Abstract
Children, especially infants, are highly vulnerable to iron-deficiency anemia because of their rapid growth of the brain and the rest of the body. The objectives of this study were to compare the prevalence of iron-deficiency anemia in infants born from HIV-positive mothers and HIV-negative mothers and to identify the determinants of iron-deficiency anemia in infants.
A comparative cross-sectional study was conducted in Bahir Dar city. Simple random sampling technique was used to select the study participants. Mothers were interviewed; blood samples were collected from mothers and infants to measure the hemoglobin level and anthropometric indicators were obtained from the infants using world health organization standards. Descriptive statistics were used to estimate the prevalence of infantile anemia. Binary logistic regression and multiple linear regressions were used to identify the determinants of infant anemia.
A total of 1459 infants born from HIV-positive and HIV-negative mothers were included. The prevalence of iron-deficiency anemia in infants born from HIV-positive and HIV-negative mothers was 41.9% (95% CI: 39–44). Infantile iron-deficiency anemia was associated with maternal HIV infection (adjusted odds ratio [AOR] 2.54 [95% CI: 1.65–3.9]), stunting (AOR 3.46 [95% CI: 2.41–4.97]), low income (AOR 2.72 [95% CI: 2–3.73]), maternal malaria during pregnancy (AOR 1.81 [95% CI: 1.33–2.47]), use of cow milk before 6 month (AOR 1.82 [95% CI: 1.35–2.45]), residence (AOR 0.09 [95% CI: 0.06–0.13]), history of cough or fever 7 days preceding the survey (AOR 2.71 [95% CI: 1.99–3.69]), maternal hemoglobin (B 0.65 [95% CI: 0.61–0.68]), educational status of mother (B 0.22 [95% CI: 0.2–0.23]), age of the mother (B –0.03 [95% CI: –0.03, –0.02]), and family size (B –0.14 [95% CI: –0.18,–0.11]).
Keywords: anemia, determinants, HIV infection, infants, prevalence
1. Introduction
Red blood cells are one of the formed elements of blood and they perform transportation of nutrients and waste products.[1] The total numbers of red blood cells present in human beings vary by genders and age. In children hemoglobin count <11 mg/dL is defined as anemia.[2–4] Anemia is a condition in which the hemoglobin level of the individual is low and one of the great burdens of the world. Globally >1.5 billion people are affected by anemia.[5] Children especially infants are highly vulnerable to anemia because of their rapid growth of brain and the rest of the body.[6–8] In developing country 42% of under 5 years of age children are anemic.[9]
Iron-deficiency anemia in early childhood has numerous effects that are nonreversible at later age. Anemia in infancy can lead to impairment in brain development, delay in brain maturation, thereby reducing the cognitive function of the child, decrease in the growth of the child, and increase in the risk of infection because of its effect on immunity.[10–18]
The burden of childhood anemia differs from continent to continent: in North America 17% to 50% of children are anemic.[19–21] In South America, the prevalence of anemic in children ranges from 13% to 40.9%.[21–23] In Europe, 6% to 14% of children are anemic.[21,24] In Asia, 19.7% to 60.8%, [25–27] in Africa, 12% to 78.6%,[21,28–30] and in Ethiopia, 25% to 75% of under 5 years of children are anemic.[31–33]
Different findings across the globe revealed that childhood iron-deficiency anemia is associated with nutritional status, duration of breast feeding, maternal education, overweight, residence, use of cow milk before 6 months, low birth weight (<3 kg), gender (higher in male), child or maternal age, maternal underweight, family size, income, dietary diversity, HIV infection, serum ferritin level, fever and cough in the preceding 7 days, frequent morbidity and low maternal CD4 count at enrolment, placental malaria and maternal anemia.[20–25,27,28,30,32,34]
Information regarding infantile anemia was scarce and this led to failure to plan effective anemia prevention in infants. Many children suffer from irreversible harm because of improper anemia prevention activities during their infancy period. This study will fill these gaps and will alert decision makers to prioritize infantile anemia prevention and control intervention. This study will also benefit scholars to investigate more into infantile anemia by acting as a baseline document.
The objectives of this study were to compare the prevalence of iron-deficiency anemia in infants born from HIV-positive mothers and HIV-negative mothers and to identify the determinants of iron-deficiency anemia in infants.
2. Methods and materials
A community-based comparative cross-sectional study was conducted in Bahir Dar city, the capital of the Amhara national regional state, located at the geographical coordinates of 11° 38′ north latitude and 37° 15′ east longitude, which is located ∼560 km northwest of Addis Ababa. The city contains 10 governmental health centers. The target population consisted of infants born from HIV-positive and HIV-negative mothers. Mothers who were unable to communicate, absent during house-to-house visit, and of unknown HIV status were excluded from the study. The sample size was calculated using Epi Info software version 7 with the assumption of 95% CI, power of 90%, 50% prevalence of iron-deficiency anemia in infants born from HIV-free mothers, ratio of infants born from HIV-free mothers to HIV-infected mothers of 3:1, odds ratio of 1.5 and 10% nonresponse rate gives 395 infants born from HIV-positive mothers and 1185 infants born from HIV-free mothers.
Infants were selected from the community of Bahir Dar using prevention of mother-to-child transmission of HIV (PMTCT) log book of the health centers as a sampling frame. Simple random sampling technique was used to select these infants. Study participants were traced from the community based on the address written in the PMTCT log book. The data were collected from January 2014 to June 2015. Mothers were interviewed and blood samples were collected both from the mothers and infants to measure the hemoglobin level. Length and weight of the infant were measured using world health organization (WHO) standards. An infant was gently placed on the recumbent board; we have assured that the infant was looking up and that the head, body, and toes were in a straight line; legs held together, with the other hand, slide the footboard against the infants feet until the heels of both feet touch the footboard with toes pointed upward; measurement was taken to the nearest 0.1 cm. The digital weight scale was used to measure the weight of each infant and weight was measured to the nearest 0.1 kg.[35] Infants who scored <–2 standard deviation on length for age were considered as stunted. For the interview part, first the questionnaire was prepared in English then translated to Amharic language and then back to English to keep its consistency. The interview was conducted by 17 nurse professionals and supervised by 6 health officers. The blood sample was collected by 9 laboratory technologists and close supervision was conducted by 4 second degree holder medical microbiologist. One milliliter (mL) blood sample was collected from each mother and infant following standard operational procedures to measure the hemoglobin level of mother and infant using the Mindray hematology analyzer. To maintain the quality of the data, pretest was conducted in 50 mothers, training was given for data collectors and supervisors, and the whole data collection process was closely supervised. The collected data were checked for completeness. The data were entered into the computer using Epi-info software and analyzed using SPSS software version 20 and WHO Antro plus software. Descriptive statistics were used to estimate the prevalence of infantile anemia. Binary logistic regression and multiple linear regressions were used to identify the determinants of infant anemia. A variable with P-value <0.05 was declared as determinants of anemia.
This study was ethically approved by Amhara National Regional State Health Bureau ethical committee. Permission was obtained from the respective authorities. Written informed consent was obtained from each mother. The confidentiality of the data was kept at all steps. Study participants having the right to withdraw from the study at any point was respected. Infants or mothers with low hemoglobin counts were referred to the nearby health center for further management.
3. Results
3.1. Population profile of infants born from HIV-negative mothers
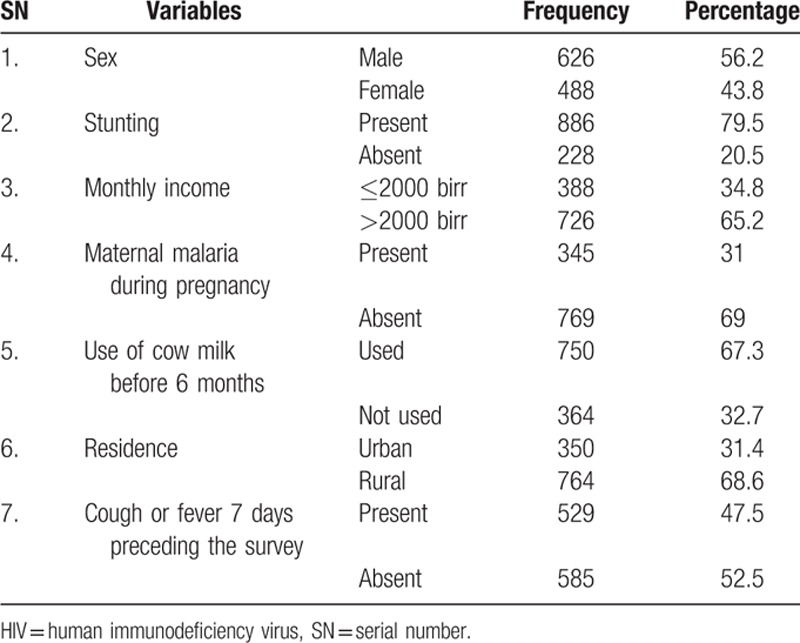
A total of 1114 infants were included with a response rate of 94%. The mean age of infants in this group was 8.11 months (SD [standard deviation] 1.80 months). The mean maternal hemoglobin count was 11.07 mg/dL (SD 1.09). The prevalence of iron-deficiency anemia among infants born from HIV-negative mothers was 46.7% (95% CI: 44–50) (Table 1).
Table 1.
Population profile of infants born from HIV-negative mothers (n = 1114).
3.2. Population profile of infants born from HIV-positive mothers
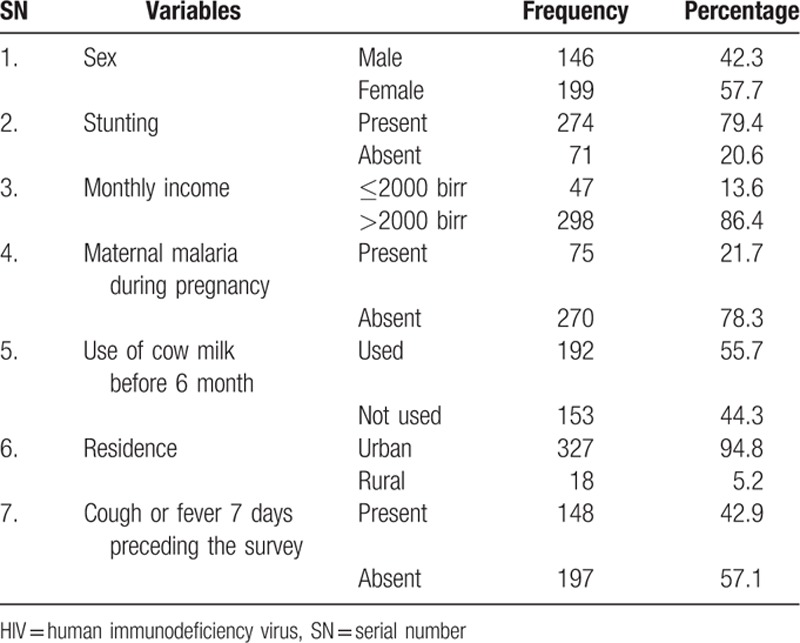
A total of 345 infants were included for a response rate of 87.34%. The mean age of infants born from HIV-positive mothers was 8.72 months (SD 1.66 months). The mean maternal hemoglobin count was 11.28 mg/dL (SD 0.73 mg/dL). The prevalence of iron-deficiency anemia among infant born from HIV-positive mothers was 26.7% (95% CI: 22–31) (Table 2).
Table 2.
population profile of infants born from HIV-positive mothers (n = 345).
3.3. Anemia in infants born from HIV-positive and HIV-negative mothers
A total of 1459 infants born from HIV-positive and HIV-negative mothers were included for a response rate of 92.34%. The mean age of the infants was 8.26 months (SD 1.79 months). The prevalence of iron-deficiency anemia in infants born from HIV-positive and HIV-negative mothers was 41.9% (95% CI: 39–44).
After adjusting for gender, age of infant, maternal HIV status, stunting, residence, history of maternal malaria during pregnancy, history of cough or fever 7 days preceding the survey, income, use of cow milk before 6 months, family size, age of the mother, educational status, and maternal hemoglobin level, infantile anemia was associated with maternal HIV status, stunting, residence, history of maternal malaria during pregnancy, history of cough or fever 7 days preceding the survey, income, use of cow milk before 6 months, family size, age of the mother, educational status of the mother, and maternal hemoglobin level (Tables 3 and 4).
Table 3.
logistic regression output of determinants of anemia in infants born from HIV-positive and HIV-negative mothers (n = 1459).
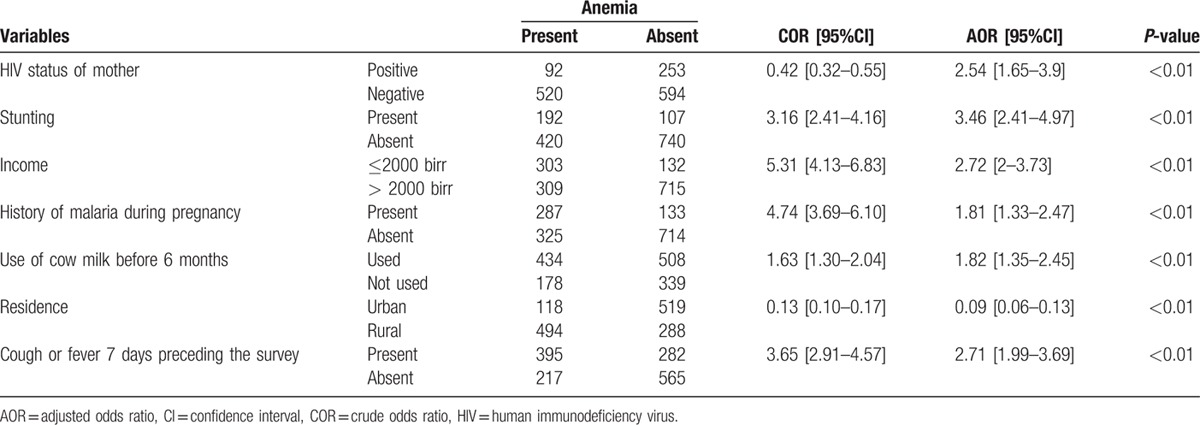
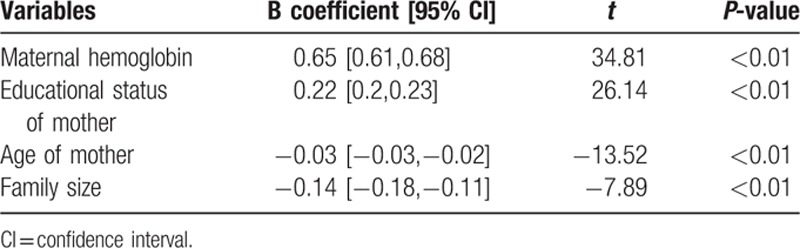
Table 4.
linear regression output for predictors of hemoglobin level in infants (dependent variable=hemoglobin level in mg/dL).
4. Discussion
The prevalence of anemia in infants born from HIV-positive and HIV-negative mothers was 41.9% (95% CI: 39–44). This result was higher than those from Montreal, Estonia, and Sweden,[23,24,34] similar to those from Nepal,[27,36] and lower than those from Indonesia.[25] This is because of the different attention given for prevention and control of infantile anemia in different countries.
The odds of anemia for infants born from HIV-positive mothers were 2.54 times higher than those born from HIV-negative mothers (AOR 2.54 [95% CI: 1.65–3.9]). This finding agrees with those from Zimbabwe and Indonesia.[25,37] This is because of the fact that maternal HIV decreases her hemoglobin level, thereby decreasing the iron transferred to infants.[37]
Stunted infants had 3.46 times higher risk of anemia than normal infants (AOR 3.46 [95% CI: 2.41–4.97]). This finding agrees with those from Mexico.[20] This is because malnourished children lack food diversity and the serum ferritin contents of malnourished children are low.[36]
Infants born from low-income family had 2.72 higher risks of anemia than those from medium-income family (AOR 2.72 [95% CI: 2 –3.73]). This finding agrees with those from Indonesia and Kenya.[25,28] This is because low-income families didn’t have access to different varieties of foods rich in iron and other nutrients necessary for red blood cell production.
Infants born from malaria-infected mother during pregnancy had 1.81 higher risk of anemia than those born from malaria-free mother during pregnancy (AOR 1.81 [95% CI: 1.33–2.47]).
This finding agrees with those from other parts of the world.[21] This is because malaria parasite decreases the hemoglobin level of the mother, thereby decreasing the amount of iron transferred from mothers to the infants.
Infants who used cow milk before 6 months had 1.82 times higher risk of anemia than those who didn’t used cow milk before 6 months (AOR 1.82 [95% CI: 1.35–2.45]). This is because the bioavailability of cow milk is less than human milk.[3,38]
Infants from urban residence were 91% protected from anemia than those from rural residence (AOR 0.09 [95% CI: 0.06–0.13]). This finding agrees with those from Mexico.[20] This is because the rural area anemia control and prevention activities are limited as compared with urban area.
Infants who had a history of cough or fever 7 days preceding the survey had 2.71 higher risk of malaria than infants who were free from cough or fever 7 days preceding the survey (AOR 2.71 [95% CI: 1.99–3.69]). This finding agrees with those from Zimbabwe.[36] This is because of the effects of acute childhood illness on the blood cell production.
The hemoglobin of infant is directly related to the hemoglobin of mother. The hemoglobin of infant increases as the hemoglobin of her/his mother increases. This signals that intervention against maternal anemia also benefits in fighting infantile anemia. This result agrees with those from other parts of the world.[39,40] This is because mothers are the principal source of iron for infants.
The hemoglobin of infant increases as the educational status of his/her mother increases. This finding agrees with those from Indonesia, Mexico, and Ethiopia.[20,25,32] This implies that women's education can act as one of anemia prevention strategies for the next generation.
The hemoglobin level of infant was negatively associated with maternal age; infants born from old-aged mothers are most likely to have low hemoglobin level. This finding agrees with those from Indonesia and Kenya.[25,28] This is because old-aged mothers are most likely to have high parity and gravidity making them a risky group for hemorrhage.
The hemoglobin counts of infants were negatively associated with family size. Infants from large family sizes are most likely to have low hemoglobin level. This finding agrees with finding from Indonesia.[25] This is because infants from large family sizes are not able to get enough quantity of nutrients including iron.
This research was implemented on a representative sample of infants born from HIV-positive and HIV-negative mothers so can be generalized safely to the population of infants born from HIV-positive and HIV-negative mothers to determine the burden and determinants of anemia.
The main limitation of this study is that the HIV status of mothers was assessed using antibody tests. This test will not detect HIV in its window period because in this period antibody against HIV is not prepared by the body defense mechanism so mothers declared HIV-negative might be HIV positive. However, the number of mothers with a window period in the community is so small that this limitation will not create much problem.
5. Conclusion
The burden of iron-deficiency anemia was highest in infants. Infantile anemia was associated with maternal HIV status, stunting, residence, history of maternal malaria during pregnancy, history of cough or fever 7 days preceding the survey, income, use of cow milk before 6 months, family size, age of the mother, educational status of the mother, and maternal hemoglobin level.
5.1. Recommendation
Decision makers should give special emphasis on intervention against infantile anemia. Both maternal and infant factors should be considered in targeting anemia control and prevention in infants. Multisectoral intervention should be considered in infantile anemia prevention and control programs.
Acknowledgments
The author wants to acknowledge the Ministry of Health for financially sponsoring this research. He would also like to acknowledge the Amhara Regional State Health Bureau for giving the necessary information. Also he wants to acknowledge all organizations and individuals that have contributed to make this practical research.
Footnotes
Abbreviations: AOR = adjusted odds ratio, CI = confidence interval, COR = crude odds ratio, Epi Info = epidemiological information, HIV = human immunodeficiency virus, kg = kilogram, mg/dL = milligram per deciliter, mL = milliliter, PMTCT = prevention of mother to child transmission, SD = standard deviation, SPSS = statistical package for social science, WHO = world health organization.
Funding: This work was financially sponsored by the Federal Democratic Republic of Ethiopia Ministry Of Health.
Author contribution: BEF conceived and designed the experiment; BEF performed the experiment, analyzed, and interpreted the data. BEF wrote the manuscript.
The author has no conflict of interest to disclose.
References
- 1.Blann A. Functions and diseases of red and white blood cells. Nurs Times 2014; 110:16–18. [PubMed] [Google Scholar]
- 2.Lutter CK. Iron deficiency in young children in low-income countries and new approaches for its prevention. J Nutr 2008; 138:2523–2528. [DOI] [PubMed] [Google Scholar]
- 3.Michaelsen K, Weaver L, Branca F, et al. Control of Iron Deficiency. Copenhagen: WHO; 2000. [Google Scholar]
- 4.WHO. Iron Deficiency Anaemia: Assessment, Prevention and Control. Geneva: World Health Organization; 2001. [Google Scholar]
- 5.Trowbridge F, Martorell R. Forging effective strategies to combat iron deficiency. Summary and recommendations. J Nutr 2002; 132:875S–879S. [DOI] [PubMed] [Google Scholar]
- 6.Bamberg R. Occurrence and detection of iron-deficiency anemia in infants and toddlers. Clin Lab Sci 2008; 21:225–231. [PubMed] [Google Scholar]
- 7.DS, JRMA S, AM The silent burden of anemia in Tanzanian children: a community-based study. Bull World Health Organ 2003; 81:581–590. [PMC free article] [PubMed] [Google Scholar]
- 8.Pasricha S-R, Drakesmith H, Black J, et al. Control of iron defi ciency anemia in low- and middle-income countries. Blood 2013; 121:2607–2619. [DOI] [PubMed] [Google Scholar]
- 9.Tolentino K, Friedman J. An update on anemia in less developed countries. Am J Trop Med Hyg 2007; 77:44–51. [PubMed] [Google Scholar]
- 10.Hunt J, Zito C, Erjavec J, et al. Severe or marginal iron deficiency affects spontaneous physical activity in rats. Am J Clin Nutr 1994; 59:413–418. [DOI] [PubMed] [Google Scholar]
- 11.Peirano PD, Algarin CR, Garrido MI, et al. Iron deficiency anemia in infancy is associated with altered temporal organization of sleep states in childhood. Pediatr Res 2007; 62:715–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lozoff B, Jimenez E, Wolf AW. Long term developmental outcomes of infants with iron deficiency. N Engl J Med 1991; 325:687–695. [DOI] [PubMed] [Google Scholar]
- 13.Booth I, Aukett M. Iron deficiency anaemia in infancy and early childhood. Arch Dis Child 1997; 76:549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stivelman J. Benefits of anaemia treatment on cognitive function. Nephrol Dial Transplant 2000; 15 suppl 3:29–35. [DOI] [PubMed] [Google Scholar]
- 15.Nelson M. Anaemia in adolescent girls: effects on cognitive function and activity. Proc Nutr Soc 1996; 55:359–367. [DOI] [PubMed] [Google Scholar]
- 16.Freire W. La anemia por deficiencia de hierro, estrategias de OPS/OMS para combatirla. Salud Publica Mex 1998; 40:199–205. [PubMed] [Google Scholar]
- 17.Moore RD. Human immunodeficiency virus infection, anemia, and survival. Clin Infect Dis 1999; 29:44–49. [DOI] [PubMed] [Google Scholar]
- 18.Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr 2001; 131:649S–666S. [DOI] [PubMed] [Google Scholar]
- 19.Willows ND, Morel J, Gray-Donald K. Prevalence of anemia among James Bay Cree infants of northern Quebec. CMAJ 2000; 162:323–327. [PMC free article] [PubMed] [Google Scholar]
- 20.Villalpando S, Shamah-Levy T, Ramírez-Silva CI, et al. Prevalence of anemia in children 1 to 12 years of age. Results from a nationwide probabilistic survey in Mexico. Salud Pública de México 2003; 45 suppl 4:S490–S498. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z, Lönnerdal B, Adu-Afarwuah S, et al. Prevalence and predictors of iron deficiency in fully breastfed infants at 6 mo of age: comparison of data from 6 studies. Am J Clin Nutr 2009; 89:1433–1440. [DOI] [PubMed] [Google Scholar]
- 22.Osório MM, Lira PIC, Batista-Filho M, et al. Prevalence of anemia in children 6-59 months old in the state of Pernambuco, Brazil. Pan Am J Public Health 2001; 10:101–107. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann F, Gray-Donald K, Mongeon M, et al. Iron deficiency anemia in 1-year-old children of disadvantaged families in Montreal. CMAJ 1992; 146:1571–1577. [PMC free article] [PubMed] [Google Scholar]
- 24.Vendt N, Grunberg H, Leedo S, et al. Prevalence and causes of iron deficiency anemias in infants aged 9 to 12 months in Estonia. Medicina 2007; 43:947–952. [PubMed] [Google Scholar]
- 25.Semba RD, Moench-Pfanner R, Sun K, et al. Iron-fortified milk and noodle consumption is associated with lower risk of anemia among children aged 6-59 mo in Indonesia. Am J Clin Nutr 2010; 92:170–176. [DOI] [PubMed] [Google Scholar]
- 26.Kadivar M, Yarmohammadi H, Mirahmadizadeh A, et al. Prevalence of iron deficiency anemia in 6 months to 5 years old children in Fars, Southern Iran. Med Sci Monit 2003; 9:CR100–CR104. [PubMed] [Google Scholar]
- 27.Siegel E, Stoltzfus R, Khatry S, et al. Epidemiology of anemia among 4- to 17-month-old children living in south central Nepal. Eur J Clin Nutr 2006; 60:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Eijk AM, Ayisi JG, Ter Kuile FO, et al. Malaria and human immunodeficiency virus infection as risk factors for anemia In infants in Kisumu, Western Kenya. Am J Trop Med Hyg 2002; 67:44–53. [DOI] [PubMed] [Google Scholar]
- 29.Totin D, Ndugwa C, Mmiro F, et al. Iron deficiency anemia is highly prevalent among human immunodeficiency virus–infected and uninfected infants in Uganda. J Nutr 2002; 132:423–429. [DOI] [PubMed] [Google Scholar]
- 30.Cornet M, Hesran J-YL, Fievet N, et al. Prevalence of and risk factors for anemia in young children in Southern Cameroon. Am J Trop Med Hyg 1998; 58:606–611. [DOI] [PubMed] [Google Scholar]
- 31.Beard J. Why iron deficiency is important in infant development. J Nutr 2008; 138:2534–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Habte D, Asrat K, Magafu MG, et al. Maternal risk factors for childhood anaemia in Ethiopia. Afr J Reprod Health 2013; 17:110–118. [PubMed] [Google Scholar]
- 33.Adish A, Esrey S, Gyorkos T, et al. Risk factors for iron deficiency anemia in preschool children in Northern Ethiopia. Public Health Nutr 1999; 2:243–252. [DOI] [PubMed] [Google Scholar]
- 34.Nead KG, Halterman JS, Kaczorowski JM, et al. Overweight children and adolescents: a risk group for iron deficiency. Pediatrics 2004; 114:104–108. [DOI] [PubMed] [Google Scholar]
- 35.WHO. Measuring Length/Height and Weight. Switzerland, Geneva: World Health Organization Department of Nutrition Health and Development; 2008. [Google Scholar]
- 36.Miller MF, Stoltzfus RJ, Iliff PJ, et al. Effect of maternal and neonatal vitamin A supplementation and other postnatal factors on anemia in Zimbabwean infants: a prospective, randomized study. Am J Clin Nutr 2006; 84:212–222. [DOI] [PubMed] [Google Scholar]
- 37.Ticconi C, Mapfumo M, Dorrucci M, et al. Effect of maternal HIV and malaria infection on pregnancy and perinatal outcome in Zimbabwe. J Acquir Immune Defic Syndr 2003; 34:289–294. [DOI] [PubMed] [Google Scholar]
- 38.Oski F. Iron deficincy in infancy and childhood. N Engl J Med 1993; 329:190–194. [DOI] [PubMed] [Google Scholar]
- 39.Geltman PL, Meyers AF, Mehta SD, et al. Daily multivitamins with iron to prevent anemia in high-risk infants: a randomized clinical trial. Pediatrics 2004; 114:86–93. [DOI] [PubMed] [Google Scholar]
- 40.Stoltzfus RJ. Research needed to strengthen science and programs for the control of iron deficiency and its consequences in young children. J Nutr 2008; 138:2542–2546. [DOI] [PubMed] [Google Scholar]


