google Scholar]
- 34.Matsuoka T, Matsushita K, Katayama Y, et al. C-terminal tails of sulfonylurea receptors control ADP-induced activation and diazoxide modulation of ATP-sensitive K(+) channels. Circ Res. 2000;87:873–880. [DOI] [PubMed] [Google Scholar]
- 35.Isomoto S, Kondo C, Yamada M, et al. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem. 1996;271:24321–24324. [DOI] [PubMed] [Google Scholar]
- 36.Hambrock A, Loffler-Walz C, Kloor D, et al. ATP-Sensitive K+ channel modulator binding to sulfonylurea receptors SUR2A and SUR2B: opposite effects of MgADP. Mol Pharmacol. 1999;55:832–840. [PubMed] [Google Scholar]
- 37.Aziz Q, Thomas AM, Gomes J, et al. The ATP-sensitive potassium channel subunit, Kir6.1, in vascular smooth muscle plays a major role in blood pressure control. Hypertension. 2014;64:523–529. [DOI] [PubMed] [Google Scholar]
- 38.Antoine MH, Berkenboom G, Fang ZY, et al. Mechanical and ionic response of rat aorta to diazoxide. Eur J Pharmacol. 1992;216:299–306. [DOI] [PubMed] [Google Scholar]