Abstract
In older adults, reduced social participation increases the risk of poor health-related quality of life, increased levels of inflammatory markers and cardiovascular disease, and increased mortality. Older adults frequently present to primary care, which offers the potential to deliver interventions at the point of care to increase social participation. The aim of this prospective study was to identify the key modifiable exposures that were associated with reduced social participation in a primary care population of older adults.
The study was a population-based prospective cohort study. Participants (n = 1991) were those aged ≥65 years who had completed questionnaires at baseline, and 3 and 6-year follow-ups. Generalized linear mixed modeling framework was used to test for associations between exposures and decreasing social participation over 6 years.
At baseline, 44% of participants reported reduced social participation, increasing to 49% and 55% at 3 and 6-year follow-up. Widespread pain and depression had the strongest independent association with reduced social participation over the 6-year follow-up period. The prevalence of reduced social participation for those with widespread pain was 106% (adjusted incidence rate ratio 2.06, 95% confidence interval 1.72, 2.46), higher than for those with no pain. Those with depression had an increased prevalence of 82% (adjusted incidence rate ratio 1.82, 95% confidence interval 1.62, 2.06). These associations persisted in multivariate analysis.
Population ageing will be accompanied by increasing numbers of older adults with pain and depression. Future trials should assess whether screening for widespread pain and depression, and targeting appropriate treatment in primary care, increase social participation in older people.
Keywords: cohort, community dwelling, depression, older adults, pain, primary care, social participation
1. Introduction
In older adults, reduced social participation increases the risk of poor health-related quality of life, increased levels of inflammatory markers and cardiovascular disease, and increased mortality.[1–5] Sixty per cent of adults aged 65 years and over experience reduced social participation, increasing to 80% in those aged 85 years and over.[6] With population ageing, the number of older adults experiencing reduced social participation will increase.
Developing and delivering interventions to increase social participation has been problematic because the key modifiable risk factors associated with reduced social participation have yet to be identified. Older adults frequently present to primary care and this offers the potential to deliver interventions at the point of care to increase social participation.[7] Physicians could assess and target modifiable exposures to increase social participation. These modifiable exposures should ideally be relatively easy to screen for and easy to change.[8]
The aim of this prospective study was to identify the key modifiable exposures of reduced social participation in a primary care population of older adults. The study specifically sought to determine the relative contribution of exposures that were common in older people, that theoretically reduced social participation, could be easily assessed and identified at the point of care (i.e., during consultation to primary care), and are amenable to change.[9–11]
2. Methods
2.1. Design overview, setting, and participants
In the United Kingdom, 98% of the population are registered with a general practice, and these registers provide convenient sampling frames of the local general population and allow survey data to be linked to medical record data.[12] This study draws on a well-established population-based cohort (the North Staffordshire Osteoarthritis Project [NorStOP]) of older people.[13] A total of 4670 individuals aged ≥65 years, registered with 6 general practices in North Staffordshire, United Kingdom, formed the cohort to be followed up 3 (2005) and 6 years (2008) later. The North Staffordshire Local Research Ethics Committee granted approval, and all participants gave written consent to participate.[13]
2.2. Measuring social participation
Social participation was measured using the Keele Assessment of Participation (KAP)[14] at baseline, 3, and 6-year follow-up. This short self-complete instrument was designed to measure social participation from the perspective of the individual in 11 aspects of life (e.g., looking after dependents, attending social activities). Items are phrased to capture performance (“I have”), individual judgment, and the nature and timeliness of participation (“as and when I have wanted”).[14] Responses are on a 5-point ordinal scale (all/most/some/a little/none of the time). The reliability and validity of individual KAP items have been established as adequate for providing estimates of reduced social participation in population studies.[14,15] Participants were classified as experiencing reduced participation in an aspect of life if they did not participate in it “as and when I wanted” for “all” or “most of the time”.[14] The resulting 11 binary items were then summed to give a total score ranging from 0 to 11.[14] Higher KAP scores indicate increasing reduction in social participation. KAP scores were calculated at all 3 time points.
2.3. Exposures of reduced social participation
The exposures included in this study are common in older people, theoretically reduce social participation, and could be easily assessed and identified at the point of contact (i.e., during consultation to primary care and are amenable to change).[9–11] These were musculoskeletal pain, multimorbidity, anxiety, depression, cognitive impairment, body mass index (BMI), demographic (age, sex) and socioeconomic factors, and physical function, and were measured at all 3 time points.
2.3.1. Musculoskeletal pain
Participants were asked “During the last month have you had an ache or pain which has lasted for 1 day or longer?” Those subjects who answered positively were asked to indicate on a 2-view (front and back) blank body manikin the location of their pain. At each time point, participants’ reports of pain were categorized into one of 3 pain groups: “widespread pain,” “some pain,” or “no pain.” Widespread pain was classified according to the American College of Rheumatology (ACR) criteria used in their definition for fibromyalgia,[16] which require pain to be present in the left and right hand sides of the body, above and below the waist, and in the axial skeleton. The “some pain” group were those participants who reported pain that did not satisfy the ACR criteria for widespread pain. These methods to determine the location and extent of pain are commonly used in population-based studies of pain, and have been shown to be valid and reliable.[17,18]
2.3.2. Multimorbidity
A count of morbidities was generated from participants’ general practice records. During consultations, general practitioners used the Read system to code all morbidity.[19] Morbidity data (i.e., symptoms and diseases) in this system are grouped under 18 main chapters (Appendix 1). Consultation for at least 1 morbidity in each of the 18 chapters was collected for 3 time periods; 18 months before baseline, between baseline and 3-year follow-up, and between 3 and 6-year follow-up. Participants with at least 4 (of 18) recorded morbidities within each time period were classified as “multimorbid.”[20]
2.3.3. Anxiety and depression
The Hospital Anxiety and Depression Scale (HADS) assessed anxiety and depression.[21] HADS consists of 14 items each scored on a Likert scale of 0 to 3: 7 items ask about symptoms of anxiety and give a total score of 0 to 21, and 7 items ask about symptoms of depression and give a total score of 0 to 21. Based on their scores separately for anxiety and depression, participants were classified as noncases (score 0–7), possible cases (8–10), and probable cases (11–21).[21]
2.3.4. Cognitive impairment
Participants completed the Cognitive and Alertness Behaviour Subscale of the Functional Limitations Profile.[22] This scale has 10 items that ask about alertness and ability to concentrate. Items are scored as 0 (no cognitive complaint) or 1 (cognitive complaint), with raw scores categorized to indicate “no cognitive complaint” (score 0) and “cognitive complaint” (score >0).
2.3.5. BMI
Calculated from self-reported height and weight (weight [kg]/height [m2]) and categorized into standard BMI groups normal weight (BMI 20–24.9 kg/m2), underweight (BMI <20 kg/m2), overweight (BMI 25–29.9 kg/m2), and obese (BMI ≥30 kg/m2).[23]
2.3.6. Physical function
The physical function is measured using the physical functioning scale of the Medical Outcomes Study Short Form-36. Ten items measured the limitation in the individual's capacity to complete basic tasks such as lifting and walking; scores range from 0 to 100, and higher scores indicate better function.[24]
2.3.7. Socioeconomic characteristics
Current occupational class (nonmanual, manual)[25] and educational attainment (finished education on leaving school; gone onto full-time education including college or university) were assessed.
2.4. Statistical analysis
Analysis was performed in subjects who had complete outcome data at all 3 time points. The baseline characteristics were described overall and stratified by baseline social participation status (no restrictions, one or more restrictions). Between-group differences for age and physical function were tested by a Kruskal–Wallis test, and for all other variables, by a chi-square test. To test for associations between exposures and decreasing social participation over 6 years, a generalized linear mixed modeling (GLMM) framework was used. As restricted social participation data were characterized by excess zeros (at each time point, more than 50% of the sample had a score of 0), a “2-part joint mixed model” was used to model data and provide coefficient estimates (see Appendix 2 for rationale). Coefficient estimates produced by the 2-part joint mixed model were transformed and expressed as incidence rate ratios (IRRs) with 95% confidence intervals (CI) and are interpreted in the same way as in a Poisson mixed model (e.g., exp (0.62) = IRR 1.86 = increased event prevalence of 86% for a particular characteristic to its reference).[26]
Initially, univariate models were constructed to examine the association between exposures and reduced social participation across the 3 time points, adjusting for age and sex. A multivariate model was then constructed that included all exposures except physical function, because it has previously been found to mediate the impact of health conditions on restricted social participation. Physical function was then included in a final multivariate model. Except for sex, occupational class, and educational attainment, all covariates were modeled as time-dependent. The use of a mathematical function to model the development of the KAP score over time always assumes a particular shape such as linear or quadratic term. Time was included in the multivariate model as a categorical variable using appropriate dummy variables and has the added advantage of not having to assume a certain shape of development.
Model goodness of fit was assessed by Akaike Information Criteria (AIC) and Bayesian Information Criteria (BIC), with lower values indicating improved model fit. All analyses were performed using Stata (v 12)[27] Generalized Linear Latent and Mixed Model (GLLAMM) program with adaptive quadrature[28] was used to implement the models.
Sensitivity to participant attrition and missing data was examined by imputing data using Multivariate Imputation Chained Equations (MICEs) using the “countimp” command in R; 100 imputations were used.[29] There were no differences in the strength or significance of associations when the analysis was conducted on the imputed data and compared with the complete case analysis. In this study, we have presented the results from the complete case analysis.
3. Results
3.1. Participant flow
At 3 years, of the 4670 eligible subjects, 436 (9.3%) had died and 505 (10.8%) were no longer at their mailing address (Fig. 1). Of the remaining 3729 subjects, 3066 (82.4%) responded. At 6 years, a further 244 (8.0%) had died, 143 (4.7%) had moved address, leaving 2679 (71.8%) eligible participants. Of those, 1991 (74.3%) responded. Compared with those subjects who had moved address or withdrew from the study (n = 1999), those who were included in the analysis were younger (mean age 71.4 vs 74.3 years; P < 0.001), were more likely to be men (52.1% vs 48.2%), had higher mental and physical health-related quality-of-life scores (mean SF-12 mental component score: 51.6 vs 48.8; P < 0.001; mean SF-12 physical component score: 40.7 vs 37.3; P < 0.001), had higher levels of participation (no restriction: 55.9% vs 38.6%; P < 0.001), and were less likely to be depressed (possible/probable cases of depression: 15.9% vs 26.6%; P < 0.001) or anxious (34.7% vs 39.6%; P < 0.001). There was no difference in pain prevalence (some pain: 46.6% vs 46.9%, widespread pain: 25.2% vs 24.9%; P = 0.97) or education level (gone on to further education: 8.0% vs 9.7%; P = 0.06).
Figure 1.
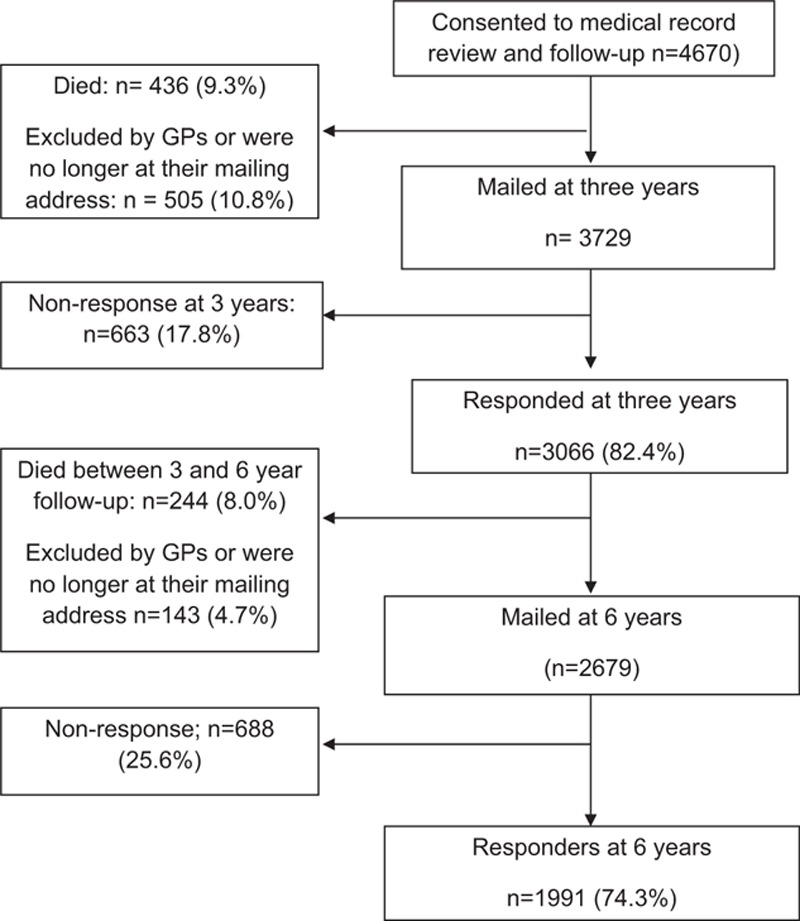
Flow of study participants.
3.2. Subject characteristics
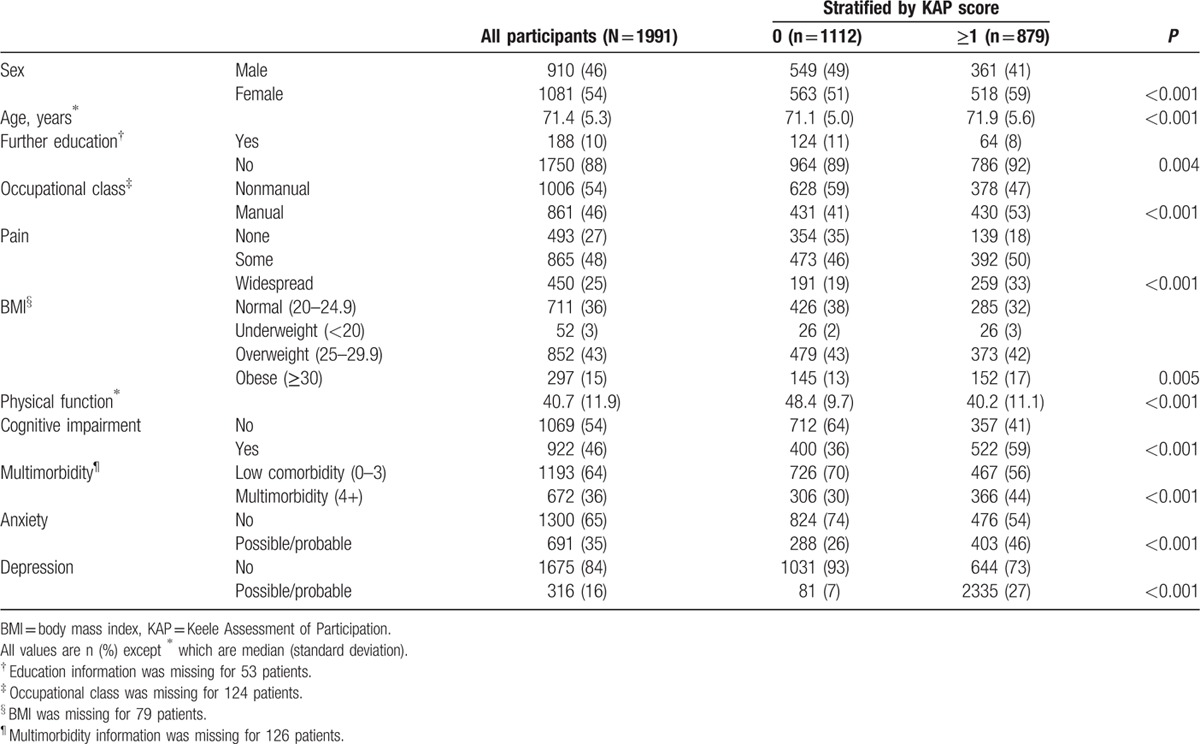
Of the 1991 participants, mean age was 71.4 (standard deviation [SD] 5.3) years, 54% were women, and 88% had finished education on leaving school (Table 1). At baseline, 44% had a KAP score of ≥1 restrictions in social participation; this increased to 49% at 3 years and 55% at 6 years. The prevalence of 1 or more restrictions at baseline was higher in participants who were female, had finished education on leaving school, were in manual occupational class, were outside the normal range of BMI, were classified as having a possible or probable case of anxiety or depression, and had cognitive impairment and multimorbidity (Table 1).
Table 1.
Participant characteristics at baseline for all subjects and stratified by participation restriction at baseline (N = 1991).
3.3. Predictors of restricted social participation
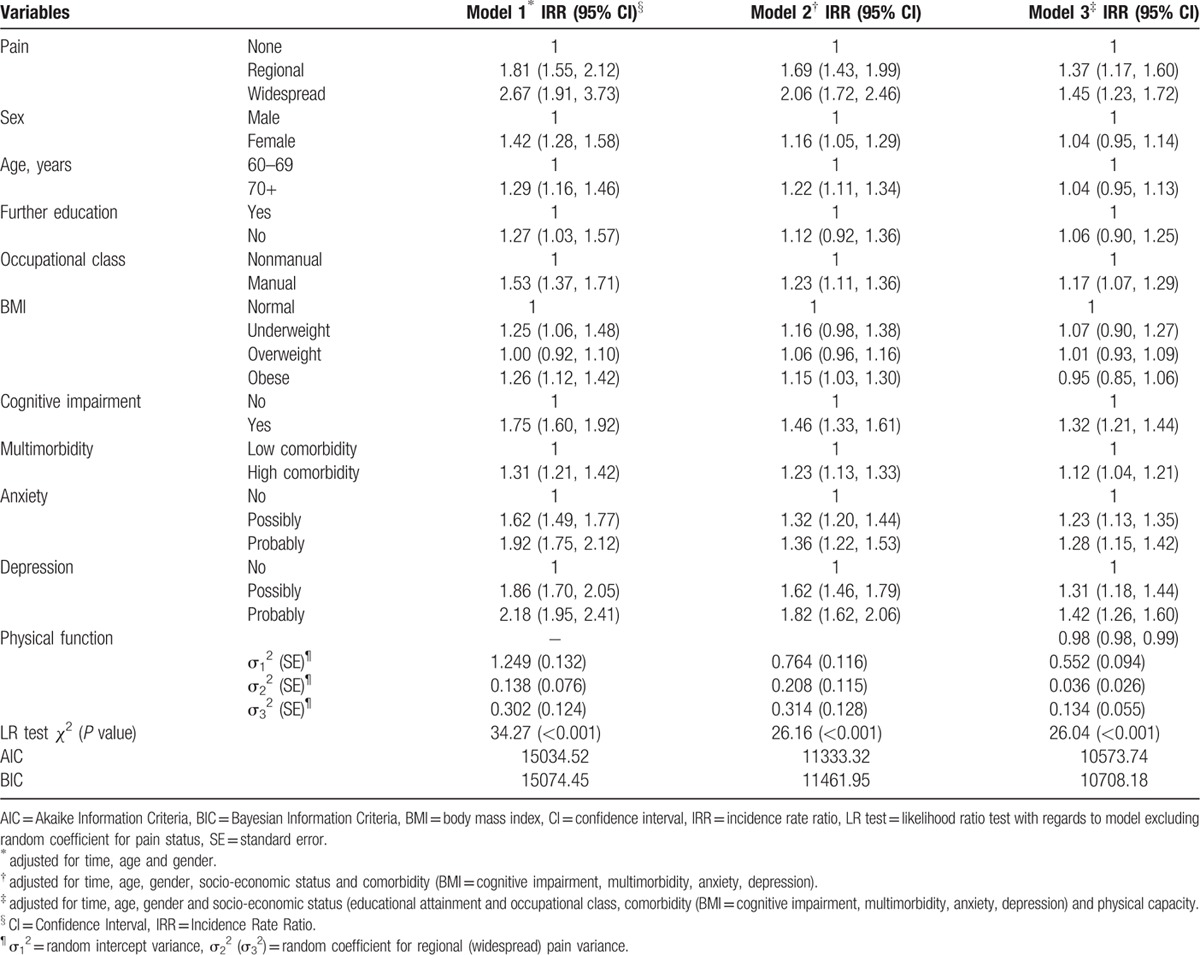
The results from the 2-part joint mixed models are presented in Table 2. After adjusting for age and sex, all exposure variables were associated with increased restriction in social participation. Across the 6 years, the strongest association in the univariate analysis was with widespread pain; the prevalence of increased restriction in those with widespread pain was 167% (IRR 2.67, 95% CI 1.91, 3.73) compared with those with no pain. In a multivariate model (Table 2, model 2), some pain (IRR 1.69, 95% CI 1.43, 1.99), widespread pain (IRR 2.06, 95% CI 1.72, 2.46), anxiety (possible: IRR 1.32, 95% CI 1.20, 1.44; and probable: IRR 1.36, 95% CI 1.22, 1.53), depression (possible: IRR 1.62, 95% CI 1.46, 1.79); and probable: IRR 1.82, 95% CI 1.62, 2.06), cognitive impairment (IRR 1.46, 95% CI 1.33, 1.61), multimorbidity (IRR 1.23, 95% CI 1.13, 1.33), and manual occupation (IRR 1.23, 95% CI 1.11, 1.36) were independently associated with increased restriction in social participation across the 6 years. Finally (Table 2, model 3), physical function was added and was found to be protective against restricted social participation (IRR 0.98, 95% CI 0.98, 0.99).
Table 2.
Risk factors of increasing participation restriction.
4. Discussion
In a large primary care population of older adults, this study has identified widespread pain and depression as the key modifiable factors associated with reduced social participation. The presence of both of these conditions in potential primary care consulters is common; in this study, 1 in 4 adults at baseline had widespread pain. Worldwide changing demographics mean that there will be increasing numbers of older adults with pain and depression. These are identifiable in primary care and can be targeted to increase social participation and prevent subsequent health problems. Case-finding questions for depression are validated[30,31] and could be used by the family physician to detect depression, and manage appropriately.[32] There are barriers, however, to the identification of depression in older people and those with multimorbidities.[33,34] There are evidence-based interventions, both pharmacological and nonpharmacological, for managing depression in primary care, which could be delivered by the family physician or delivered by professionals in the wider primary care team.[35] In addition, sign-posting older people with depression to third-sector services may reduce mental health symptoms and increase social participation.[36]
Assessing pain and its impact, and offering appropriate analgesia and advice about activity, including referral to local activity groups, are simple interventions that could be delivered in primary care. However, if pain persists, our results suggest that targeting physical function may maintain social participation. In additional analysis, the extent of mediation of physical function on the effect of musculoskeletal pain on restricted social participation was calculated using the product of the coefficients method; physical function explained 48.9% of the effect of pain status on restricted social participation. Physiotherapy, exercise classes, and psychological interventions that address the barriers to physical function are example treatments that may improve physical functioning despite the presence of pain.[37] Future research should focus on case-finding for pain and depression in older adults in primary care and examine if it maintains social participation and subsequently prevents physical and mental health problems.[38]
This study has a number of strengths. It was a large population-based study of older community-dwelling individuals. Data were collected prospectively and were allowed the contemporaneous relationship between exposures and participation restriction to be determined across a 6-year period. Although some of the exposures (e.g., depression) may be a consequence of reduced social participation, in additional sensitivity analysis using regression modeling, the patterns of association between baseline exposures and the onset of reduced social participation at 3 years (i.e., moving from no restriction at baseline to 1 or more at 3-year follow-up) were similar to those presented using the 2-part joint mixed model. The KAP is the first instrument to be developed and used to measure social participation restriction in population studies.[39] The statistical approach (i.e., use of 2-part joint mixed model) to modeling produced subject-specific coefficients which are considered to be more accurate estimates of the underlying mechanisms than simpler approaches that do not acknowledge that the equi-dispersion assumption is not met, which may result in biased estimates and misleading conclusions.[26]
Study limitations were as follows: social participation was measured by self-report. Although this is susceptible to measurement error, it is the most appropriate method to capture an individual's performance in social activities.[39] The self-report of impairments and functional problems has been shown to be accurate in older adults,[40] and validated instruments (e.g., Hospital Anxiety and Depression scale[21] and Short Form-36[24]) were used to measure anxiety, depression, and physical function. As is common in population-based longitudinal studies, there was attrition and missing data throughout the 6 years. The extent of missing exposure data during the entire 6 years was small, with typically 3% to 6% of patients missing data on BMI, physical function, multimorbidity, education, and/or occupational class. Our analyses comprised of only those subjects who had participation measured at all 3 time points, which resulted in 1999 subjects being excluded as a result of being lost to follow-up. However, reanalyzing the data after imputation of missing data for such participants who were still alive at the end of 6-year follow-up led to similar conclusions to those based on complete data analysis. The generalizability of the study may be limited by the characteristics of the study sample; the area covered by the study is more deprived on health, education, and employment, but with fewer barriers to housing and services, than England as a whole. The 3-year gap between time points may miss some of the changes in status of predictors and social participation. Finally, there may be other predictors (e.g., social networks) which may be important, but which were not included in this study.
The study identifies that widespread pain and depression are the key modifiable factors associated with reduced social participation. The sheer frequency of widespread pain in older adults means that there will be a large number of primary care consulters at risk of reduced social participation, which puts them more at risk of developing further health problems. Both widespread pain and depression are identifiable in primary care and modifiable. Further studies that screen for widespread pain and/or depression could intervene to potentially increase social participation and prevent further physical and mental health problems in older adults.
Acknowledgment
We would like to thank Professor Peter Croft for his comments on previous drafts of the manuscript.
Appendix 1
During consultations to general practice, general practitioners use the Read system to code all morbidity to eighteen diagnoses codes (http://www.gp-training.net/it/synergy_archive/synergy/readcode.htm). These are (A) infectious/parasitic diseases (b) Neoplams, (c) endocrine/metabolic (D) blood diseases (E) mental disorders, (F) nervous system/senses (G) circulatory system (H) respiratory diseases (J) digestive diseases (K) genito-urinary system (M) skin/subcutaneous tissue (N) musculoskeletal (P) congenital abnormalities (Q) perinatal conditions (R) Ill-defined conditions/working diagnoses (S) Injury/poisoning (T) causes of injury poisoning.
Appendix 2
Participation data was characterised by excess zeros (at each time point, more than 50% of the sample had a score of 0) and hence application of standard Poisson count model would result in violation of model assumption of equidispersion. The alternative is to employ a two-part model such as zero inflated Poisson model,[26] which is an extension of the standard Poisson model, and models simultaneously the odds of an individual having one or more restrictions (Binomial process) and the relative risk of an increase in participation score (Poisson process). This model however produces two sets of coefficient estimates, one for each process, rendering reporting and interpretation of results somewhat cumbersome. An extension is the two-part joint model, where the distribution of the dependent variable is split according to Binomial/Poisson distribution, resulting in only one set of coefficient estimates.
To account for repeated measures design of our study, random effects (assumed to be normally distributed with mean zero and fixed variance) are incorporated within the Binomial/Poisson linear predictor component. This two-part joint mixed model, which is used in this paper, has been advocated to be a better method of analysing data with excess zeros in terms of model fit compared to linear or Poisson mixed models.[27] Initially we model only the random intercept, followed by inclusion of random coefficients for time-dependent covariates; using likelihood ratio tests, significance of variances of random coefficients assigned to time dependent variables was determined. Only the effect of pain status on participation over time varied significantly between individuals, hence random coefficients pertaining to some and widespread pain were the only ones retained in the models. The single coefficients produced by the two-part joint mixed model were transformed and expressed as Incidence Rate Ratios (IRR) with 95% confidence interval (CI) and are interpreted in the same way as a Poisson mixed model (e.g., exp (0.62) = IRR 1.86 = increased prevalence of 1.86).[27]
Footnotes
Abbreviations: 95% CI = 95% confidence interval, ACR = American College of Rheumatology, AIC = Akaike Information Criteria, BIC = Bayesian Information Criteria, BMI = body mass index, exp = exponential, GLLAMM = Generalized Linear Latent and Mixed Models, GLMM = generalized linear mixed modeling, HADS = Hospital Anxiety and Depression Scale, IRR = incidence rate ratio, KAP = Keele Assessment of Participation, MICE = Multivariate Imputation Chained Equations, n = number, SF-12 = Medical Outcomes Study Short-Form 12.
Funding: This study is supported financially by the Medical Research Council, UK (grant code: G9900220 and G0501798) and by the North Staffordshire Primary Care R&D Consortium.
The authors report no conflicts of interest.
References
- 1.Glass TA, Mendes De Leon C, Marottoli RA, et al. Population based study of social and productive activities as predictors of survival among elderly Americans. BMJ 1999; 319:478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thraen-Borowski KM, Trentham-Dietz A, Edwards DF, et al. Dose-response relationships between physical activity, social participation, and health-related quality of life in colorectal cancer survivors. J Cancer Surviv 2013; 7:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamiya Y, Whelan B, Timonen V, et al. The differential impact of subjective and objective aspects of social engagement on cardiovascular risk factors. BMC Geriatr 2010; 10:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dale C, Prieto-Merino D, Kuper H, et al. Modelling the association of disability according to the WHO International Classification of Functioning, Disability and Health (ICF) with mortality in the British Women's Heart and Health Study. J Epidemiol Commun Health 2012; 66:170–175. [DOI] [PubMed] [Google Scholar]
- 5.Julien D, Gauvin L, Richard L, et al. The role of social participation and walking in depression among older adults: results from the VoisiNuAge study. Can J Aging 2013; 32:1–12. [DOI] [PubMed] [Google Scholar]
- 6.Wilkie R, Peat G, Thomas E, et al. The prevalence of person-perceived participation restriction in community-dwelling older adults. Qual Life Res 2006; 15:1471–1479. [DOI] [PubMed] [Google Scholar]
- 7.O’Malley PG. First things first: getting primary care right. JAMA Intern Med 2013; 173:13–14. [DOI] [PubMed] [Google Scholar]
- 8.Mant D. Health checks and screening: what works in general practice? Br J Gen Pract 2014; 64:493–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkie R, Peat G, Thomas E, et al. Factors associated with participation restriction in community-dwelling adults aged 50 years and over. Qual Life Res 2007; 16:1147–1156. [DOI] [PubMed] [Google Scholar]
- 10.Davis AM, Perruccio AV, Ibrahim S, et al. Understanding recovery: changes in the relationships of the International Classification of Functioning (ICF) components over time. Soc Sci Med 2012; 75:1999–2006. [DOI] [PubMed] [Google Scholar]
- 11.Gignac MA, Backman CL, Davis AM, et al. Understanding social role participation: what matters to people with arthritis? J Rheumatol 2008; 35:1655–1663. [PubMed] [Google Scholar]
- 12.Bowling A. Research Methods in Health: Investigating Health and Health Services (3rd edition). Maidenhead: Open University Press; 2009. [Google Scholar]
- 13.Wilkie R, Peat G, Thomas E, et al. The Keele Assessment of Participation: a new instrument to measure participation restriction in population studies. Combined qualitative and quantitative examination of its psychometric properties. Qual Life Res 2005; 14:1889–1899. [DOI] [PubMed] [Google Scholar]
- 14.Thomas E, Wilkie R, Peat G, et al. The North Staffordshire Osteoarthritis Project-NorStOP: prospective, 3-year study of the epidemiology and management of clinical osteoarthritis in a general population of older adults. BMC Musculoskelet Disord 2004; 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermsen LA, Terwee CB, Leone SS, et al. Social participation in older adults with joint pain and comorbidity; testing the measurement properties of the Dutch Keele Assessment of Participation. BMJ Open 2013; 3:e003181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990; 33:160–172. [DOI] [PubMed] [Google Scholar]
- 17.Margolis RB, Chibnall JT, Tait RC. Test–retest reliability in the pain drawing instrument. Pain 1988; 33:49–51. [DOI] [PubMed] [Google Scholar]
- 18.Lacey RJ, Lewis M, Jordan K, et al. Interrater reliability of scoring of pain drawings in a self-report health survey. Spine 2005; 30:E455–E458. [DOI] [PubMed] [Google Scholar]
- 19.NHS Information Authority. The Clinical Terms Version 3 (The Read Codes). Birmingham: NHS Information Authority; 2000. [Google Scholar]
- 20.Kadam UT, Croft PR. North Staffordshire GP Consortium Group. Clinical multimorbidity and physical function in older adults: a record and health status linkage study in general practice. Fam Pract 2007; 24:412–419. [DOI] [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67:361–370. [DOI] [PubMed] [Google Scholar]
- 22.Bergner M, Bobbitt RA, Carter WB, et al. The Sickness Impact Profile: development and final revision of a health status measure. Med Care 1981; 19:787–805. [DOI] [PubMed] [Google Scholar]
- 23.Erens B, Primatesta P, Prior G. (eds). Health Survey for England ‘99. Series HS no. 9. London: The Stationery Office; 2001. [Google Scholar]
- 24.Ware JE, Snow KK, Kosinski M, et al. SF-36® Health Survey: Manual and Interpretation Guide. Rhode Island: QualityMetric Incorporated; 1993. [Google Scholar]
- 25.Office for National Statistics. Standard Occupational Classification 2000, Vol. 2. London: The Coding Index; 2000. [Google Scholar]
- 26.Spriensma AS, Hajos TRS, de Roer MR, et al. A new approach to analyse longitudinal epidemiological data with an excess of zeros. BMC Med Res Methodol 2013; 13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.StataCorp. Stata Statistical Software: Release 12. College Station, Texas: StataCorp LP; 2011. [Google Scholar]
- 28.Rabe-Hesketh S, Skrondal A, Pickles A. Reliable estimation of generalized linear mixed models using adaptive quadrature. Stata J 2002; 2:1–21. [Google Scholar]
- 29.Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed?. Some practical clarifications of multiple imputation theory. Prev Sci 2007; 8:206–213. [DOI] [PubMed] [Google Scholar]
- 30.Whooley MA, Avins AL, Miranda J, et al. Case-finding instruments for depression: two questions are as good as many. J Gen Intern Med 1997; 12:439–445.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003; 41:1284–1292. [DOI] [PubMed] [Google Scholar]
- 32.National Institute for Health and Care Excellence. Depression in adults with a chronic physical health problem: treatment and management. Available from: www.nice.org.uk/guidance/cg91 Accessed September 2014. [Google Scholar]
- 33.Coventry PA, Hays R, Dickens C, et al. Talking about depression: a qualitative study of barriers to managing depression in people with long term conditions in primary care. BMC Fam Pract 2011; 12:10.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chew-Graham C, Kovandžić M, Gask L, et al. Why may older people with depression not present to primary care? Messages from secondary analysis of qualitative data. Health Soc Care Commun 2012; 20:52–60. [DOI] [PubMed] [Google Scholar]
- 35.National Institute for Health and Care Excellence. Osteoarthritis: Care and management in older adults. Available from: http://www.nice.org.uk/guidance/cg177 Accessed September 2014. [Google Scholar]
- 36.Mead N, Lester HE, Gask L, et al. A meta-analysis of befriending in the treatment of depression. Br J Psychiatry 2010; 196:96–101. [DOI] [PubMed] [Google Scholar]
- 37.Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev 2010; 20: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gask L, Bower P, Lamb J, et al. the AMP group. Improving access to psychosocial interventions for common mental health problems in the United Kingdom: review and development of a conceptual model. BMC Health Serv Res 2012; 12:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkie R, Jordan J, Muller S, et al. A review of instruments to measure social function and participation in musculoskeletal populations. Arthr Rheum 2011; 63:S325–S336. [DOI] [PubMed] [Google Scholar]
- 40.Thinggaard M, Jacobsen R, Jeune B, et al. Is the relationship between BMI and mortality increasingly U-shaped with advancing age? A 10-year follow-up of persons aged 70-95 years. J Gerontol A Biol Sci Med Sci 2010; 65:526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]


