Supplemental Digital Content is available in the text
Keywords: C difficile, epidemiology, long-term care, mortality, nursing home, prevalence, risk factors
Abstract
The elderly population is particularly vulnerable to Clostridium difficile infection (CDI), but the epidemiology of CDI in long-term care facilities (LTCFs) is unknown.
We performed a retrospective cohort study and used US 2011 LTCF resident data from the Minimum Data Set 3.0 linked to Medicare claims. We extracted CDI cases based on International Classification of Diseases-9 coding, and compared residents with the diagnosis of CDI to those who did not have a CDI diagnosis during their LTCF stay. We estimated CDI prevalence rates and calculated 3-month mortality rates.
The study population consisted of 2,190,613 admissions (median age 82 years; interquartile range 76–88; female to male ratio 2:1; >80% whites), 45,500 of whom had a CDI diagnosis. The nationwide CDI prevalence rate was 1.85 per 100 LTCF admissions (95% confidence interval [CI] 1.83–1.87). The CDI rate was lower in the South (1.54%; 95% CI 1.51–1.57) and higher in the Northeast (2.29%; 95% CI 2.25–2.33). Older age, white race, presence of a feeding tube, unhealed pressure ulcers, end-stage renal disease, cirrhosis, bowel incontinence, prior tracheostomy, chemotherapy, and chronic obstructive pulmonary disease were independently related to “high risk” for CDI. Residents with a CDI diagnosis were more likely to be admitted to an acute care hospital (40% vs 31%, P < 0.001) and less likely to be discharged to the community (46% vs 54%, P < 0.001) than those not reported with CDI during stay. Importantly, CDI was associated with higher mortality (24.7% vs 18.1%, P = 0.001).
CDI is common among the elderly residents of LTCFs and is associated with significant increase in 3-month mortality. The prevalence is higher in the Northeast and risk stratification can be used in CDI prevention policies.
1. Introduction
Clostridium difficile is the most common cause of acute infectious diarrhea in the hospital setting as well as in long-term care facilities (LTCFs),[1] and disproportionately affects individuals who are >65 years old.[2] Although the incidence of other healthcare-associated infections has declined, the incidence of C difficile infections (CDIs) has increased and is the most common hospital infection, representing 12.1% of healthcare-related infections in 2011.[3] The burden and medical care costs of CDIs have reached historic heights and the estimated number of deaths attributed to CDI, based on multiple cause-of-death mortality data, increased from 3000 deaths per year in 1999 to 2000 to 14,000 in 2006 to 2007 with >90% of deaths among persons aged ≥65 years.[4] In 2009, the annual economic burden of CDI in the United States was $8.2 billion,[5] or 2.3% of all hospital costs. These figures also seem to have increased, with other estimations ranging up to $3.2 billion.[6–8] As a result, the Centers for Disease Control and Prevention has categorized C difficile as 1 of the 3 microorganisms with a “Threat Level of Urgent.”[9]
LTCF residents represent a subset of elderly people particularly vulnerable to CDI. Environmental factors, such as residence in close, shared quarters, shared toilet facilities, and limited ability to isolate infected residents, as well as the intrinsic characteristics of this population, such as the advanced age, immune and physiologic senescence, and multiple comorbid conditions, all contribute to their increased susceptibility to CDI.[10] As data on the epidemiology and risk factors of CDI among elderly residents are limited, we drew on data from Minimum Data Set (MDS) 3.0, the federally mandated nursing home resident assessment questionnaire, linked to Medicare claims to describe the prevalence and correlates of CDI in LTCF residents admitted in 2011.
2. Methods
We used data from the MDS 3.0 linked to Medicare claims to study the epidemiology of C difficile in 2011 among LTCF residents >65 years old. MDS is a federally mandated resident assessment tool (available at: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/index.html).[11–13] Resident data (including demographics, diagnosis, and functioning) are recorded on admission and at least quarterly thereafter by LTCF nurses, with high interobserver reliability.[14] The MDS data are available in a national repository, which we accessed through a data use agreement with the Centers for Medicare & Medicaid Services (DUA #28056) for this project. The Institutional Review Board approved the study (Brown University IRB #1410001151).
We summarized the characteristics of the study population using descriptive methods of data analysis. Resident data were retrieved from the pertinent MDS 3.0 sections to include demographics (Section A), active diagnoses (Section I), special treatments and procedures (Section O), swallowing/nutritional status (Section K), bladder and bowel (Section H), and skin conditions (Section M). We a priori sought to retrieve the following specific information: patient demographics (age, sex, race, length of LTCF stay) and medical comorbidities present (including diabetes mellitus, hypertension, chronic obstructive pulmonary disease [COPD], coronary artery disease, Parkinson disease, stroke, dementia, cirrhosis, end-stage renal disease [ESRD], and prior exposure to chemotherapy and/or irradiation). We also included the presence of a feeding tube, bowel/urine incontinence, prior tracheostomy, and the presence of unhealed pressure ulcers as comorbidities that reflect the residents’ performance status. On MDS assessments, CDI reporting relies on the healthcare practitioner to mark the condition as “additional active diagnosis” and there is no checkbox for the exclusive documentation of CDI. CDI diagnosis was based on the International Classification of Diseases (ICD)-9-CM code for “an intestinal infection with C difficile” (008.45). Each additional diagnosis (if present) is coded by LTCF personnel in the appropriate box according to ICD-9 classification. We extracted the specific code from the pertinent box, as originally coded, to ascertain CDI.
Comparison of residents with and without CDI during their LTCF stay was performed using the χ2 test for categorical variables and the Wilcoxon rank-sum test for continuous data. We performed logistic regression modeling to adjust for significant confounders associated with CDI. We further addressed the impact of CDI on 3-month mortality (defined as death from any cause within the next 90 days after first CDI assessment), using Medicare enrollment data.
CDI prevalence was defined as the proportion of CDI cases per LTCF admissions in 2011. Stratified CDI rates were presented by state and by geographic region (Northeast, Midwest, South, West) according to US Census Bureau grouping and definitions in the National Nursing Home Survey.[15] Stata v.14 (College Station, TX) was used for data analysis. The study is STROBE-compliant and provides the pertinent checklist as per journal requirements.
3. Results
Overall, 2,190,613 admissions (1,806,900 unique individuals) aged 65 or older were included in the analysis. Specifically 1,488,605 individuals had a single admission in 2011, whereas 318,295 individuals had >1 LTCF admission during 2011, totaling 702,008 admissions (average 2.2 admissions for individuals with multiple admissions). The study profile is illustrated in Fig. 1. Their median age was 82 years (interquartile range 76–88) and females outnumbered male residents (approximately 2:1 ratio). Approximately 82% were white, followed by African American (9%), Hispanic (4%), and mixed or other origin (2%). Comorbidities included, in descending order, hypertension (69%), diabetes mellitus (29%), dementia (26%), coronary artery disease (24%), COPD (21%), ESRD (13%), stroke (12%), Parkinson disease (4%), and cirrhosis (0.5%). The median length of resident stay was 33 days (interquartile range, 19–90 days). Approximately 16% had unhealed pressure ulcers, 18% had urinary incontinence, 10% had bowel incontinence, and 4% had a feeding tube. Less than 1% had prior tracheostomy or received chemotherapy or irradiation (Table 1).
Figure 1.

Profile of the present study. CDI = C difficile infection, LTCF = long-term care facility.
Table 1.
Demographics and clinical characteristics of surveyed residents.
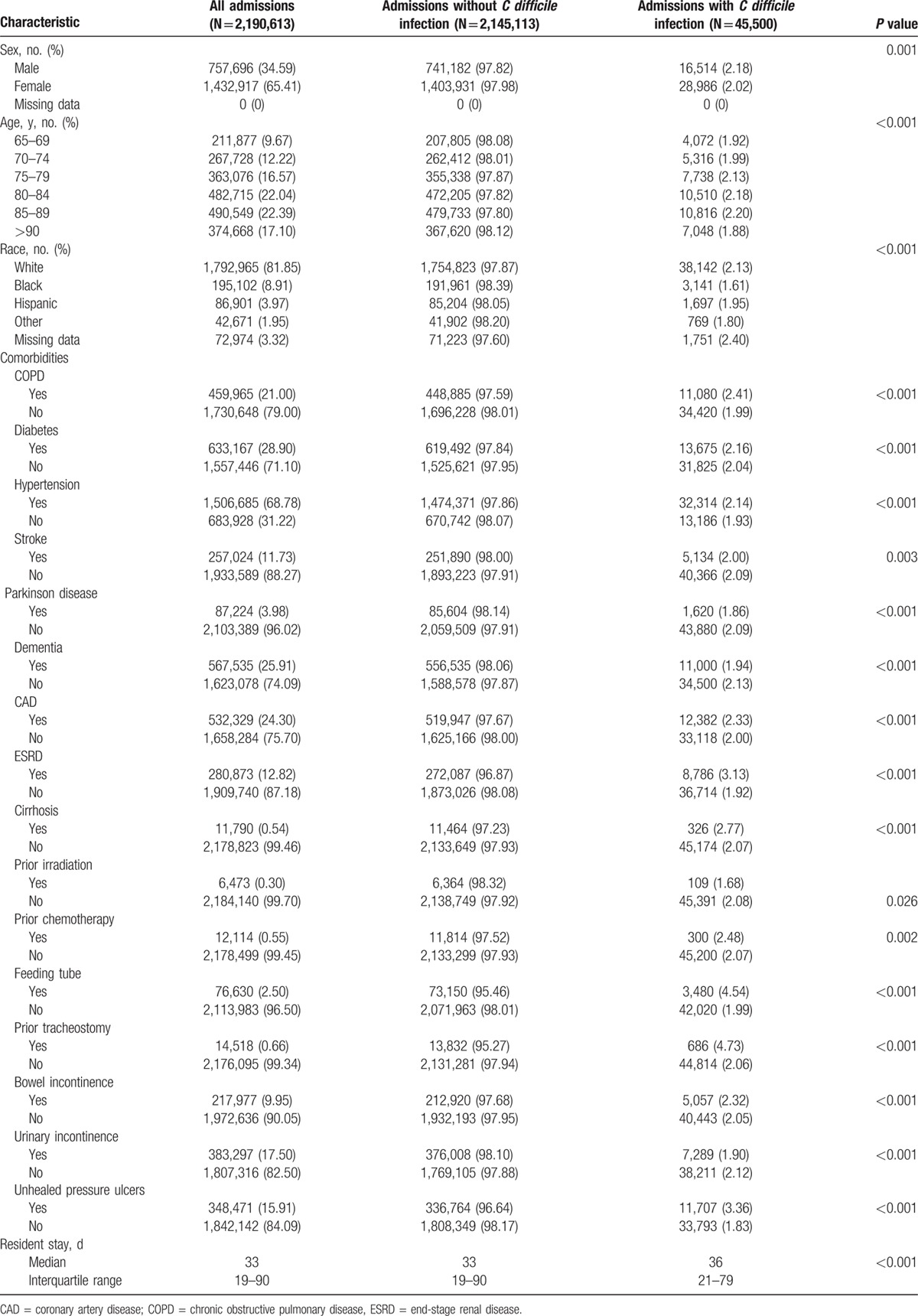
CDI was reported in 45,500 of admissions during LTCF stay (2.08%), representing 41,565 unique individuals. The stratified data (by demographics and comorbidities) are summarized in Table 1. The rate was higher among whites (2.13%) and lowest among African Americans (1.61%). Across medical comorbidities, the highest rates were noted among residents with tracheostomy (4.73%), feeding tube (4.54%), unhealed pressure ulcers (3.36%), ESRD (3.13%), and cirrhosis (2.77%). CDI varied across different age strata, being higher (2.20%) for the 85 to 89 age group.
In multivariable analysis for risk stratification (full model in Table S1 in Supplementary Appendix), the CDI risk was lower among nonwhite populations and increased with age. No sex differences were noted. Across comorbidities, the presence of a feeding tube, unhealed pressure ulcers, ESRD, cirrhosis, prior chemotherapy, bowel incontinence, prior tracheostomy, and COPD were influential factors associated with CDI (Fig. 2).
Figure 2.
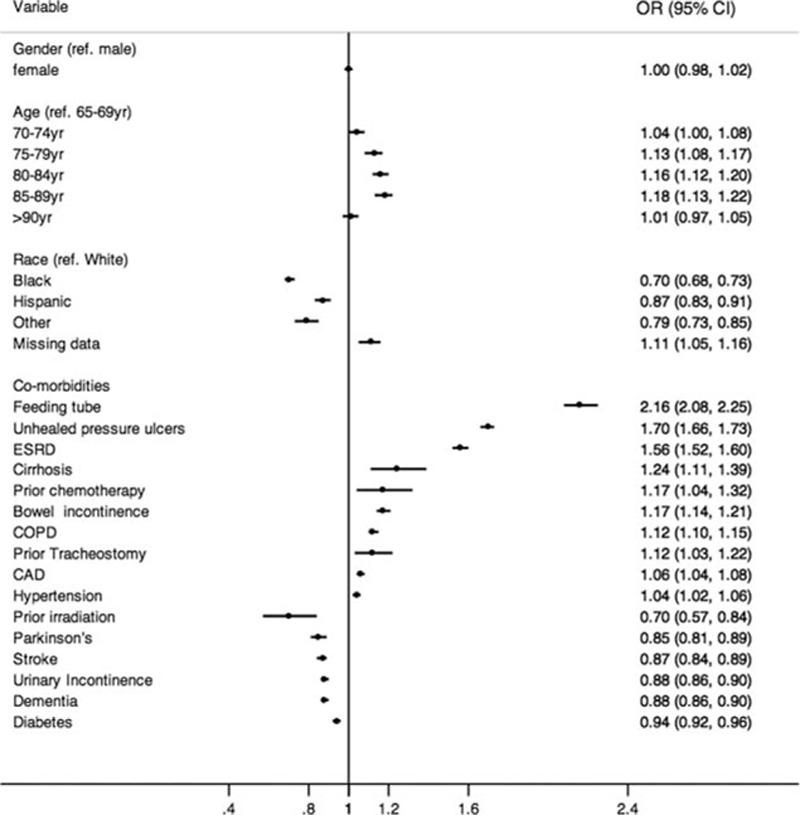
Multivariable analysis. CDI is the dependent outcome. CDI = C difficile infection, CI = confidence interval, COPD = chronic obstructive pulmonary disease, ESRD = end-stage renal disease, OR = odds ratio.
The nationwide 2011 prevalence rates, stratified by state, appear in Table S2, and are graphically illustrated in Fig. 3. The crude CDI rate was 1.85% (95% confidence interval [CI] 1.83–1.87) and the rate was higher in the Northeast (2.29%; 95% CI 2.25–2.33) and lower in the South (1.54%; 95% CI 1.51–1.57) (Table 2). Overall, 26,268 of 41,565 individuals (63%) with CDI in LTCF had recent hospitalization (within the prior 30 days) and/or had been discharged with CDI from the hospital within the previous 90 days, which leaves a total of 37% CDI cases that are not clearly related to prior healthcare exposure and may represent “true” LTCF-related cases. CDI was reported more than once in 3935 of 16,705 (24%) individuals who had >1 LTCF admission in 2011.
Figure 3.

US choropleth map illustrating CDI rates by state (quartile distribution of point estimates). Template state map derived as Environmental Systems Research Institute shapefile from the National Weather Service (available at: http://www.nws.noaa.gov/geodata/catalog/national/html/us_state.htm). Hawaii and Alaska territories (not seen in the map) belong to the lowest quartile. CDI = C difficile infection.
Table 2.
CDI prevalence (%) rates in long-term care facilities grouped by region (as used by the US Census Bureau).

Table 3 displays the cumulative discharge data. LTCF residents with a CDI diagnosis were more likely to be admitted to an acute care hospital (40% vs 31%, P < 0.001) and less likely to be discharged to the community (46% vs 54%, P < 0.001) than those not reported with CDI during stay. The crude LTCF mortality rates were 9.0% and 8.7% (P = 0.01), respectively.
Table 3.
Outcome of long-term care facility stay.

Survival data were available for 1,754,553/1,806,900 (97%) of individuals included in the study. The 3-month mortality rates were 24.7% (8924/36,164) for residents with a CDI diagnosis at LTCF compared to 18.1% (310,358/1,718,389) for those without CDI (P < 0.001). Importantly, CDI was independently associated with mortality (Fig. 4), in multivariable analysis (full model in Table S3 in Supplementary Appendix), with the odds being 27% higher for those with reported CDI (adjusted odds ratio 1.27, 95% CI 1.24–1.30).
Figure 4.
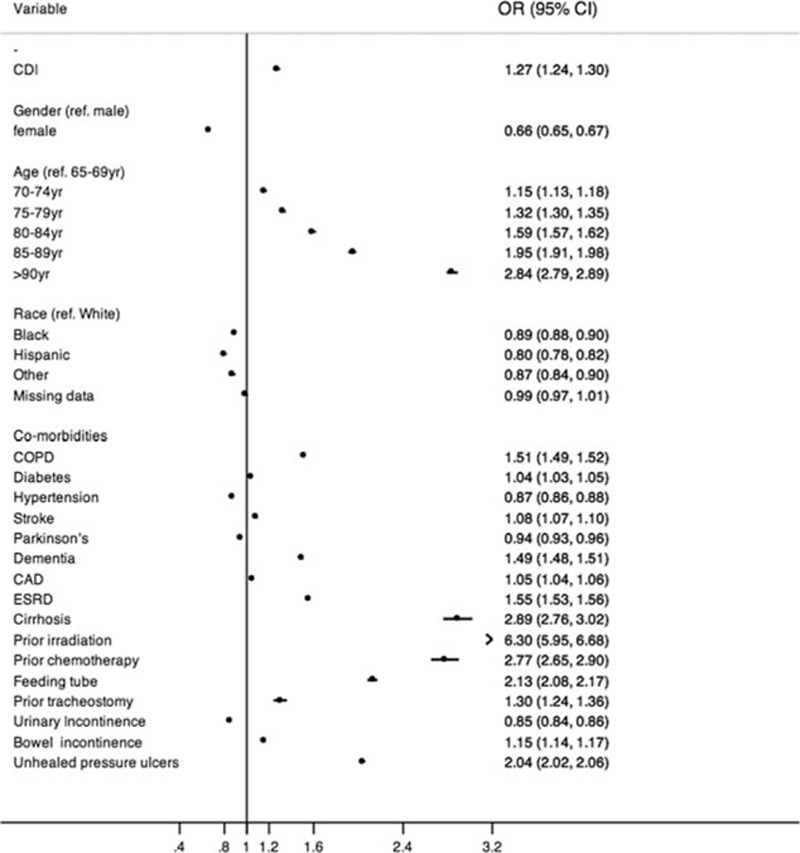
Multivariable analysis. The 3-month mortality is the dependent outcome. CDI = C difficile infection, CI = confidence interval, COPD = chronic obstructive pulmonary disease, ESRD = end-stage renal disease, OR = odds ratio.
4. Discussion
Our results indicate that approximately 1 out of every 50 residents aged 65 or older admitted in a Medicare-certified LTCFs was diagnosed with CDI during his or her stay. The nationwide CDI estimate in this setting is reported for the first time and the presence of geographic differences across the United States is depicted. Importantly, more than one-third of CDI cases appear to be independent of recent hospitalization to an acute care facility. CDI recorded at LTCF was independently associated with specific risk factors, and is correlated with a higher overall mortality.
Hospital discharge data are suggestive of an increasing proportion of patients with CDI[16] and more than half of LTCF admissions derive from acute care hospitals and hospital-based facilities.[15] Taken together these findings outline the need to evaluate the burden of CDI in LTCFs. Defining the origin of CDI is important, because imported cases will reflect poor prevention policies and antibiotic misuse in the hospital setting, rather than in LTCF. Indeed, we showed that almost two-thirds of CDI cases in LTCFs had been previously discharged with CDI from hospital or had recent hospitalization in an acute care facility, which underscores the fact that the majority of CDI that develop in the LTCF setting may “originate” in the acute hospital setting.[17] However, LTCF environment should be regarded as a major component of CDI epidemics as more than one-third of CDI cases may be regarded as “true” LTCF-related cases.
On the other hand, the distinction of LTCF-associated cases may reflect transmission patterns and inadequacy of infection control in the LTCF setting. Transmission in the LTCFs likely occurs by direct spread from the hands of personnel, fomites, and LTCF environment[18–20] and is facilitated by the fact that residents live in shared quarters, including sleeping, eating, and toileting facilities, and that the assignment of patients to private rooms does not seem to be feasible in most cases.[21] The fact that management of CDI is challenging in these facilities[10,22–24] highlights the importance of primary prevention of CDI in this patient population.
Identifying factors that are associated with increased risk of CDI may indicate a subpopulation for future preventive strategies. To date, limited data exist regarding risk factors for CDI in LTCF and the pertinent information was derived from a number of studies conducted in the hospital setting.[10] The presence of comorbid conditions,[25] the prolonged length of stay,[26] the use of feeding tubes,[25] overexposure to acid-suppressant medications,[26] and frailty[27,28] have been sporadically implicated in CDI epidemiology in LTCF. We found that indicators of more debilitated individuals and specific comorbidities including cirrhosis and ESRD may also contribute to CDI susceptibility. Plausible explanations are the frequent hospitalizations and interaction of these patients with the hospital environment (e.g., patients with ESRD), the use of broad-spectrum antibiotics for treatment of infections (e.g., decubitus ulcers) and for prophylaxis (e.g., for patients with cirrhosis), and the bypassing of the gastric barrier through nasogastric/enteral feeding or the increased use of antacid medications.[29–33] Of note is the fact that the association of fecal incontinence with increased CDI rates may be partially inflated by more frequent CDI testing in this subgroup of individuals.[34]
CDI is a worldwide epidemic with worrisome expansion, and a growing challenge for health experts. It is not confined to LTCFs in USA but also extends in several geographic areas overseas, affecting the elderly and LTCF setting.[35–38] The adoption of prevention policies across the continuum of care is necessary in order to control the epidemic, but it is unlikely that antibiotic stewardship programs[39,40] and infection control policies will suffice to solve the problem across the healthcare system.[10,22] Moreover, bacteria can continue to adapt.[41,42] In a bigger picture, the profile related to CDI in the elderly LTCF population documented in the present study unfolds the adverse impact of chronic disease and increased contact with the healthcare system on CDI epidemic. Inevitably, prevention policies should expand beyond the static viewpoint of disease management, aiming to promoting healthy living. Therefore, interventions that promote healthy living or alter environmental influences before disease develops can be more effective in controlling chronic illness[43] and limiting healthcare exposure. For example, COPD can be prevented by successful campaigns favoring tobacco cessation, and cirrhosis by alcohol restriction legislation; obesity and related disorders such as coronary artery disease and hypertension may require calorie restriction policies including fiscal measures as a public health tool (using the example of Mexico on imposing tax on sweetened beverages).[44,45] Living in healthy cities[46] that adopt evidence-informed health strategies and put health high in their social, political, and environmental priorities may also contribute to disease control, as urbanization and socioeconomic status differences impact population health.[47–49] CDI epidemic in the LTCF is a paradigm that justifies a shift from unidimensional, disease-based prevention policies to multidimensional and dynamic healthcare.[50]
4.1. Limitations
Analysis of official registries does not provide detailed individual data, but the large sample size and the nationwide coverage add to the importance and validity of the present study. Moreover, socioeconomic status, such as income, education level, and social status that may influence disease profile and outcome were not available in the present study. Also, pertinent data on antimicrobial drug use, proton pump inhibitors/histamine H2-receptor antagonists, and individual data such as body mass index or obesity were also not available.
Our definition of CDI was based on the ICD-9-CM code for “an intestinal infection with C difficile,” whose sensitivity and specificity are largely unknown, with previous studies showing that it may underestimate or overestimate the true burden of the disease.[51,52] However, in the absence of a national surveillance system for CDI, ICD-9 code provides an easily accessible tool for estimating the burden of this infection.[51] Moreover, for the estimation of CDI episodes during the LTCF stay we used the MDS v3.0 forms, in which there is no checkbox for the documentation of CDI exclusively. On MDS assessments, the identification of CDI relies on the healthcare practitioner to input the diagnosis, as opposed to check a box. This may result in an underreporting of CDI in LTCFs and may induce selection bias in the form of selecting patients with the most severe clinical characteristics. As such, our estimations probably underestimate the actual burden of CDI. Along with the potential insensitivity of coding, limitations involve the different methods used for isolation of C difficile in different LTCFs and hospitals all over the United States. Furthermore, the analysis data are representative only of patients in Medicare fee-for-service and do not reflect the population enrolled in Medicare-managed care, for whom insurance claims are not collected. The data set does not contain information regarding services not covered by, or billed to, Medicare and how that might affect the results. Finally the ratio of “true” LTCF-related cases is likely overestimated, because a number of CDI cases categorized as such may have acquired C difficile in the community and become symptomatic in LTCF. Additionally, the analysis of MDS form does not permit the distinction between primary CDI episode and CDI recurrences, which might significantly affect estimates on LTCF-acquired CDI. Such analysis would require longitudinal follow-up on a per patient basis that should also cover the community setting.
5. Conclusions
Our study estimated the nationwide burden of CDI infection among the elderly population in LTCF and provided the rationale for targeted prevention policies. Identification of LTCF residents who are at high risk for CDI may provide insights into interventions for future prevention strategies studied exclusively in this patient population. These efforts should focus on LTCF residents who are at high risk, such as those having a feeding tube or pressure ulcers, and those with ESRD and cirrhosis. Importantly, we report that both CDI in the LTCF and the hospital setting appear to feed the CDI epidemic, as up to two-thirds of CDI cases may be related to hospitalization in acute care facilities, but a significant proportion of CDI is related to LTCF. Further research could benefit from the addition of a separate checkbox in MDS form to uniquely code for CDI, allowing opting for primary infection or recurrences. Acute care facilities and LTCFs should collaborate in reducing CDI rates across the continuum of care.
Supplementary Material
Footnotes
Abbreviations: CDI = C difficile infection, CI = confidence interval, COPD = chronic obstructive pulmonary disease, ESRD = end-stage renal disease, ICD = International Classification of Diseases, LTCF = long-term care facility, MDS = Minimum Data Set.
Authorship: PDZ and NJ were involved in study concept and design, data analysis, data interpretation, manuscript drafting, critical revision for important intellectual content, and approval of the final version; IMZ and FNZ were involved in study concept and design, data interpretation, manuscript drafting, critical revision for important intellectual content, and approval of the final version; RWB, VM, and EM were involved in study concept and design, data interpretation, manuscript drafting, critical revision for important intellectual content, study supervision, and approval of the final version.
Funding: The study was supported by the SURDNA Foundation 2015 Fellowship Program from the Center for Gerontology and Healthcare Research, Brown University (available at: http://brown.edu/research/projects/gerontology-healthcare-research/fellowship-programs/surdna-fellowship-program). Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number T32MH019733.
PDZ is a 2015 awardee of SURDNA foundation fellowship by the Center for Gerontology and Healthcare Research, Brown University. NJ is an awardee of NIH postdoctoral fellowship by the National Institute of Mental Health of the National Institutes of Health under Award Number T32MH019733. VM's research is in a related area to that of several different paid activities. He periodically serves as a paid speaker at national conferences where he discusses trends and research findings in long-term and post–acute care. Each of these is enumerated in the following. VM holds stock of unknown value in PointRight, Inc, an information services company providing advice and consultation to various components of the long-term care and post–acute care industry, including suppliers and insurers. PointRight sells information on the measurement of nursing home quality to nursing homes and liability insurers. VM was a founder of the company but has subsequently divested much of his equity in the company and relinquished his seat on board. In addition, VM chairs the Independent Quality Committee for HRC Manor Care, Inc, a nursing home chain, for which he receives compensation in the $20,000 to $40,000 range. He also serves as chair of a Scientific Advisory Committee for NaviHealth, a post–acute care service organization, for which he also receives compensation in the $20,000 to $40,000 per year range. VM serves as a Technical Expert Panel member on several Center for Medicare/Medicaid quality measurement panels. He is a member of the board of directors of Tufts Health Plan Foundation, Hospice Care of Rhode Island, and the Jewish Alliance of Rhode Island. The remaining authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med 2008; 359:1932–1940. [DOI] [PubMed] [Google Scholar]
- 2.Laffan AM, Bellantoni MF, Greenough WB, 3rd, et al. Burden of Clostridium difficile-associated diarrhea in a long-term care facility. J Am Geriatr Soc 2006; 54:1068–1073. [DOI] [PubMed] [Google Scholar]
- 3.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370:1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall AJ, Curns AT, McDonald LC, et al. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis 2012; 55:216–223. [DOI] [PubMed] [Google Scholar]
- 5.Lucado J, Gould C, Elixhauser A. Clostridium difficile Infections (CDI) in Hospital Stays, 2009: Statistical Brief #124. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; 2006. [PubMed] [Google Scholar]
- 6.Kyne L, Hamel MB, Polavaram R, et al. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis 2002; 34:346–353. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien JA, Lahue BJ, Caro JJ, et al. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol 2007; 28:1219–1227. [DOI] [PubMed] [Google Scholar]
- 8.Dubberke ER, Wertheimer AI. Review of current literature on the economic burden of Clostridium difficile infection. Infect Control Hosp Epidemiol 2009; 30:57–66. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States; 2013. Available at: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013–508.pdf [accessed May 24, 2016]. [Google Scholar]
- 10.Simor AE. Diagnosis, management, and prevention of Clostridium difficile infection in long-term care facilities: a review. J Am Geriatr Soc 2010; 58:1556–1564. [DOI] [PubMed] [Google Scholar]
- 11.Gozalo PL, Pop-Vicas A, Feng Z, et al. Effect of influenza on functional decline. J Am Geriatr Soc 2012; 60:1260–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips CD, Morris JN, Hawes C, et al. Association of the Resident Assessment Instrument (RAI) with changes in function, cognition, and psychosocial status. J Am Geriatr Soc 1997; 45:986–993. [DOI] [PubMed] [Google Scholar]
- 13.Mor V. A comprehensive clinical assessment tool to inform policy and practice: applications of the minimum data set. Med Care 2004; 42:III50–III59. [DOI] [PubMed] [Google Scholar]
- 14.Mor V, Angelelli J, Jones R, et al. Inter-rater reliability of nursing home quality indicators in the U.S. BMC Health Serv Res 2003; 3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Vital and Health Statistics (2009). The National Nursing Home Survey: 2004. Overview. Available at: http://www.cdc.gov/nchs/data/series/sr_13/sr13_167.pdf [ accessed May 24, 2016]. [Google Scholar]
- 16.McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis 2006; 12:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mylotte JM, Russell S, Sackett B, et al. Surveillance for Clostridium difficile infection in nursing homes. J Am Geriatr Soc 2013; 61:122–125. [DOI] [PubMed] [Google Scholar]
- 18.Brooks SE, Veal RO, Kramer M, et al. Reduction in the incidence of Clostridium difficile-associated diarrhea in an acute care hospital and a skilled nursing facility following replacement of electronic thermometers with single-use disposables. Infect Control Hosp Epidemiol 1992; 13:98–103. [DOI] [PubMed] [Google Scholar]
- 19.Fawley WN, Wilcox MH. Molecular epidemiology of endemic Clostridium difficile infection. Epidemiol Infect 2001; 126:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samore MH, Venkataraman L, DeGirolami PC, et al. Clinical and molecular epidemiology of sporadic and clustered cases of nosocomial Clostridium difficile diarrhea. Am J Med 1996; 100:32–40. [DOI] [PubMed] [Google Scholar]
- 21.Quinn LK, Chen Y, Herwaldt LA. Infection control policies and practices for Iowa long-term care facility residents with Clostridium difficile infection. Infect Control Hosp Epidemiol 2007; 28:1228–1232. [DOI] [PubMed] [Google Scholar]
- 22.Simor AE, Bradley SF, Strausbaugh LJ, et al. Clostridium difficile in long-term-care facilities for the elderly. Infect Control Hosp Epidemiol 2002; 23:696–703. [DOI] [PubMed] [Google Scholar]
- 23.Wilcox MH, Fawley WN, Wigglesworth N, et al. Comparison of the effect of detergent versus hypochlorite cleaning on environmental contamination and incidence of Clostridium difficile infection. J Hosp Infect 2003; 54:109–114. [DOI] [PubMed] [Google Scholar]
- 24.Boyce JM, Pittet D. Healthcare Infection Control Practices Advisory Committee. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol 2002; 23:S3–S40. [DOI] [PubMed] [Google Scholar]
- 25.Simor AE, Yake SL, Tsimidis K. Infection due to Clostridium difficile among elderly residents of a long-term-care facility. Clin Infect Dis 1993; 17:672–678. [DOI] [PubMed] [Google Scholar]
- 26.Al-Tureihi FI, Hassoun A, Wolf-Klein G, et al. Albumin, length of stay, and proton pump inhibitors: key factors in Clostridium difficile-associated disease in nursing home patients. J Am Med Dir Assoc 2005; 6:105–108. [DOI] [PubMed] [Google Scholar]
- 27.Strausbaugh LJ, Sukumar SR, Joseph CL. Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin Infect Dis 2003; 36:870–876. [DOI] [PubMed] [Google Scholar]
- 28.Davies EA, O’Mahony MS. Adverse drug reactions in special populations—the elderly. Br J Clin Pharmacol 2015; 80:796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 2002; 136:834–844. [DOI] [PubMed] [Google Scholar]
- 30.Donskey CJ. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin Infect Dis 2004; 39:219–226. [DOI] [PubMed] [Google Scholar]
- 31.Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis 2008; 28:26–42. [DOI] [PubMed] [Google Scholar]
- 32.Lodato F, Azzaroli F, Di Girolamo M, et al. Proton pump inhibitors in cirrhosis: tradition or evidence based practice? World J Gastroenterol 2008; 14:2980–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eddi R, Malik MN, Shakov R, et al. Chronic kidney disease as a risk factor for Clostridium difficile infection. Nephrology (Carlton) 2010; 15:471–475. [DOI] [PubMed] [Google Scholar]
- 34.Leung FW, Rao SS. Approach to fecal incontinence and constipation in older hospitalized patients. Hosp Pract (1995) 2011; 39:97–104. [DOI] [PubMed] [Google Scholar]
- 35.Marwick CA, Yu N, Lockhart MC, et al. Community-associated Clostridium difficile infection among older people in Tayside, Scotland, is associated with antibiotic exposure and care home residence: cohort study with nested case–control. J Antimicrob Chemother 2013; 68:2927–2933. [DOI] [PubMed] [Google Scholar]
- 36.Arvand M, Moser V, Schwehn C, et al. High prevalence of Clostridium difficile colonization among nursing home residents in Hesse, Germany. PLoS One 2012; 7:e30183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birgand G, Blanckaert K, Carbonne A, et al. Investigation of a large outbreak of Clostridium difficile PCR-ribotype 027 infections in northern France, 2006–2007 and associated clusters in 2008–2009. Euro Surveill 2010; 15: pii:19597. [DOI] [PubMed] [Google Scholar]
- 38.Szabó R, Böröcz K. Infections and antimicrobial use among institutionalized residents in Hungary: increasing need of microbiological surveillance. Acta Microbiol Immunol Hung 2015; 62:29–44. [DOI] [PubMed] [Google Scholar]
- 39.Morrill HJ, Caffrey AR, Jump RL, et al. Antimicrobial stewardship in long-term care facilities: a call to action. J Am Med Dir Assoc 2016; 17:183.e1–183.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giannella M, Tedeschi S, Bartoletti M, et al. Prevention of infections in nursing homes: antibiotic prophylaxis versus infection control and antimicrobial stewardship measures. Expert Rev Anti Infect Ther 2016; 14:219–230. [DOI] [PubMed] [Google Scholar]
- 41.Research Highlights. Evolution: bacteria cannot stop adapting. Nature 2015; 528:439. [Google Scholar]
- 42.Lenski RE, Wiser MJ, Ribeck N, et al. Sustained fitness gains and variability in fitness trajectories in the long-term evolution experiment with Escherichia coli. Proc Biol Sci 2015; 282:20152292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diet, nutrition and the prevention of chronic diseases. Report of the joint WHO/FAO expert consultation. WHO Technical Report Series, No. 916 (TRS 916); 2003:1–149. Available at: http://www.who.int/dietphysicalactivity/publications/trs916/download/en/ [accessed May 24, 2016]. [PubMed] [Google Scholar]
- 44.Sassi F. Taxing sugar. BMJ 2016; 352:h6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cabrera Escobar MA, Veerman JL, Tollman SM, et al. Evidence that a tax on sugar sweetened beverages reduces the obesity rate: a meta-analysis. BMC Public Health 2013; 13:1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simos J, Spanswick L, Palmer N, et al. The role of health impact assessment in phase V of the Healthy Cities European Network. Health Promot Int 2015; 30 suppl 1:i71–i85. [DOI] [PubMed] [Google Scholar]
- 47.Moore M, Gould P, Keary BS. Global urbanization and impact on health. Int J Hyg Environ Health 2003; 206:269–278. [DOI] [PubMed] [Google Scholar]
- 48.Wilkinson RG, Pickett KE. Income inequality and population health: a review and explanation of the evidence. Soc Sci Med 2006; 62:1768–1784. [DOI] [PubMed] [Google Scholar]
- 49.Ribeiro AI, Krainski ET, Carvalho MS, et al. Where do people live longer and shorter lives? An ecological study of old-age survival across 4404 small areas from 18 European countries. J Epidemiol Community Health 2016; 70:561–568. [DOI] [PubMed] [Google Scholar]
- 50.Leischik R, Dworrak B, Strauss M, et al. Plasticity of health. German J Med 2016; 1:1–17. [Google Scholar]
- 51.Dubberke ER, Reske KA, McDonald LC, et al. ICD-9 codes and surveillance for Clostridium difficile-associated disease. Emerg Infect Dis 2006; 12:1576–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kralovic SM, Danko LH, Simbartl LA, Roselle GA. Clostridium difficile infection in VA medical centers nationwide. In: Proceedings of the 15th Annual Scientific Meeting of the Society for Healthcare Epidemiology of America, Los Angeles, CA, April 9–12, 2005. Abstract 284. Alexandria, VA: Slank Inc; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


