Supplemental Digital Content is available in the text
Keywords: Acinetobacter baumannii, meningitis, mortality, neurosurgery, Staphylococcus aureus, trauma
Abstract
Postcraniotomy meningitis (PCM) is a major challenge in neurosurgery, and changing patterns of infectious agents in PCM have been noted. The limited epidemiological data and urgent clinical needs motivated this research. We conducted this study to determine a risk assessment for PCM and the current pattern of infectious agents.
We performed a retrospective case-control study of significant cases of postcraniotomy meningitis in the Changhua Christian Hospital System between January 1, 2008, and December 31, 2012. Postcraniotomy meningitis was diagnosed in 22 out of 4392 surgical patients; this data was reviewed for risk assessment.
This study assessed the risk factors for postcraniotomy meningitis and found that it was more frequently seen in patients who were elderly (OR = 1.57, 95% CI = 1.32–2.98, P = 0.013), underwent emergency procedures (OR = 4.82, 95% CI = 1.50–14.53, P = 0.008), had leak of cerebrospinal fluid (OR = 4.62, 95% CI = 2.03–10.50, P = 0.012), had external ventricular drainage (OR = 4.68, 95% CI = 2.46–8.87, P = 0.006), were admitted to the intensive care unit (OR = 2.41, 95% CI = 1.53–8.08, P = 0.012), had used drain placement >72 hours (OR = 2.66, 95% CI = 1.04–4.29, P = 0.007), had surgery >4.5 hours (OR = 2.38, 95% CI = 1.39–4.05, P = 0.005), had repeat operations (OR = 2.74, 95% CI = 1.31–5.73, P = 0.018), endured trauma (OR = 5.97, 95% CI = 1.57–17.61, P = 0.007), or had 30-days mortality (OR = 5.07, 95% CI = 2.20–11.48, P = 0.001). The predominant pathogens isolated from cerebrospinal fluid were Staphylococcus aureus in 8 patients (36.7%) and Acinetobacter baumannii in 7 patients (31.8%). In our study, the mortality rate was 5.1% among all postcraniotomy patients.
Accurate risk assessment, early diagnosis, and choice of appropriate antibiotics in accordance with epidemiologic information are the cornerstones of reducing mortality and morbidity in PCM. The changing pattern of infectious agents in PCM over time suggests the necessity of further studies to provide the most up-to-date insight to physicians.
1. Introduction
Postcraniotomy meningitis (PCM) is an important challenge in neurosurgery, resulting in severe morbidity and mortality.[1–3] The rates of PCM that have been reported vary from 0.3% to 10%, and Staphylococcus aureus (S aureus) is the major pathogen.[3–5] Some studies reveal that the rate of PCM due to Acinetobacter calcoaceticus–Acinetobacter baumannii complex (A baumannii) has been increasing;[2,6–8] however, the limited epidemiological data can be perplexing for physicians, in both making accurate diagnoses and choosing effective antibiotics.
PCM results in a high mortality rate and a longer hospital stay;[1] the most commonly isolated etiologic agents vary between Gram-negative bacilli[1,9,10] and S aureus.[3,11,12] After Mombelli's report in 1983, Gram-negative bacillary PCM related to traumatic cranial neurosurgery was more widely diagnosed.[9]A baumannii has emerged as a significant nosocomial pathogen in hospitalized patients worldwide, and A baumannii meningitis (ABM) causes high mortality and morbidity rates.[7,13,14] In addition, the rate of PCM due to A baumannii has been growing in China, Iran, and Turkey.[2,6–8]
There have been few studies examining a risk assessment for ABM, especially those investigating the initial clinical features of patients with PMC.[15] The limited epidemiological data and urgent clinical needs are the motivations for performing this study.
To understand the demographic features of patients with PCM, we conducted this study to determine (i) a risk assessment of PCM and (ii) the current pattern of its infectious agents in our healthcare system.
2. Materials and methods
2.1. Clinical setting and study population
The Changhua Christian Hospital System (CCHS) provides care to most of the Changhua population in Taiwan and has maintained comprehensive clinical records since 1867. Changhua Christian Hospital (CCH) is a member of the CCHS; it is an 1800-bed tertiary referral medical center located in northern Changhua County. More than 1500 craniotomies are performed in our healthcare system every year (Appendix 1). Ours is a retrospective case-control study and included 22 patients who developed post-neurosurgical meningitis among the 4392 patients who underwent craniotomy procedures between January 1, 2008, and December 31, 2012. Case group was defined as those patients who developed postneurosurgical meningitis among the 4392 patients who underwent craniotomy procedures between January 1, 2008, and December 31, 2012. Control group was defined as those patients who did not develop meningitis among the 4392 patients who underwent craniotomy procedures between January 1, 2008, and December 31, 2012.
2.2. Case findings and the validity and reliability of methods
Cases of post-craniotomy meningitis were identified from microbiological databases and medical records at CCH in central Taiwan, using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). We used computerized indices to find cases with the following ICD-9-CM codes: 320–324, 326, 038, 038.11, 038.19, 785.59, and procedures involving operation of the nervous system (01.21–01.25, 01.28). We performed a small-scale validity study on our case-finding procedures. We examined the reliability of our case-finding procedure by randomly selecting 20 records of potential cases and having them reviewed independently by 2 infectious disease specialists (CCH and CCY).
2.3. Definitions
The diagnosis of meningitis was based on the following inclusion criteria: a micro-organism must have been isolated either from cerebrospinal fluid (CSF) cultures or from blood cultures taken during the initial diagnostic workup; clinical presentation with acute new onset of fever, headache, stiff neck, meningeal signs, cranial nerve signs, irritability, or turbid CSF in drainage bag; elevated white blood cell count and elevated CSF protein and/or decreased glucose; and organisms seen on Gram stain of CSF. The exclusion criteria were incomplete data; inconsistency between the data from the ICD-9 code and the clinical dataset; and diagnosis of a CNS infection, brain abscess, or meningitis on the first neurosurgical day. The definition of surgical site infection (SSI) was based on Centers for Disease Control and Prevention/National Healthcare Safety Network surveillance definitions.[16] Polymicrobial meningitis was considered when the etiology was >1 pathogen. We only considered the predominant culturable pathogen of each case when performing statistical analysis. The disease incidence was calculated by considering the entire admitted patient population of CCH that was at risk for infection. Recurrent meningitis was considered when the patient had >1 episode of meningitis, and only the first episode of PCM in each patient during the study period was included in the statistical analysis. Mortality was defined as the patients with PCM who died within 30 days after diagnosis of PCM. The effective duration of antibiotics was based on clinical presentation and microbiological susceptibility tests. The relationship between a patient's mortality and postoperative meningitis was based on clinical presentation and laboratory data.
2.4. Patient care and protocol for patients with suspected meningitis
All surgical patients received prophylactic antibiotics (cefazolin) before the induction of anesthesia. Lumbar puncture was performed in all patients who did not have external ventricular drainage (EVD) or lumbar drainage and were suspected to have postoperative meningitis. CSF and blood cultures were taken at the same time. Infection of other sites was excluded by taking various cultures. All isolates were routinely examined at the medical laboratory department of CCH and were routinely stored at the bacterial bank of CCH during the study period. The Vitek 2 System (BioMerieux, Marcy l’Etoile, France) was used to identify the phenotype of isolates. Antimicrobial susceptibility was determined using the automated Vitek 2 (BioMerieux) according to the recommendations of the clinical and laboratory standards institute.[17]
2.5. Statistical analysis
Descriptive statistics were calculated using SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL) in order to analyze the case characteristics and laboratory parameters for each group. The demographic data and clinical characteristics were compared by using the χ2 test or Student's t-test when appropriate. Results were analyzed using the χ2 test. The multivariate analysis model was used to evaluate the risk factors that affected mortality and were significant according to univariate analysis. Odds ratio (OR) with 95% confidence interval (CI) was calculated. A 2-sided test with a P value < 0.05 was considered as statistically significant.
3. Results
3.1. Demographics of postcraniotomy patients
The study flowchart showing the selection of subjects during the study period is shown in Fig. 1. During the study period, PCM was diagnosed in 22 out of 4392 (1.4%) surgical patients (Table 1). The period prevalence of PCM in CCHS was 134.4 ± 76.8 per 100,000 patient-years. The incidence of PCM was 1.4% among 4392 surgical patients in CCHS. A total of 3455 (3455/4392, 78.7%) patients were male and the mean age [± SD] was 45 ± 13.8 years. The craniotomies were performed as emergency procedures in 2524 (72.3%) patients. In total, 1256 (28.6%) patients had EVD, and 1160 (26.4%) patients were admitted to the intensive care unit (ICU). The medium period from admission to diagnosis of PCM was 10.5 ± 2.1 days (range, 2–30 days). The mean hospitalization duration was 18.6 ± 3.1 days (range, 2–69 days) and the mean postcraniotomy hospitalization duration was 6.32 ± 1.5 days (range, 1–35 days). The overall mortality rate of patients who received craniotomy was 5.5% (242/4392).
Figure 1.
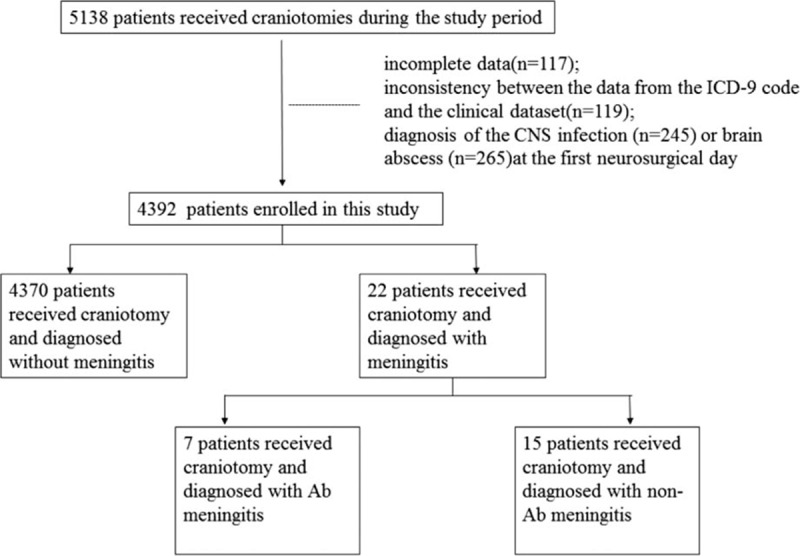
Study flowchart showing the selection of subjects.
Table 1.
Demography and clinical characteristics of patients who received postcraniotomy operation between those with meningitis and those without meningitis.
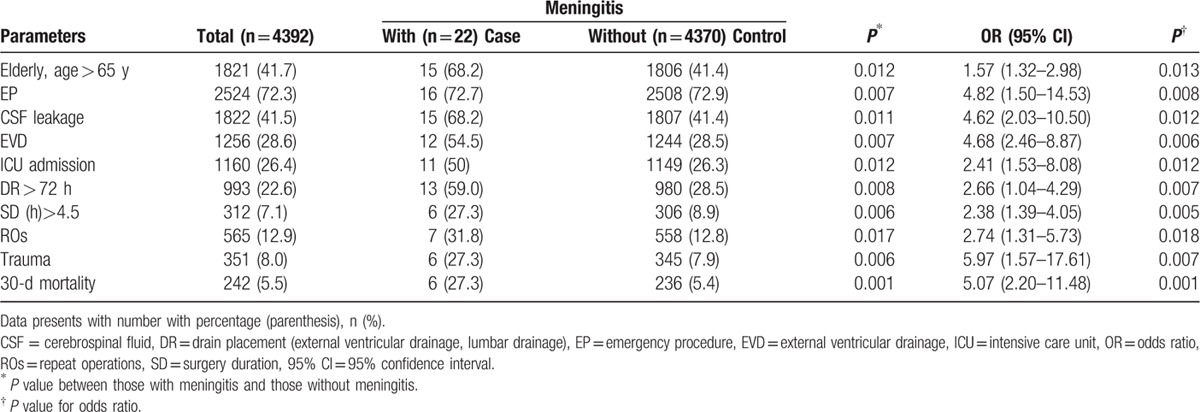
3.2. Comparison of postcraniotomy meningitis
The variables used to compare patients with PCM to patients without PCM in the univariate analysis are listed in Table 1. Factors with a P value of < 0.05 included age >65 years (OR = 1.57, 95% CI = 1.32–2.98, P = 0.013), emergency procedure (OR = 4.82, 95% CI = 1.50–14.53, P = 0.008), CSF leak (OR = 4.62, 95% CI = 2.03–10.50, P = 0.012), EVD (OR = 4.68, 95% CI = 2.46–8.87, P = 0.006), ICU admission (OR = 2.41, 95% CI = 1.53–8.08, P = 0.012), duration of drain placement longer than 72 hours (OR = 2.66, 95% CI = 1.04–4.29, P = 0.007), surgery duration longer than 4.5 hours (OR = 2.38, 95% CI = 1.39–4.05, P = 0.005), repeat operations (OR = 2.74, 95% CI = 1.31–5.73, P = 0.018), trauma (OR = 5.97, 95% CI = 1.57–17.61, P = 0.007), and 30-days mortality (OR = 5.07, 95% CI = 2.20–11.48, P = 0.001).
Six patients who had traumatic head injury and received a craniotomy suffered from meningitis; 3 of these had mixed pathogens and the predominant bacteria of those mixed pathogens were analyzed in this study. The predominant pathogens among those 6 head injury patients with postcraniotomy meningitis were A baumannii in 5 and S aureus in 1.
3.3. Demographics of patients with postcraniotomy meningitis
Twenty-two (0.5%) patients had PCM. The 5-year longitudinal analysis of HAI, SSI, total number of craniotomies, and total number of PCM during the study period is shown in Fig. 2. Seven of 22 PCM patients did not meet the criteria of SSI because they had open wounds on admission. Fever was observed in 15 (68.2%) patients. There was CSF leak in 15 (68.2%) patients. Four (18.2%) patients had bacteremia. Nineteen (86.4%) patients had leukocytosis with left shift and 19 (86.4%) patients had elevated C-reactive protein at the initial assessment. CSF cell count was > 5 elements/mm3 in 22 (100%) examinations. The CSF protein level was > 500 mg/dL in 6 (27.2%) cases, and the ratio of CSF glucose level to serum glucose was < 0.5 in 19 (86.4%) examinations.
Figure 2.
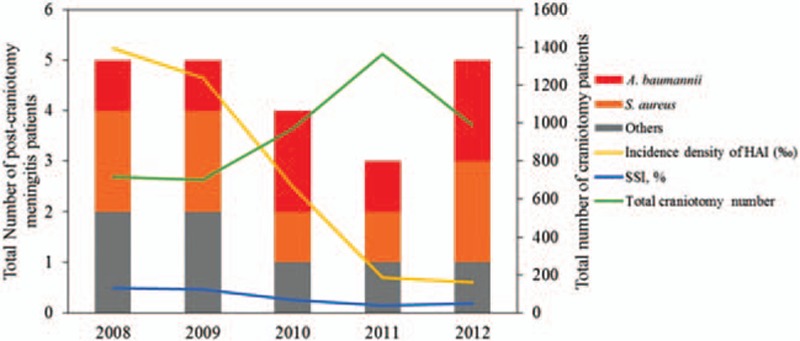
The correlation among healthcare-associated infection, surgical site infection, and total craniotomy number and total postcraniotomy meningitis during the study period.
The pathogens isolated from CSF were S aureus in 8 patients (36.7%), A baumannii in 7 patients (31.8%), P aeruginosa in 2 patients (9.1%), K pneumoniae in 2 patients (9.1%), E cloacae in 1 patient (4.5%), E coli in 1 patient (4.5%), and E faecalis in 1 patient (4.5%). Six (75%) of 8 S aureus isolates were methicillin resistant. The median time from diagnosis to effective treatment (treatment with antibiotics to which the organisms were susceptible) was 3.2 ± 0.6 days (range, 1–5 days) during the empirical therapy. All patients received appropriate antibiotic therapy based on susceptibility tests. Among patients with ABM, carbapenem with colistin was used in 5 patients (71.4%) and ceftriaxone with colistin was used in 2 (28.6%). Two (28.6%) of the patients with ABM died. Four of them were given meropenem treatment. For K pneumoniae meningitis, ceftriaxone was used in 2 patients. Vancomycin was given to patients with methicillin-resistant S aureus (n = 6) and E faecalis (n = 1). One (16.7%) of the 6 patients with methicillin-resistant S aureus meningitis died.
Of the 22 patients with PCM, 14 (63.6%) of the patients had at least 1 concurrent HAI. The distribution of these infections were as follows: SSI in 15 patients (68.2%), pneumonia in 4 (28.6%), bacteremia in 4 (28.6%), urinary tract infection in 2 (14.3%), and central venous catheter-related infection in 1 (7.1%). Of the 22 patients with PCM, 6 (27.3%) died. Of the 6 patients who died, 5 (83.3%) had ABM. The clinical information for ABM is listed in Appendix 2.
4. Discussion
4.1. Risk assessment for PCM
This study assessed the risk factors for PCM. PCM was more frequently seen in patients who had the following risk factors: old age, emergency procedure, CSF leak, EVD, ICU admission, duration of drain placement >4.5 hours, surgery duration >4.5 hours, repeat operations, and trauma. In our study, the 30-day mortality rate of 22 PCM patients was 0.14 % among 4392 postcraniotomy patients.
In our study, the period prevalence of PCM in CCHS was 134.4 ± 76.8 per 100,000 patient-years and the incidence of PCM was 1.4% among 4392 surgical patients in CCHS. The reported incidence of PCM is quite variable in the literature, ranging from 0.5% to 5.5%.[3,8,12,18] The incidences of PCM in studies by Kourbeti et al (5.5%)[18] and Srinivas et al (2.2%)[12] were remarkably higher than in our study (1.4%). The reasons for the lower incidence of PCM in our study included prophylactic antibiotics given to all patients after the surgery, few SSI events, few traumatic patients, the low use of drainage, and the low number of trans-sphenoidal surgeries. Although the risk factors for PCM are well established, it is nearly impossible to reach the goal of zero HAIs in clinical practice. PCM is still a threat in daily practice. Physicians must pay attention to PCM as it can result in severe morbidity and mortality.[1–3]
4.2. Changing patterns in pathogens of post-traumatic patients with PCM
This study showed that AMB emerged among post-traumatic patients with PCM. Many studies have described that HAI and postneurosurgical ABM are becoming increasingly more common.[6,8,9,13,19–23] In the present study, the most commonly isolated agents for PCM were A baumannii and S aureus, and there was no institutional outbreak of these organisms in CCHS during the study period. The predominance of these PCM pathogens may be related to global emergence. A baumannii has emerged as an important healthcare-associated pathogen because it can survive a wide range of environmental conditions and persist for.[24,25] And, the extensive use of medical equipment in modern institutes has contributed to the emergence of A baumannii as a predominant pathogen.[24,25] An increase of A baumannii colonization and infection results from skin or wound colonization, invasive procedures, entry through traumatic wounds, or a type of predominant cluster. In our results, S aureus was still the most common pathogen in patients with PCM, but we emphasize that physicians should also consider A baumannii when caring for PCM patients.
4.3. Considerations for post-traumatic patients with PCM
In our study, 6 of 22 (27.37%) patients with PCM died. Of the 6 patients who died, 5 (83.3%) had ABM. Five (71.4%) of 7 ABM patients had a history of traumatic injury and died. Regarding the risk factor of traumatic head injury in ABM, there has been a remarkable increase in the incidence of A baumannii.[8,24–26] In this study, we found 8 patients with postcraniotomy S aureus meningitis and 7 with postcraniotomy ABM, six of whom suffered from traumatic head injuries. However, the predominant pathogens among patients with PCM in this study were different from those of other studies.[6,8,9,13,19–23,27] The reasons for this could be due to multiple factors, including geographic difference, local predominant epidemiological features, population, and compliance with infection control guidelines. Thus, we concluded that post-traumatic patients were easily susceptible to A baumannii and S aureus infection. Because the clinical symptoms of ABM vary and some of causative pathogens include multiple-drug resistant A baumannii, it is very important to determine the most effective antibiotic strategy as well as ensure accurate and effective diagnosis and treatment of ABM. Physicians should be alert to the changing patterns of infectious agents in specific individuals with PCM, and promptly prescribe accurate antibiotics at the initial stages.
The present study has several strengths. The most important strength was that we accumulated a complete longitudinal dataset covering 5 years. We had access to excellent data for evaluating the demographic features of PCM and ABM. Secondly, patients with culture positive meningitis were included for analysis, so our results have representational faithfulness. Thirdly, >1500 craniotomies are performed in our healthcare system annually (Appendix 1), and this ensures that we have enough power in our analysis. Overall, the present findings provide invaluable epidemiological information about PCM in central Taiwan.
Nevertheless, this study has several limitations. The individual effect of each intervention was not evaluated, because interventions were performed either individually and simultaneously or sequentially according to the complexity of the clinical conditions. Moreover, compliance with the various measures for infection control during the study period could not be verified because part of study was performed retrospectively, and only the patients with culturable pathogens were enrolled and analyzed. However, these cases are not likely to constitute a large proportion of PCM cases. As this is a retrospective study, history information on antibiotics was possibly inaccurate for the numerous patients who had received antibiotics within 1 month prior to their PCM episode. Due to a lack of adequate workforce, the defined daily doses of the some antibiotics administered during the study period could not be calculated. In addition, we did not perform molecular typing of A baumannii isolates owing to no evidence of an outbreak during the study period. Further causal relationship studies and nationwide studies are necessary to elucidate ABM among patients with PCM.
5. Conclusion
In conclusion, PCM should be suspected in patients with new onset fever or unexplained deterioration in neurologic status. Early diagnosis and choice of appropriate antibiotics according to epidemiologic information are the cornerstones of reducing mortality and morbidity. Along with S aureus, we found a high rate of PCM due to A baumannii among patients with traumatic head injury. The choice of empirical therapy for post-traumatic patients with suspected PCM should cover S aureus and A baumannii. The changing pattern of infectious agents in PCM over time suggests the necessity of other studies to give the most up-to-date insight to physicians.
Acknowledgments
The authors thank the staffs at the Clinical Microbiological Laboratory, the Department of Medical Records, and the Department of Computer at Changhua Christian Hospital for the case findings. The authors thank Professor Min-Chi Liu from Chung Shan Medical University for proofreading this article. This research project would not have been possible without the support of many people. The authors wish to express their gratitude to the staffs of the Department of Neurosurgery, the Department of Critical Care, the Department of Trauma, Infection Control Committee, the Division of Infectious Diseases, the Department of Medical Radiology, the Department of Nursing, the Department of Healthcare Quality, and the Department of Pharmacology at Changhua Christian Hospital who were extremely helpful and provided invaluable assistance and support. They thank Fu-Chou Chen from Taichung Veterans General Hospital for reviewing this article.
Supplementary Material
Footnotes
Abbreviations: A baumannii = Acinetobacter calcoaceticus–Acinetobacter baumannii complex, ABM = A baumannii meningitis, CCH = Changhua Christian Hospital, CCHS = Changhua Christian Hospital System, CI = confidence interval, CSF = cerebrospinal fluid, EVD = external ventricular drainage, HAI = healthcare-associated infection, OR = Odds ratio, PCM = postcraniotomy meningitis, S aureus = Staphylococcus aureus.
Funding: Changhua Christian Hospital (grant 103-CCH-IPR-001, 104-CCH-IPR-001, and 105-CCH-IPR-001).
Ethical approval: The study was approved by the institutional review board of Changhua Christian Hospital (CCH IRB No. 160604) with waiver for informed consents.
The authors have no conflicts of interest to disclose.
Competing interests: All authors declare that they have no competing interests.
Supplemental Digital Content is available for this article.
References
- 1.Reichert MC, Medeiros EA, Ferraz FA. Hospital-acquired meningitis in patients undergoing craniotomy: incidence, evolution, and risk factors. Am J Infect Control 2002; 30:158–164. [DOI] [PubMed] [Google Scholar]
- 2.Chen C, Zhang B, Yu S, et al. The incidence and risk factors of meningitis after major craniotomy in China: a retrospective cohort study. PLoS One 2014; 9:e101961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClelland S, 3rd, Hall WA. Postoperative central nervous system infection: incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis 2009; 45:55–59. [DOI] [PubMed] [Google Scholar]
- 4.van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med 2010; 362:146–154. [DOI] [PubMed] [Google Scholar]
- 5.Chang SM, Parney IF, McDermott M, et al. Glioma Outcomes Investigators. Glioma outcomes investigators. Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the glioma outcome project. J Neurosurg 2003; 98:1175–1181. [DOI] [PubMed] [Google Scholar]
- 6.Yadegarynia D, Gachkar L, Fatemi A, et al. Changing pattern of infectious agents in postneurosurgical meningitis. Caspian J Intern Med 2014; 5:170–175. [PMC free article] [PubMed] [Google Scholar]
- 7.Metan G, Alp E, Aygen B, et al. Carbapenem-resistant acinetobacter baumannii: an emerging threat for patients with post-neurosurgical meningitis. Int J Antimicrob Agents 2007; 29:112–113. [DOI] [PubMed] [Google Scholar]
- 8.Kourbeti IS, Vakis AF, Ziakas P, et al. Infections in patients undergoing craniotomy: risk factors associated with post-craniotomy meningitis. J Neurosurg 2015; 122:1113–1119. [DOI] [PubMed] [Google Scholar]
- 9.Mombelli G, Klastersky J, Coppens L, et al. Gram negative bacillary meningitis in neurosurgical patients. J Neurosurg 1983; 58:634–641. [DOI] [PubMed] [Google Scholar]
- 10.Briggs S, Ellis-Pegler R, Raymond N, et al. Gram-negative bacillary meningitis after cranial surgery or trauma in adults. Scand J Infect Dis 2009; 36:165–173. [DOI] [PubMed] [Google Scholar]
- 11.Logigan C, Mihalache D, Dorneanu O, et al. Study of nosocomial bacillary meningitis admitted in the Clinic of Infectious Diseases Iasi on a 20 years period. Rev Med Chir Soc Med Nat Iasi1 2009; 13:721–726.[Romanian]. [PubMed] [Google Scholar]
- 12.Srinivas D, Veena Kumari HB, Somanna S, et al. The incidence of postoperative meningitis in neurosurgery: an institutional experience. Neurol India 2011; 59:195–198. [DOI] [PubMed] [Google Scholar]
- 13.Gulati S, Kapil A, Das B, et al. Nosocomial infections due to Acinetobacter baumannii in a neurosurgery ICU. Neurol India 2001; 49:134–137. [PubMed] [Google Scholar]
- 14.Wroblewska MM, Dijkshoorn L, Marchel H, et al. Outbreak of nosocomial meningitis caused by Acinetobacter baumannii in neurosurgical patients. J Hosp Infect 2004; 57:300–307. [DOI] [PubMed] [Google Scholar]
- 15.deFreitas DJ, McCabe JP. Acinetobacter baumanii meningitis:a rare complication of incidental durotomy. J Spinal Disord Tech 2004; 17:115–116. [DOI] [PubMed] [Google Scholar]
- 16.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definitions for specific types of infections. Am J Infect Control 2008; 36:309–332. [DOI] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Twenty-second informational supplement (M100-S22). Wayne, PA: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 18.Kourbeti IS, Jacobs AV, Koslow M, et al. Risk factors associated with postcraniotomy meningitis. Neurosurgery 2007; 60:317–325. [DOI] [PubMed] [Google Scholar]
- 19.Briggs S, Ellis-Pegler R, Raymond N, et al. Gram-negative bacillary meningitis after cranial surgery or trauma in adults. Scand J Infect Dis 2004; 36:165–173. [DOI] [PubMed] [Google Scholar]
- 20.Howard A, O’Donoghue M, Feeney A, et al. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence 2012; 3:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krcmery V, Paradisi F. Nosocomial bacterial and fungal meningitis in children: an eight year national survey reporting 101 cases. Int J Antimicrob Agents 2000; 15:143–147. [DOI] [PubMed] [Google Scholar]
- 22.O’Neill E, Humphreys H, Phillips J, et al. Third-generation cephalosporin resistance among Gram-negative bacilli causing meningitis in neurosurgical patients: significant challenges in ensuring effective antibiotic therapy. J Antimicrob Chemother 2006; 57:356–359. [DOI] [PubMed] [Google Scholar]
- 23.Huang CR, Lu CH, Chuang YC, et al. Adult Pseudomonas aeruginosa meningitis: high incidence of underlying medical and/or postneurosurgical conditions and high mortality rate. Jpn J Infect Dis 2007; 60:397–399. [PubMed] [Google Scholar]
- 24.Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis 2006; 42:692–699. [DOI] [PubMed] [Google Scholar]
- 25.Chen CH, Lin LC, Chang YJ, et al. Long-term effectiveness of infection and antibiotic control programs on the transmission of carbapenem-resistant Acinetobacter calcoaceticus–Acinetobacter baumannii complex in central Taiwan. Med Mal Infect 2015; 45:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moiyadi AV, Shetty P, Biswas S. Fulminant post-craniotomy wound infection and meningitis with pneumocephalus caused by Acinetobacter baumannii: an unusual presentation. J Neurosci Rural Pract 2013; 4 suppl 1:S136–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaynes R, Edwards JR. National Nosocomial Infections Surveillance System. Overview of Nosocomial Infections Caused by Gram-Negative Bacilli. Clin Infect Dis 2005; 41:848–854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


