Abstract
Solitary large hepatocellular carcinomas (SLHCC) form a heterogeneous group of patients with different survival probabilities. The aim of our study was to develop a simple prognostic index for identifying prognostic subgroups of SLHCC patients.
A retrospective analysis of clinical data from 268 patients with operable SLHCC was conducted to investigate prognostic factors and to construct a score system based on risk factors. A Cox proportional hazard regression analysis was used to evaluate the variables associated with prognosis. Survival analyses were performed using Kaplan–Meier survival curves.
Three variables remained in the final multivariate model: platelet to lymphocyte ratio (PLR), microvascular invasion (MVI), and tumor size with hazard ratios equal to 1.004 (95% confidence interval: 1.001–1.006), 1.092 (1.044–1.142), and 2.233 (1.125–2.233), respectively. A score of 1 was assigned to each risk factor. Patient scores were determined based on these risk factors; thus, the scores ranged between 0 and 3. Ultimately, three categories (0, 1–2, 3) were defined. Patients with scores of 3 had a 5-year survival rate of 25.4%, whereas patients with a score of 0 had a 5-year survival rate of 52.1%. The prognosis significantly worsened as the score increased. Similar results were found among cirrhotic and noncirrhotic patients.
Our simple prognostic index successfully predicts SLHCC survival.
Keywords: HCC, hepatectomy, prognosis, prognostic score system
1. Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide and has a rising incidence rate. In Asia, HCC mainly occurs in patients with hepatitis B virus (HBV) infection and HBV-associated cirrhosis and accounts for half of all HCC cases worldwide.[1] Although awareness of HCC surveillance has been reinforced in clinical practice, HCC with a tumor size larger than 5.0 cm in diameter is quite common. Surgical resection is widely used for curative treatment. The American Association for the Study of Liver Diseases (AASLD) does not recommend hepatectomy for a tumor size >5 cm. Previous studies have suggested that hepatectomy should be considered for SLHCC patients with well-preserved liver function because it offers patients the chance of a long-term cure.[2–4] Over the past several decades, hepatic resection for HCC has advanced and results in low operative mortality, which is less than 2% at large liver surgery centers.[5] Furthermore, development of the associating liver partition with portal vein ligation for staged hepatectomy (ALPPS) technique has enabled the careful removal of more SLHCCs.[6]
According to the Barcelona clinic liver cancer (BCLC) algorithm, solitary large HCC is classified as early-stage with a survival rate of 50% to 70%. In previous studies, the 5-year survival rate for SLHCC was 28.6% to 47%.[7–11] However, other studies have reported that the SLHCC outcome after hepatic resection was comparable to that of small HCC.[12,13] The staging of solitary large HCC remains controversial. The BCLC system was derived from the experience of the Barcelona group with allocating the best candidates to the best available treatments rather than using a statistical prognostic model. Patients with large tumors commonly had more risk factors, such as microvascular invasion (MVI), and were more likely to suffer from HCC recurrence.[14–17] Moreover, not all solitary HCC patients can undergo curative treatment. The BCLC stage system may not be completely accurate for the allocation of treatment options and prediction of prognosis for SLHCC patients. Indeed, the treatment outcomes for HCC patients are affected by multiple variables, including tumor characteristics, performance status of the patient, and treatment methods. Thus, a prognostic score system to stratify the prognoses of operable SLHCC patients is needed.
The role of tumor size on patient prognosis remains controversial. Some researchers have suggested that large HCC has oncological behavior similar to small HCC.[12] In certain current stage systems, size is not a determinant factor for tumor stage.[18,19] However, other investigators insist that tumor size is an important predictor of prognosis in patients with solitary HCC.[4,16,17] For patients with non-B and non-C hepatocellular carcinoma, larger HCC did not correlate with a poorer prognosis, suggesting that tumor size does not reflect tumor behavior in specific settings.[20] In contrast, HCC tended to occur in the setting of underlying liver disease in HBV endemic areas. Tumor oncological behavior may be closely related to tumor size.[17] Recently, the role of MVI on long-term survival has been confirmed in various cancers.[21,22] Patients commonly developed MVI, which indicates a more aggressive tumor behavior. Lim et al[21] suggested that the presence of MVI can be used to discriminate between prognosis subgroups after surgical resection for HCC and surpasses the Milan criteria. However, few staging systems incorporate MVI as a variable for guiding treatments and predicting prognosis. Inflammation-based prognostic indicators, such as the platelet to lymphocyte ratio (PLR) and neutrophil to lymphocyte ratio (NLR), can be used as prognostic factors for HCC and other cancers.[14,23,24] In clinical settings, these cost-effective biomarkers could be utilized to provide information regarding patient treatment outcomes.
To the best of our knowledge, the integration of MVI, inflammation-based prognostic indicators (PLR), and tumor size to improve outcome predictions for operable SLHCC has not yet been studied. Our study aimed to analyze multiple prognostic factors and construct a novel prognostic scoring system for the identification of SLHCC patient subgroups with different prognoses.
2. Materials and methods
This study was approved by the Ethics Committee of West China Hospital at Sichuan University. A total of 268 qualified patients who underwent curative liver resection between 2009 and 2013 in the Department of Liver Surgery and Liver Transplantation Center of West China Hospital were included and retrospectively analyzed in this study. Routine blood examinations, liver function tests, and alpha-fetoprotein (AFP) tests were completed 2 d before the operation. NLR and PLR were calculated as the absolute neutrophil count divided by the absolute lymphocyte count and the absolute platelet count divided by the absolute lymphocyte count, respectively. The HCC diagnosis was confirmed by liver pathologists in West China Hospital. Pathological characteristics, including tumor size, MVI, and tumor differentiation, were collected. We used two inclusion criteria: single HCC (>5 cm); and appropriate liver reserve function (Child–Pugh grade A/B). We used four exclusion criteria: recurrent HCC; macrovascular invasion or extrahepatic spread; the presence of other malignancies; and unreliable follow-up data.
Postoperative therapy included transcatheter arterial chemoembolization, chemotherapy (doxifluridine capsules and sorafenib), and immunoenhancer therapy (thymopeptide and a Chinese patented drug, such as Huaier granule). Follow-ups were conducted every 3 months for the first 2 years, 6 months for the subsequent 3 years, and annually in the following years. A serum AFP test, HBV-DNA measure, liver ultrasound, computed tomography or magnetic resonance imaging, and chest radiography were performed for all patients. Bone scintigraphy was completed when HCC recurrence was suspected. Postoperative recurrence was defined as positive imaging findings compared with the preoperative examination values and a newly elevated AFP level. Antiviral drugs were administered to patients with positive HBV-DNA before and after the operation. The overall survival (OS) was calculated from the operation date to either the day of death or the last follow-up. The disease-free survival (DFS) was calculated from the operation date to the day of HCC recurrence. We identified three risk factors related to long-term survival: PLR, MVI, and tumor size. Next, we calculated the optimal cutoff values for PLR and tumor size as 107 and 6.8 cm, respectively, using receiver operating characteristic (ROC) curve analysis. A survival analysis was performed with Kaplan–Meier survival curves stratified by the three risk factors. Each risk factor was assigned a score of 1, and patient scores ranged from 0 to 3. Four groups were identified, and a survival analysis was performed. We combined two subgroups with similar prognoses. Thus, three groups were identified according to patient scores (i.e., 0, 1–2, 3) and resulted in distinct prognoses. The prognostic score system was successfully applied to cirrhotic patients and noncirrhotic patients.
Continuous variables with normal distributions are expressed as the mean ± standard deviation (SD) and were compared using standard t tests. A Wilcoxon rank-sum test was used for continuous variables with non-normal distributions. Categorical variables are displayed as a number (percent). The χ2 test or Fisher exact test was used to analyze categorical variables. Survival analyses were performed using Kaplan–Meier survival curves, and significant differences between groups were compared by the log-rank test. A Cox proportional hazard regression was used to identify associations between outcomes and variables. Variables identified as P <0.10 in the univariate analysis were used in the multivariate analysis with forward-stepwise selection and Cox hazard proportion model. Cutoff values for PLR and tumor size were identified from a receiver operating characteristic (ROC) curve analysis.
3. Results
3.1. Patient characteristics
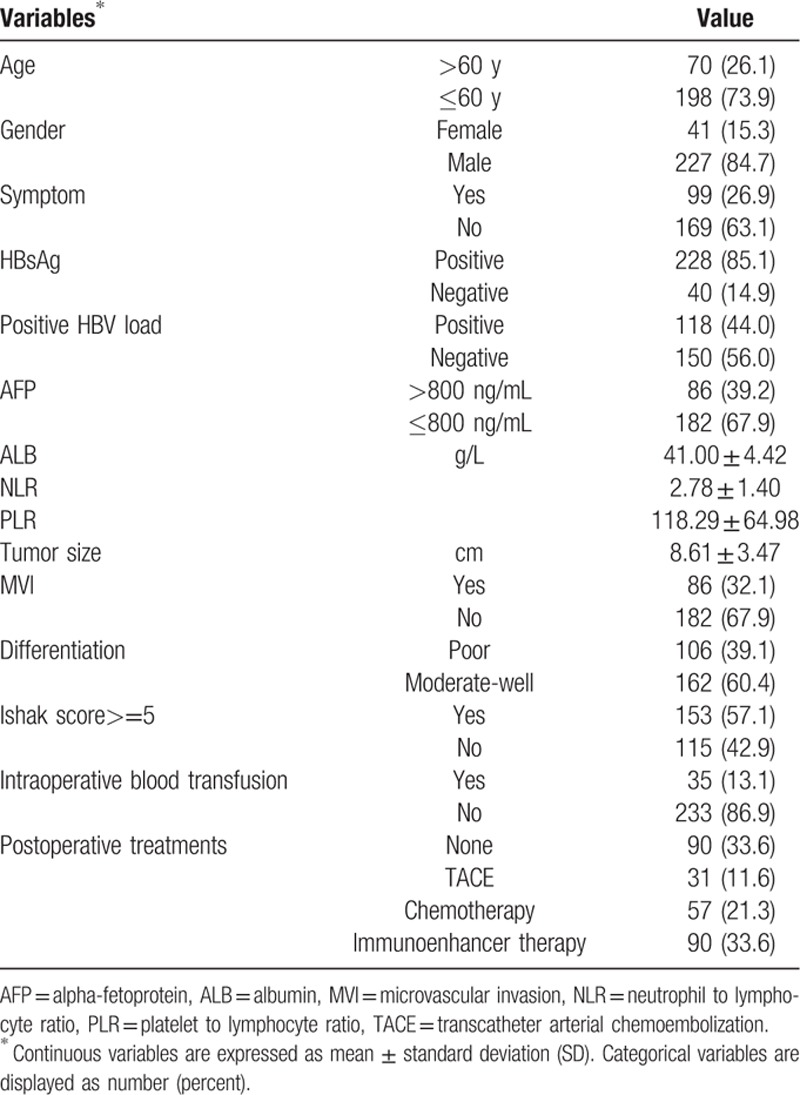
A total of 268 patients with SLHCC were included in the present study. Baseline characteristics of all patients are reported in Table 1. A total of 198 patients were diagnosed at younger than 60 years of age, and 70 patients were diagnosed after 60 years of age. At the time of the last follow-up, 194 patients had relapsed and 144 patients had died. A total of 86 (32.1%) of these cases had MVI, and 153 (57.1%) cases had an Ishak score greater than 5. The tumor size was 8.16 ± 3.47 cm, and the PLR was 118.29 ± 64.89. The median follow-up time was 33.1 months and ranged from 1 to 79 months (Table 1).
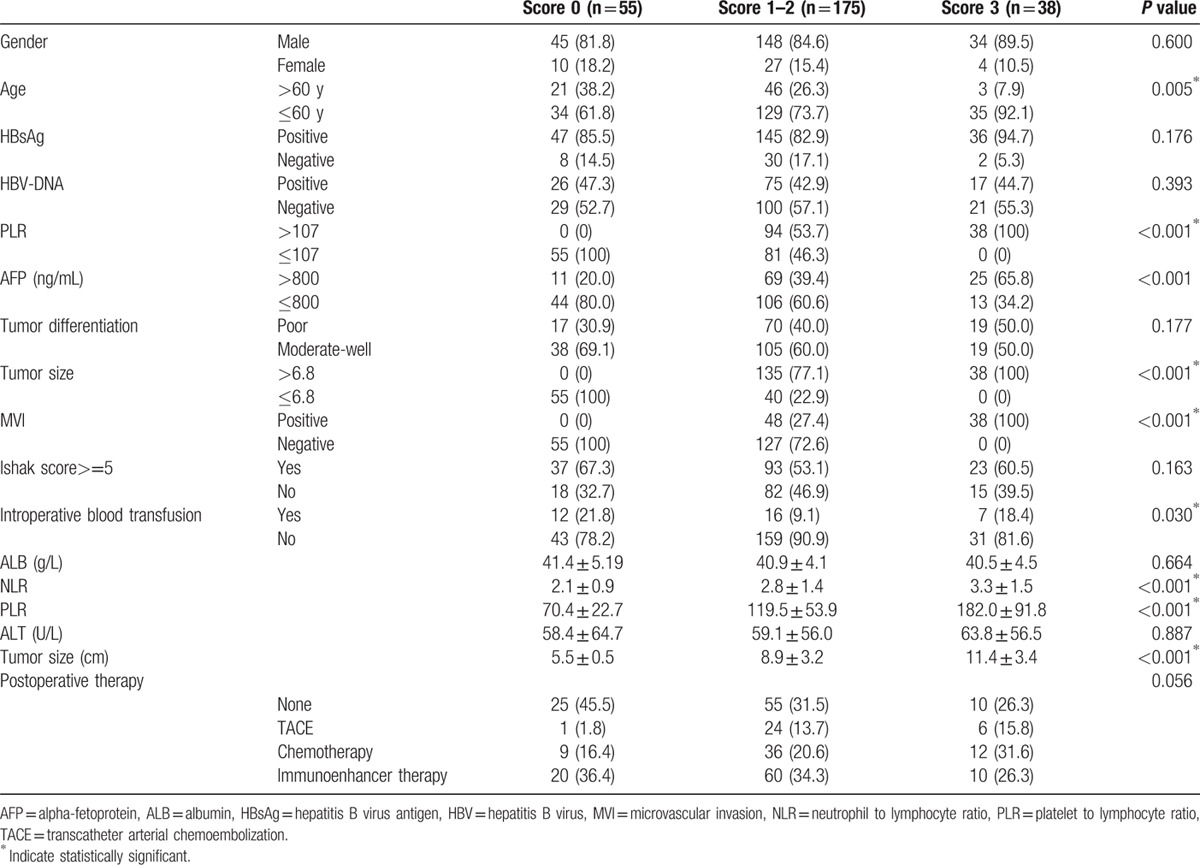
Table 1.
Demographic features of all patients.
3.2. Prognostic significance of variables and cutoff value determination
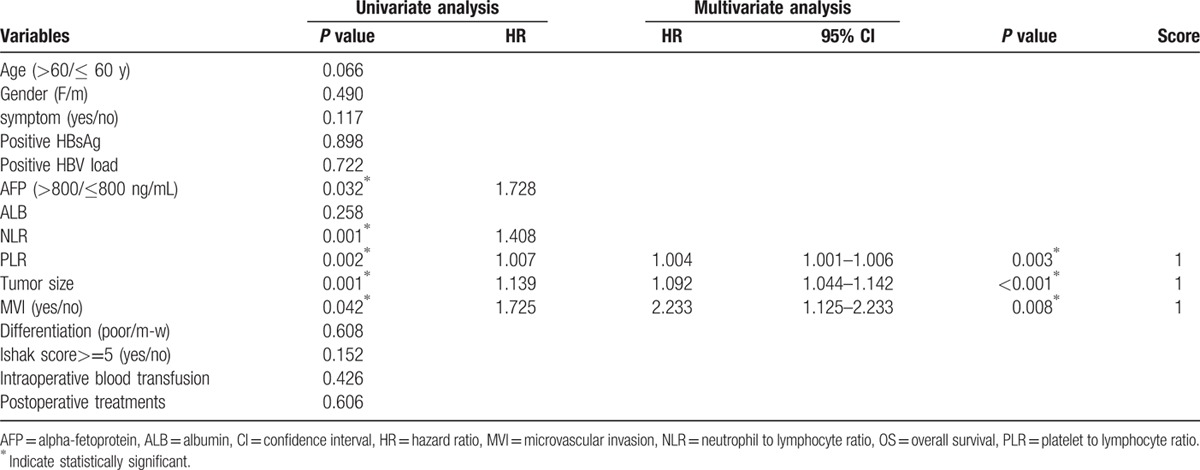
Potential risk factors were included in the univariate analysis. Factors related to DFS, including age, AFP, PLR, tumor size, and MVI, and factors related to OS, including age, AFP, NLR, PLR, tumor size, and MVI, were entered into a Cox proportional hazard model using the forward-stepwise method (P <0.10 for all factors). The multivariate analysis showed that PLR, MVI, and tumor size were independent prognostic factors for OS (hazard ratio [HR] 1.004, 95% CI 1.001–1.006, P = 0.003; HR 2.233, 95% CI 1.125–2.233, P = 0.008; HR 1.092, 95% CI 1.044–1.142, P <0.001, respectively) and DFS (HR 1.003, 95% CI 1.003–1.006, P = 0.003; HR 1.534, 95% CI 1.136–2.071, P = 0.005; HR 1.062, 95% CI 1.020–1.106, P = 0.004, respectively) (Tables 2 and 3). Based on the ROC curves, the optimal cutoff values for PLR and tumor size that corresponded to a maximum joint sensitivity and specificity were 107.0 and 6.8 cm, respectively.
Table 2.
Factors associated with DFS in univariate and multivariate analysis.
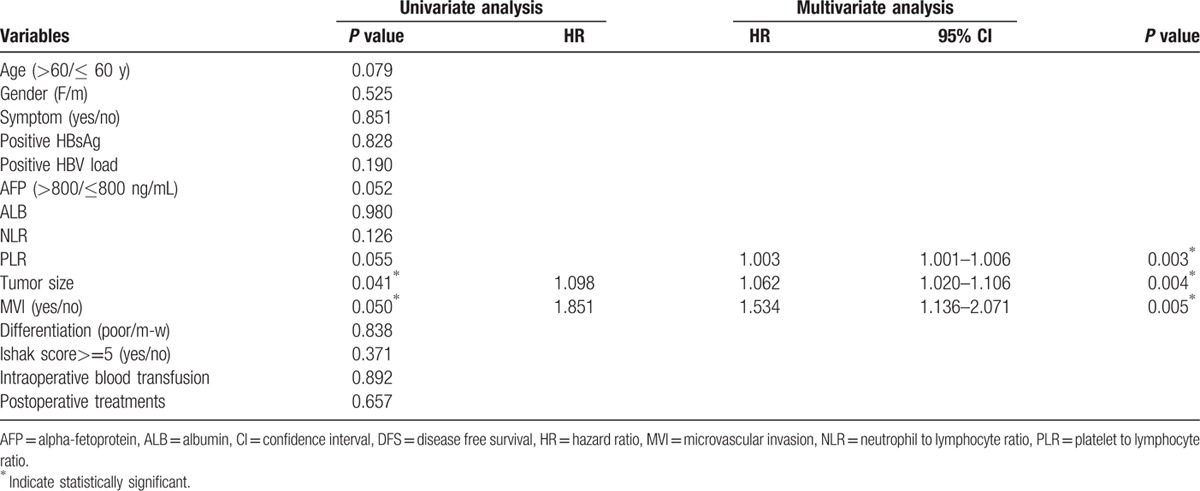
Table 3.
Factors associated with OS in univariate and multivariate analysis.
3.3. Survival analysis of patients stratified according to risk factors
Patients were divided into groups based on PLR (<107 vs ≥107), MVI (positive vs negative), and tumor size (<6.8 cm vs ≥6.8 cm). Kaplan–Meier survival curves demonstrated that an increased PLR, the presence of MVI, and a larger tumor were associated with a shorter OS (P = 0.001, P = 0.001, and P = 0.001, respectively) and DFS (P = 0.002, P < 0.001, and P < 0.001, respectively). The cumulative 3- and 5-year OS (64.2% and 40.5%, respectively) and DFS (42.8% and 24.9%, respective) rates in the low PLR group were higher than the OS (44.1% and 30.8%, respectively) and DFS (24.6% and 20.3%, respectively) rates in the high PLR group (OS P = 0.001 and DFS P < 0.001) (Fig. 1). Patients with an MVI had shorter 3- and 5-year OS (37.7% and 26.7%, respectively) and DFS (20.3% and 11.5%, respectively) rates than the non-MVI group (3- and 5-year OS rates of 61.9% and 39.4%, respectively, P = 0.001 and 3- and 5-year DFS rates of 39.8% and 26.7%, respectively, P <0.001) (Fig. 2). Patients with smaller tumor sizes had better 5-year DFS and OS rates than did those with large tumors (33.6% and 45.9% vs 15.3% and 29.8%, P <0.001 and P = 0.001, respectively) (Fig. 3).
Figure 1.
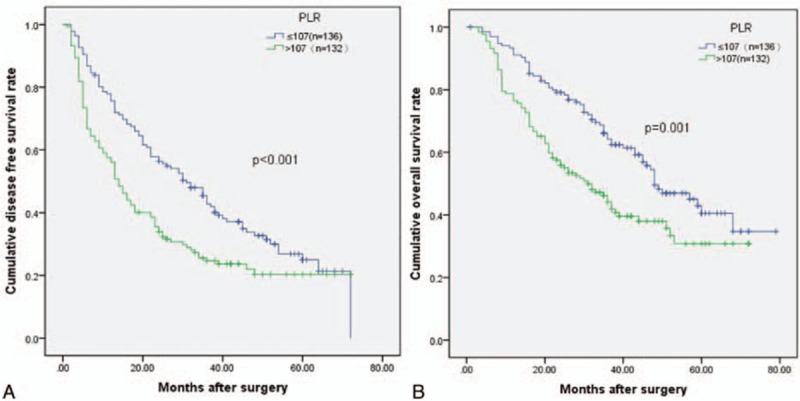
Relationship between (A) disease-free survival (DFS) and (B) overall survival (OS) in solitary large HCC patients and platelet to lymphocyte ratio (PLR). Patients were stratified into high and low PLR groups using a cutoff value of 107; patients with higher PLR (PRL ≥107) had lower DFS and OS rates (P <0.001 and P = 0.001).
Figure 2.
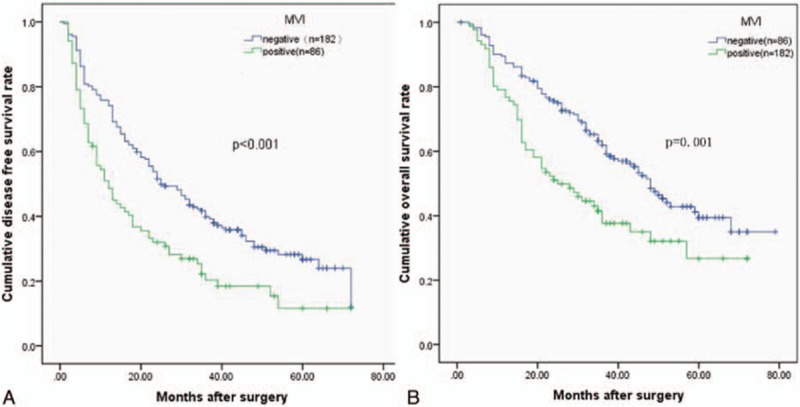
Relationship between (A) disease-free survival (DFS) and (B) overall survival (OS) in solitary large HCC patients and microvascular invasion (MVI). Patients with MVI had lower DFS and OS rates (P <0.001 and P = 0.001).
Figure 3.
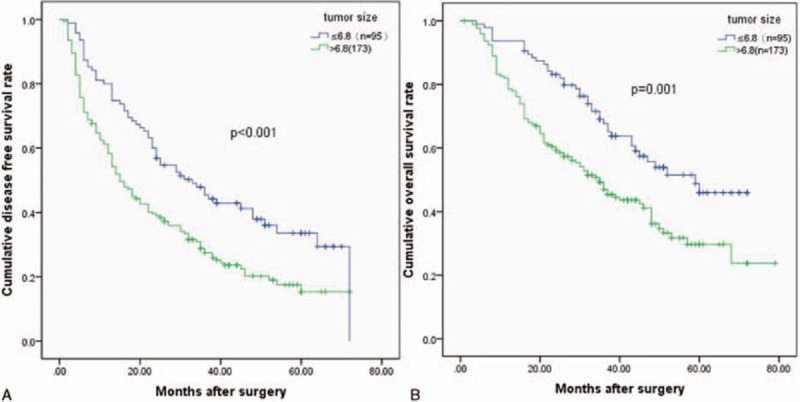
Relationship between (A) disease-free survival (DFS) and (B) overall survival (OS) in solitary large HCC patients and tumor size. Patients were stratified into tumor size (≥6.8 cm) group and tumor size (<6.8 cm) group using the cutoff value of 6.8; patients with larger tumor size had lower DFS and OS rates (P <0.001 and P = 0.001).
3.4. Integration of PLR, MVI, and tumor size to stratify prognoses
Each of the 3 risk factors was assigned a score of 1. Patient scores ranged between 0 and 3. Thus, we thus defined 4 subgroups based on their scores (0, 1, 2, and 3). A total of 55 patients without any risk factors were assigned a score of 0, and 38 patients with all 3 risk factors were assigned score of 3. A total of 73 patients with 1 risk factor were assigned a score of 1, and 102 patients with 2 risk factors were assigned a score of 2. As shown in Fig. 4, the OS and DFS rates were similar in subgroups with a score of 1 or 2 (P = 0.099 and P = 0.079, respectively). Therefore, we combined these 2 subgroups, which resulted in 175 patients with a score of 1 to 2.
Figure 4.
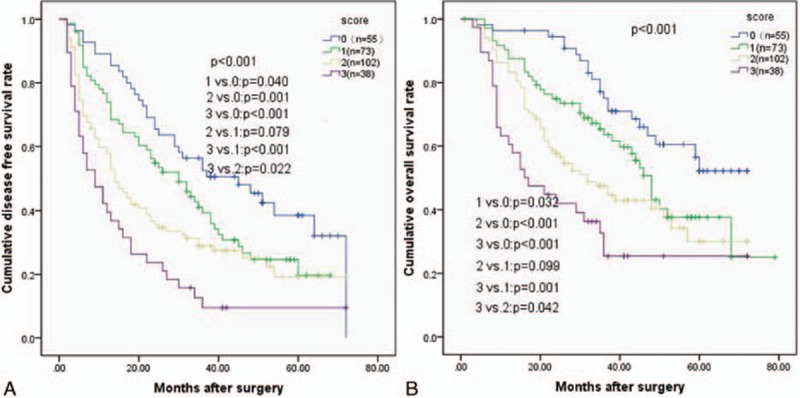
Relationship between (A) disease-free survival (DFS) and (B) overall survival (OS) in solitary large HCC patients according to patient scores. Patients were separated into 4 groups as follows: scores of 0, 1, 2, 3. Patients with score of 1 or 2 had similar DFS and OS (P = 0.079 and P = 0.099).
Kaplan–Meier curves showed that high scores were associated with shorter OS and DFS rates (P <0.001 for both). The cumulative 3-year DFS rates were 52.5%, 33.2%, and 9.5% for patients with scores of 0, 1 to 2, and 3, respectively. The 5-year DFS rates were 38.5%, 19.5%, and 9.5% for patients with scores of 0, 1 to 2, and 3, respectively. The differences in cumulative DFS rates among the 3 subgroups were statistically significant (P <0.001). For patients with scores of 0, 1 to 2, and 3 the cumulative 3-year OS rates in the corresponding subgroups were 75.1%, 54.1%, and 32.7%, respectively. The 5-year OS rates were 52.1%, 33.2%, and 25.4% for patients with scores of 0, 1 to 2, and 3, respectively. The differences in cumulative OS rates among the 3 subgroups were statistically significant (P <0.001) (Fig. 5).
Figure 5.
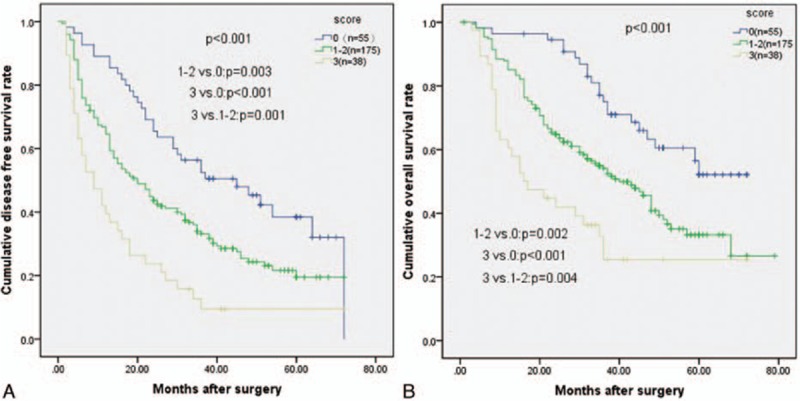
Relationship between (A) disease-free survival (DFS) and (B) overall survival (OS) in solitary large HCC patients using the simplified prognostic score system. Increasing risk score was associated with decreasing long-term survival (P <0.001 for both).
3.5. Comparison of clinicopathological features among the 3 groups (0 vs 1–2 vs 3)
Clinicopathological characteristics were compared among the 3 groups (Table 4). The low score group was older (P = 0.005) and more patients had received a blood transfusion (P = 0.030). The high score group had significantly more features of tumor invasion, such as larger tumor size, MVI, and elevated AFP levels. Moreover, the high score group had higher NLR and PLR levels. There was no significant difference in gender, HBsAg status, HBV-DNA, tumor differentiation, Ishak score, ALB, or ALT among the 3 groups (Table 4).
Table 4.
Baseline characteristics of SLHCC patients stratified by risk score.
3.6. Subgroup analysis based on liver cirrhosis status
In the current study, there were 153 patients with liver cirrhosis and 115 patients without liver cirrhosis. For noncirrhotic patients, the 3-year overall survival rate was 76.7% for patients with a score of 0, 59.4% for patients with a score of 1 to 2, and 23.3% for patients with a score of 3 (P = 0.001) (Fig. 6A). For cirrhotic patients, the 3-year overall survival rates were 74.0% for patients with a score of 0, 49.7% for patients with a score of 1 to 2, and 26.1% for patients with a score of 3. There was a significant difference among the 3 groups (P = 0.003) (Fig. 6B). The prognostic score successfully distinguished the prognoses within the subgroups.
Figure 6.
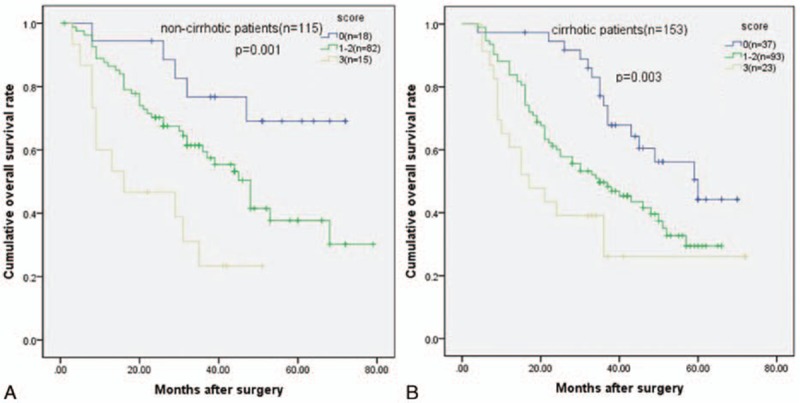
Good discriminatory power for the prognosis of solitary large HCC patients (A) without or (B) with liver cirrhosis (P = 0.001 and P = 0.003).
4. Discussion
Solitary large HCC was classified as an early-stage cancer according to the widely accepted BCLC staging system and stage T1 or T2 according to the current AJCC. The expected long-term survival rate was similar to those with small HCC. However, the prognosis varied greatly in clinical practice and some cases consisted of a large, unresectable HCC. The BCLC and American Joint Committee on Cancer (AJCC) staging systems are controversial because they do not take tumor size into account. Large HCCs appeared to exhibit a tendency toward a more aggressive behavior. When centers proposed expanded liver transplant criteria, tumor size was carefully limited to cases of a high recurrence risk and unsatisfactory long-term outcomes. The Hangzhou criteria expanded the Milan criteria and were the first expanded LT criteria to limit tumor differentiation and AFP level with tumor size or limit tumor size alone, which suggests that a large tumor size is more likely to be associated with poor oncological behavior. SLHCC can occur in patients with a heterogeneous survival probability; thus, the early stage of SLHCC is poorly defined. In the current study, we proposed a simple prognostic score for SLHCC with full consideration of tumor biological behavior. An analysis of 268 solitary large HCC patients revealed that MVI, PLR, and tumor size were independently associated with prognosis. Kaplan–Meier curves demonstrated that the 3 factors were associated with shorter OS and DFS rates. The 3 risk factors identified are consistent with previous studies. Two factors are tumor related (MVI and tumor size) and 1 reflects the immune response to the tumor (PLR). We used these factors to predict patient prognosis. For tumor morphology, tumor size is typically used to evaluate the tumor burden. For tumor biological behavior, the MVI represents a high level of invasion that is often associated with HCC recurrence and prognosis. For systematic inflammation, recent reports on the effects of inflammation on cancer prognosis indicate that inflammation and immune status are associated with tumor prognosis. Each factor identified in our study was assigned a score of 1. We combined patients with scores of 1 or 2 into 1 group because of their similar prognoses. Thus, we derived a simple prognostic score that distinguishes 3 subgroups according to their estimated risk of death. The prognostic score is simple and effective at stratifying the different prognoses. Patients with a score of 0 had a 5-year survival rate of 52.1%, whereas patients with a score of 3 had a 5-year survival rate of 25.4%. In clinical practice, patients with HCC commonly have liver cirrhosis; therefore, we applied the prognostic score to both noncirrhotic and cirrhotic patients. The scoring system was successful in these subgroups.
Microvascular invasion is an accepted risk factor in various cancers.[22,25,26] Multiple studies have reported that HCC patients with microvascular invasion have unfavorable prognoses, including a study of solitary HCC.[21,27,28] Recurrence is more common in patients with MVI than in those without MVI. Recently, a study by Shindoh et al suggested that MVI in HCC was related to the overexpression of an lncRNA termed microvascular invasion in hepatocellular carcinoma (MVIH). In turn, MVIH inhibited miR-199a (tumor suppressor) and resulted in tumor progression. It is often difficult to diagnose MVI preoperatively due to its histopathological nature. Many investigators have attempted to identify methods that can detect MVI. Banerjee et al[29] reported that contrast-enhanced computed tomography (CECT) with a biomarker-radiogenomic venous invasion noninvasively predicted histological MVI. Additionally, easily available tumor parameters, such as tumor size, were reported to be positively associated with the presence of MVI.[15,27] Patients with large HCC may also exhibit a high rate of MVI. To date, few staging systems for the prognostic classification of HCC have included MVI.
Inflammation and tumor progression greatly influence cancers.[30] Inflammation of the microenvironment plays a key role in the progression of malignancies by regulating the proliferation and survival of tumor cells. An inflammation-based score using the PLR as a prognostic factor has been investigated in various cancers.[23,31,32] Recently, a meta-analysis of a large dataset suggested that a high PLR was associated with shorter OS rates in various solid tumors.[33] Platelets guard tumor cells from immune elimination and promote their arrest at the endothelium, which contributes to tumor cell survival and spread.[34] Lymphocytes are adaptive immune cells that produce tumor-promoting chemokines and cytokines (i.e., IL-1, IL-6, tumor necrosis factor (TNF)-a, and IL-23).[35] These factors create a tumorigenic microenvironment. The combination of platelet and lymphocyte counts, which reflects the systematic inflammation of the host, improved the predictive accuracy for cancer prognoses. However, there was little consideration for associated host-related factors in the current staging systems, including the BCLC staging system and AJCC. A previous study on cervical cancer incorporated PLR to successfully stratify different prognoses of patients, suggesting that the addition of PLR may provide more accurate survival information. Similarly, our study showed that PLR enhanced the outcome prediction for SLHCC.
The effect of tumor size on prognosis remains controversial. We cannot neglect the fact that patients with larger tumor sizes were more likely to have risk factors associated with a poorer long-term survival rate. The influence of tumor size on the prognosis of patients SLHCC does not agree with previous reports suggesting that the prognosis of solitary large HCC is comparable to small HCC. Tumor size alone was not completely accurate for predicting the prognosis of SLHCC patients. Factors such as elevated AFP levels and the presence of MVI are validated predictors of prognosis.[21,36] Interestingly, in our study, tumor size was independently associated with prognosis. Similar to PLR, a tumor size greater than 6.8 cm could stratify SLHCC prognoses.
There were some limitations in our study. First, this was a retrospective study with a relatively small sample size. Second, the new prognostic score system for operable SLHCC was internally validated, but it should be externally validated in a prospective manner. Third, the frequency and dose of each postoperative therapy varied in patients and could not be assessed. The effectiveness of postoperative treatments for HCC remains unclear. Thus, a well-designed clinical trial for postoperative therapy in HCC patients could clarify this issue.
In conclusion, our newly proposed prognostic score system was successful at predicting differences prognoses in SLHCC patients. With the exception of tumor size, this study is the first to integrate PLR and MVI as prognostic factors for predicting OS and DFS rates in HCC patients. This prognostic score could aid in determining the prognosis of SLHCC patients.
Acknowledgment
The authors thank Professor Juying Zhang in the Department of Epidemiology and Biostatistics at Sichuan University for assisting with the statistical analysis.
Footnotes
Abbreviations: AASLD = American Association for the Study of Liver Disease, AFP = alpha-fetoprotein, AJCC = American Joint Committee on Cancer, ALB = albumin, ALPPS = associating liver partition with portal vein ligation for staged hepatectomy, BCLC = Barcelona clinic liver cancer, CECT = contrast-enhanced computed tomography, CI = confidence interval, DFS = disease-free survival, HBV = hepatitis B virus, HR = hazard ratio, MVI = microvascular invasion, MVIH = microvascular invasion in hepatocellular carcinoma, NLR = neutrophil to lymphocyte ratio, PLR = platelet to lymphocyte ratio, SLHCC = solitary large HCC, TNF = tumor necrosis factor.
The authors have no conflicts of interest to disclose.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Zhu SL, Ke Y, Peng YC, et al. Comparison of long-term survival of patients with solitary large hepatocellular carcinoma of BCLC stage A after liver resection or transarterial chemoembolization: a propensity score analysis. PLoS One 2014; 9:e115834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Min YW, Lee JH, Gwak GY, et al. Long-term survival after surgical resection for huge hepatocellular carcinoma: comparison with transarterial chemoembolization after propensity score matching. J Gastroenterol Hepatol 2014; 29:1043–1048. [DOI] [PubMed] [Google Scholar]
- 4.Liu L, Zhang QS, Pan LH, et al. Subclassification of patients with solitary hepatocellular carcinoma based on post-hepatectomy survival: a large retrospective study. Tumour Biol 2016; 37:5327–5335. [DOI] [PubMed] [Google Scholar]
- 5.Zhong JH, Rodriguez AC, Ke Y, et al. Hepatic resection as a safe and effective treatment for hepatocellular carcinoma involving a single large tumor, multiple tumors, or macrovascular invasion. Medicine 2015; 94:e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Haese JG, Neumann J, Weniger M, et al. Should ALPPS be used for liver resection in intermediate-stage HCC? Ann Surg Oncol 2016; 23:1335–1343. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Li C, Wen TF, et al. Is hepatectomy for huge hepatocellular carcinoma (>/= 10 cm in diameter) safe and effective? A single-center experience. Asian Pac J Cancer Prev 2014; 15:7069–7077. [DOI] [PubMed] [Google Scholar]
- 8.Jung YK, Jung CH, Seo YS, et al. BCLC stage B is a better designation for single large hepatocellular carcinoma than BCLC stage A. J Gastroenterol Hepatol 2016; 31:467–474. [DOI] [PubMed] [Google Scholar]
- 9.Pandey D, Lee KH, Wai CT, et al. Long term outcome and prognostic factors for large hepatocellular carcinoma (10 cm or more) after surgical resection. Ann Surg Oncol 2007; 14:2817–2823. [DOI] [PubMed] [Google Scholar]
- 10.Chen XP, Qiu FZ, Wu ZD, et al. Long-term outcome of resection of large hepatocellular carcinoma. Br J Surg 2006; 93:600–606. [DOI] [PubMed] [Google Scholar]
- 11.Zhou L, Rui JA, Wang SB, et al. Prognostic factors of solitary large hepatocellular carcinoma: the importance of differentiation grade. Eur J Surg Oncol 2011; 37:521–525. [DOI] [PubMed] [Google Scholar]
- 12.Yang LY, Fang F, Ou DP, et al. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg 2009; 249:118–123. [DOI] [PubMed] [Google Scholar]
- 13.Ariizumi S, Kotera Y, Takahashi Y, et al. Impact of hepatectomy for huge solitary hepatocellular carcinoma. J Surg Oncol 2013; 107:408–413. [DOI] [PubMed] [Google Scholar]
- 14.Pang Q, Zhang JY, Xu XS, et al. Significance of platelet count and platelet-based models for hepatocellular carcinoma recurrence. World J Gastroenterol 2015; 21:5607–5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao WC, Fan LF, Yang N, et al. Preoperative predictors of microvascular invasion in multinodular hepatocellular carcinoma. Eur J Surg Oncol 2013; 39:858–864. [DOI] [PubMed] [Google Scholar]
- 16.Goh BK, Teo JY, Chan CY, et al. Importance of tumor size as a prognostic factor after partial liver resection for solitary hepatocellular carcinoma: implications on the current AJCC staging system. J Surg Oncol 2016; 113:89–93. [DOI] [PubMed] [Google Scholar]
- 17.Hwang S, Lee YJ, Kim KH, et al. The impact of tumor size on long-term survival outcomes after resection of solitary hepatocellular carcinoma: single-institution experience with 2558 patients. J Gastrointest Surg 2015; 19:1281–1290. [DOI] [PubMed] [Google Scholar]
- 18.Minagawa M, Ikai I, Matsuyama Y, et al. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg 2007; 245:909–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llovet JM, Fuster J, Bruix J. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transplant 2004; 10:S115–S120. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa H, Arimoto A, Wakasa T, et al. Comparison of clinical characteristics and survival after surgery in patients with non-B and non-C hepatocellular carcinoma and hepatitis virus-related hepatocellular carcinoma. J Cancer 2013; 4:502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim KC, Chow PK, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg 2011; 254:108–113. [DOI] [PubMed] [Google Scholar]
- 22.Patel AJ, Daniel G, Naidu B, et al. The significance of microvascular invasion after complete resection of early-stage non-small-cell lung cancer. Interact Cardiovasc Thorac Surg 2016; 22:101–105. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Chen ZH, Xing YF, et al. Platelet-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma. Tumour Biol 2015; 36:2263–2269. [DOI] [PubMed] [Google Scholar]
- 24.Okamura Y, Sugiura T, Ito T, et al. Neutrophil to lymphocyte ratio as an indicator of the malignant behaviour of hepatocellular carcinoma. Br J Surg 2016; 103:891–898. [DOI] [PubMed] [Google Scholar]
- 25.Huang H, Pan XW, Huang Y, et al. Microvascular invasion as a prognostic indicator in renal cell carcinoma: a systematic review and meta-analysis. Int J Clin Exp Med 2015; 8:10779–10792. [PMC free article] [PubMed] [Google Scholar]
- 26.Grimm M. Prognostic value of clinicopathological parameters and outcome in 484 patients with oral squamous cell carcinoma: microvascular invasion (V+) is an independent prognostic factor for OSCC. Clin Transl Oncol 2012; 14:870–880. [DOI] [PubMed] [Google Scholar]
- 27.Hirokawa F, Hayashi M, Miyamoto Y, et al. Outcomes and predictors of microvascular invasion of solitary hepatocellular carcinoma. Hepatol Res 2014; 44:846–853. [DOI] [PubMed] [Google Scholar]
- 28.Tsai TJ, Chau GY, Lui WY, et al. Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery 2000; 127:603–608. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee S, Wang DS, Kim HJ, et al. A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology 2015; 62:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coffelt SB, de Visser KE. Cancer: inflammation lights the way to metastasis. Nature 2014; 507:48–49. [DOI] [PubMed] [Google Scholar]
- 31.You J, Zhu GQ, Xie L, et al. Preoperative platelet to lymphocyte ratio is a valuable prognostic biomarker in patients with colorectal cancer. Oncotarget 2016; 7:25516–25527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao QT, Yuan Z, Zhang H, et al. Prognostic role of platelet to lymphocyte ratio in non-small cell lung cancers: a meta-analysis including 3,720 patients. Int J Cancer J Int Cancer 2016; 139:164–170. [DOI] [PubMed] [Google Scholar]
- 33.Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2014; 23:1204–1212. [DOI] [PubMed] [Google Scholar]
- 34.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer 2011; 11:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140:883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang SL, Liu LP, Yang S, et al. Preoperative serum alpha-fetoprotein and prognosis after hepatectomy for hepatocellular carcinoma. Br J Surg 2016. [DOI] [PubMed] [Google Scholar]


