Abstract
Outcomes of adult patients with dengue infections requiring intensive care unit (ICU) admissions remain unclear. We assessed the clinical manifestations and prognostic factors of patients critically ill with severe dengue.
This retrospective study was done in a tertiary referral hospital with 96 adult ICU beds. All of the patients with laboratory-confirmed severe dengue infections and admitted to the ICU were enrolled between July 31 and November 31, 2015, during the large outbreak period. The medical records of all the recruited patients were reviewed for the following information: age, gender, clinical manifestations, disease severity scores, underlying conditions, laboratory examinations, and outcomes. The primary endpoint was to find the predictors of ICU mortality.
During the study period, 4787 patients with dengue infections required ICU admission. One hundred forty-three (2.99%) were critically ill (mean age: 69.7 years). Hypertension (n = 90, 62.9%) and diabetes mellitus (n = 70, 49.0%) were the 2 most common underlying diseases. Eighty critically ill patients (55.9%) had cobacterial infections, and 33 had cobacteremia. The hematologic system failed most often, followed by thoracic and cardiovascular systems. Fever was the most common presentation (n = 112; 78.3%), followed by anorexia (n = 47; 32.9%) and abdominal pain (n = 46; 32.2%). Overall, 33 patients died (mortality rate: 23.1%). Multivariate analysis showed that ICU mortality was significantly associated with lower Glasgow Coma Scale (GCS) scores, lower platelet counts before ICU discharge, and more organ failures.
The number of severe dengue patients who require ICU admission remains high. The mortality rate was associated with lower GCS scores, lower platelet counts, and more organ failures. In addition, more than half of the critically ill dengue patients had comorbid bacterial infections.
Keywords: dengue, intensive care unit, outcome
1. Introduction
Dengue has become the most common mosquito-borne viral disease worldwide.[1,2] According to estimates by the World Health Organization (WHO), the global incidence of dengue infections ranges between 50 million and 200 million every year.[1] However, the real disease burden of dengue may be underestimated because of inadequate disease surveillance, misdiagnosis, and low levels of reporting. The clinical manifestations range from asymptomatic infections to severe and fatal forms.[2,3] One recent study,[4] based on 1780 country-years of mortality data from 130 countries and 1636 country-years of dengue case reports from 76 countries, estimated an average of 9221 dengue deaths per year between 1990 and 2013. Overall, global mortality greater than 1% rates have been reported.[5,6]
Taiwan is no exception, and several dengue infection outbreaks have recently been reported here.[7–10] In 2015, a large outbreak of dengue infections caused by dengue serotype 2 developed in Tainan, Taiwan, and a significant portion of patients with severe dengue infections required intensive care unit (ICU) admission. Because the outcomes of adult patients with dengue infections requiring ICU admissions have rarely been investigated,[11–17] we did this retrospective study to assess the clinical manifestations and the prognostic factors of patients critically ill with severe dengue infections.
2. Methods
2.1. Patients and hospital setting
This study was done at Chi Mei Medical Center, a tertiary referral hospital with 96 adult ICU beds. In this retrospective study, all of the patients with laboratory-confirmed severe dengue infections and admitted to the ICU were enrolled between July 31 and November 31, 2015, during the large outbreak period. The data were routinely collected and the analyses were done retrospectively. Therefore, the study was approved by the Institutional Review Board of Chi Mei Medical Center (IRB10503-005), and informed consent was waived.
2.2. Variables measured
The following information was collected: age, gender, and severity scores—the Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Therapeutic Intervention Scoring System (TISS), and Glasgow Coma Scale (GCS) score. Underlying comorbidities included congestive heart failure, chronic lung diseases, end-stage renal disease, liver cirrhosis, diabetes mellitus, and cancer. We also measured immunocompromised conditions, associated infections and organisms, the number of multiorgan failures, the use of a mechanical ventilator and continuous renal replacement therapy (CRRT), associated laboratory data at or during admission, symptoms presented in the emergency room, and patient outcomes. The primary endpoint was to find the predictors of ICU mortality.
2.3. Definitions
Laboratory-confirmed dengue cases included those with at least one of the following positive laboratory results: nonstructural protein 1 antigen test, dengue immunoglobulin-M, dengue immunoglobulin-G, a dengue polymerase chain reaction, or viral isolation.
Thoracic failure was defined as the ratio of the partial pressure of oxygen in the patient's arterial blood to the fraction of oxygen in the inspired air less than 200. Cardiovascular failure was defined as a systolic blood pressure (SBP) of ≤90 mm Hg or a mean arterial pressure (MAP) ≤65 mm Hg for at least 1 hour despite adequate fluid resuscitation; or the need for vasoactive agents (dopamine ≥5 mg/kg/min) to maintain SBP ≥ 90 mm Hg or MAP ≥ 65 mm Hg. Metabolic acidosis was defined as a pH ≤ 7.30 or a base deficit ≥5.0 mEq/L and a plasma lactate level >3 mmol/L. Hematologic failure was considered a platelet count <80,000/mm3 or a 50% decrease in the platelet count from the highest value recorded over the previous 3 days. Kidney failure was considered oliguria with an average urine output <0.5 mL/kg/h for 4 hours despite adequate fluid resuscitation or a creatinine level ≥2 mg/dL. Hepatic failure was defined as a markedly increased serum bilirubin level ≥4 mg/dL.
According to the WHO 2009 criteria, patients were classified as having dengue with warning signs if there were reports of abdominal pain or tenderness, vomiting, clinical fluid accumulation, mucosal bleeding, lethargy or restlessness, hepatomegaly, and a rise in hematocrit concurrent with a rapid drop in the platelet count. Severe dengue (group C) criteria included the following symptoms: severe plasma leakage leading to shock or fluid accumulation with respiratory distress, severe bleeding,severe organ involvement, or transaminase levels >1000 IU/L.[18,19] Those who were diagnosed with severe dengue by emergency physicians were admitted to the ICU and included in this study. The focus of bacterial infections was diagnosed on the basis of clinical, laboratory, and radiologic findings. Patients with bacteremia were divided into 2 subgroups: secondary bacteremia caused by another primary focus, such as a respiratory tract or urinary tract infection, and if no primary focus could be identified, the bacteremia was classified as primary.
2.4. Statistical analysis
Continuous variables are expressed as means ± standard deviation. The differences between groups, and between survivors and nonsurvivors at hospital discharge, were examined using univariate analysis. Continuous data were compared using Student t test or the Wilcoxon rank-sum test. Categorical variables were analyzed using χ2 or Fisher exact tests. Significance was set at P < 0.05. Patients significantly associated with in-hospital mortality in univariate analysis (P < 0.05) were tested for interaction with multiple logistic regression analysis. Odds ratios and 95% confidence intervals were also calculated. SPSS 19.0 for Windows (SPSS, Inc., Chicago, IL) was used for all data analysis.
3. Results
During the study period, there were 4787 patients with dengue infections, 143 (2.99%) of whom were critically ill and required ICU admission (mean age: 69.7 years; age range: 6–94 years) (Table 1). Thirty-three of these patients died (mortality rate: 23.1% for ICU patients and 0.7% for all hospital patients). The average APACHE II, TISS, and GCS scores were 17.9, 22.8, and 12.0, respectively. Hypertension (n = 90; 62.9%) and diabetes mellitus (n = 70; 49.0%) were the 2 most common comorbidities. Eighty patients had cobacterial infections: the lungs (pneumonia) (n = 40) and the urinary tract (n = 33) were the most common sites. Thirty-three of the 80 patients had cobacteremia: 11 primary bacteremia cases and 22 secondary bacteremia cases; 59 (41.3%) had multiorgan failure: the hematologic system failed most frequently, followed by thoracic and cardiovascular systems. Overall, there were 50 (35.0%) patients who required mechanical ventilation and 20 (14.0%) who required CRRT. The patients who died in hospital had significantly higher APACHE II, TISS, and GCS scores, and more hypertension, bacteremia, pneumonia, and organ failure (cardiovascular, thoracic, metabolic, renal and hepatic failures, and multiorgan failure) and needed more mechanical ventilation and CRRT than did survivors.
Table 1.
Demographic and clinical variables of the dengue groups at admission.
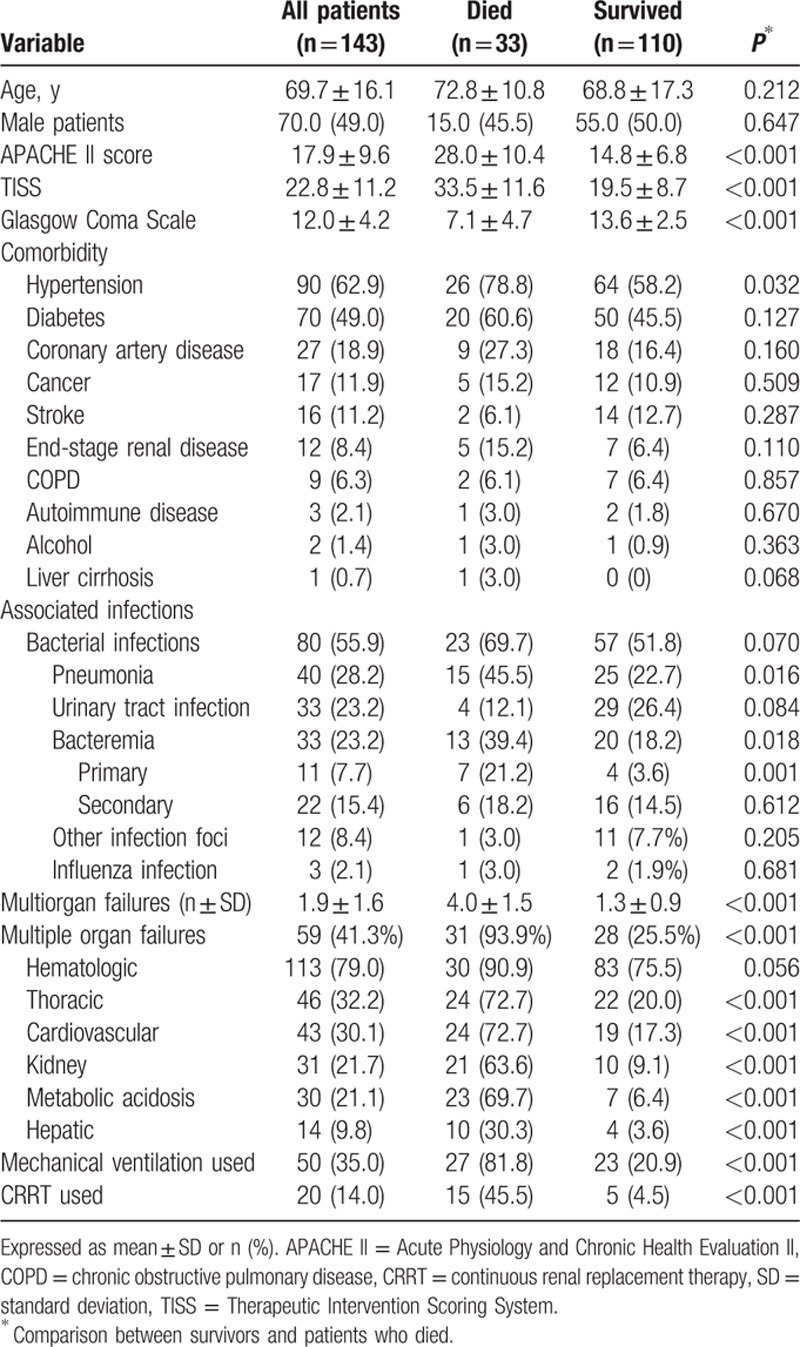
The levels of procalcitonin and C-reactive protein (CRP) were 12.3 (±34.7) μg/L and 49.0 (±72.8) mg/L, respectively (Table 2). The mean levels of the N-terminal prohormone brain natriuretic peptide and of lactate were 4788.5 (±7492.8) and 3.4 (±4.1), respectively. The lowest platelet count was 35,000 (±43,000)/mm3, and it recovered to 151,000 (±107,000)/mm3 before the patients were discharged from the ICU. The highest levels of aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) were 1126.4 (±2488.5) IU/L and 395.7 (±739.8) IU/L, respectively. The patients who died had significantly higher activated partial thromboplastin time, lactate, AST/GOT, and ALT/GPT levels than did survivors. The patients who died, however, had significantly lower albumin, hemoglobin, and hematocrit, and lower platelet counts in the trough and recovery stage before they were discharged from the ICU than did survivors.
Table 2.
Laboratory data of dengue groups at admission.
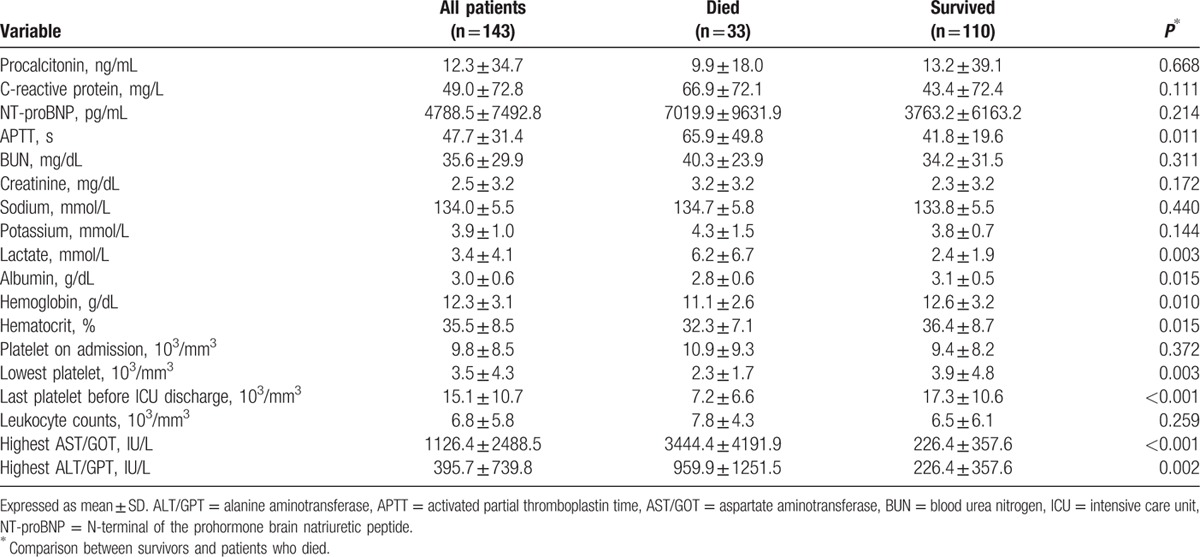
At ICU admission, fever was the most common presentation symptom (n = 112; 78.3%), followed by anorexia (n = 47; 32.9%) and abdominal pain (n = 46; 32.2%) (Table 3). The gastrointestinal tract (n = 45; 31.5%), the genitourinary tract (n = 26; 18.2%), and the gums (n = 3; 2.1%) were the most common sites, in that order, for bleeding. There were no significant differences in the symptoms, the overall ICU 7.8 (±10.3) and hospital-stay 14.7 (±16.1) days, or hospital costs between survivors and patients who died.
Table 3.
Symptoms at admission between dengue groups.
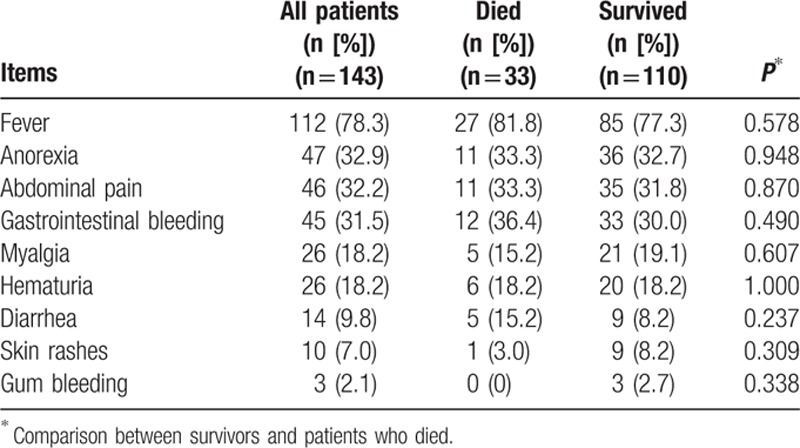
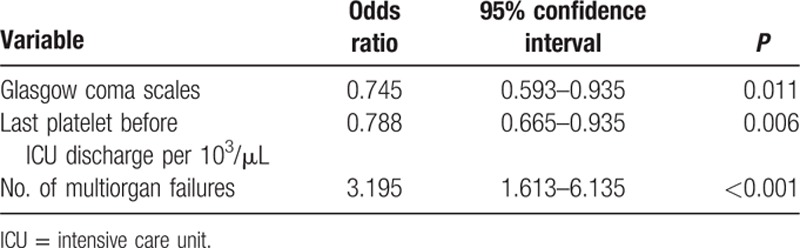
Mortality was highest for 60- to 69-year-olds (8 died [36.4%]; 14 survived), 70- to 79-year-olds (16 died [24.2%]; 50 survived), and 80- to 89-year-olds (7 died [23.3%]; 23 survived) (Fig. 1). Multiple logistic regression analysis showed that ICU mortality was significantly positively associated with the following independent predictors: lower GCSs, lower platelet counts, and more organ failures before ICU discharge (Table 4).
Figure 1.
Survival and mortality by age group.
Table 4.
The predictors of intensive care unit mortality using multiple logistic regression analysis.
4. Discussion
This is the first study that focuses on dengue patients in Taiwan who require ICU admission and is one of few studies[11–13,17] that investigates this topic anywhere in the world.
First, we found that the mortality of this specific group remains as high as 23.1%, which is higher than reported by other studies.[11–13] In 1 study[12] of 72 critically ill patients with dengue in northern India, 8 patients died (mortality: 11.1%). In another[13] of 198 ICU patients with dengue in New Delhi, 12 patients died (mortality: 6.1%). In contrast, another multicenter study[17] of 42 adults with dengue hemorrhagic fever or dengue shock syndrome admitted to ICU in India reported 8 in-ICU deaths (mortality: 19%). A study in Brazil[11] of 97 adult patients with dengue admitted to the ICU reported in-ICU and in-hospital mortality rates of 18.6% and 19.6%, respectively. The differences between our study and others might be the differences in disease severity. In our study, the mean APACHE II score was 17.9 ± 9.6, higher than the 7.5[13] and the 11[11] in 2 of the others. In addition, only group C (severe dengue patients with warning signs and organ failure) were enrolled in our study, which probably explains why the outcomes of our study were worse. In addition, the mean age of our enrolled patients was about 70 years, older than 2 of the other studies[12,13]; age itself might also partially contribute to the higher mortality rate.
Second, univariate analysis showed that the patients who died had cases of dengue that were more severe, based on APACHE II, TISS, and GCS scores, than did survivors. This is similar with other reports[11,13] that in-hospital mortality was associated with APACHE II scores and Sequential Organ Failure Assessment scores. In contrast, after multivariate analysis, only the association between lower GCS scores and in-hospital mortality remained significant in the present study. Our finding might indicate that GCS scores, in addition to other commonly used severity score systems, are good predictors of the outcomes of patients with severe dengue.
Third, a multivariate analysis showed that a higher number of organ failures was independently associated with mortality in the present study. Like another study[20] which reported that some common etiologies of multiple organ dysfunction—dengue shock syndrome and multiple organ dysfunction syndrome—directly related to the organ failure index present a significant risk of mortality, we found that patients who died had more thoracic (72.2% vs 20.0%), cardiovascular (72.7% vs 17.3%), and multiorgan (93.9% vs 25.5%) failures than did survivors (both P < 0.0001). Other studies have reported that patients who die were more likely to have cardiovascular (100% vs 12%) or respiratory (88% vs 12%) failures than were survivors (both P < 0.01),[17] that a dengue viral infection with an acute respiratory failure is a significant mortality risk,[21] and that a lower platelet count before being discharged from the ICU was associated with higher in-hospital mortality.[22]
Finally, we found that 80 (55.9%) of our patients developed bacterial infections in the ICU. Moreover, 15 (45.5%) of the 33 patients who died had pneumonia, and 13 (39.4%) had bacteremia. In another study,[11] 45 (46.4%) patients had had been treated with antibiotics, and 7 (36.8%) of 19 deaths were presumably attributed to bacterial infections associated with dengue. In the present study, the most common pathogen in the 33 cases of confirmed bacteremia was Escherichia coli, followed by Staphylococcus aureus (S aureus), and streptococcus pp. Despite our finding that S aureus was the most commonly reported copathogen, different from that of other reports,[23,24] the present study and other reports[25,26] might indicate the clinical significance of bacterial infections in patients with severe dengue. It should at least emphasize the importance of an early diagnosis of cobacterial infections and prompt antibiotic treatment of patients critically ill with dengue.
In the present study, CRP levels were nonsignificantly higher in patients who died (66.9) than in survivors (43.4), which does not support the hypothesis that CRP is an early predictor of dengue severity in adult patients.[27] The differences should be because of the different study populations. We enrolled only patients with severe dengue in the present study and found that CRP was a limited outcome predictor in patients with severe dengue. We also found that albumin levels were significantly lower (2.8 ± 0.6 vs 3.1 ± 0.5) and lactate significantly higher (6.2 ± 6.7 vs 2.4 ± 1.9) in patients who died than in survivors, which was consistent with other studies[17,28] that low serum albumin levels on ICU admission were associated with worse outcomes. Although a multivariate analysis eliminated the significance in our study, it still indicates an association between albumin levels and the outcomes in patients with severe dengue.
Our study has some limitations. First, because it is a single-center study, the number of cases is limited. Therefore, our findings may not be generalizable to other hospitals. Second, because of the study design of a retrospective investigation, our data collection might have been inadequate. Third, we did not test the serotypes of dengue virus because our laboratory cannot do that type of examination. However, several studies[28–31] have reported the association between dengue virus serotypes and the severity of dengue infection. Our findings should help clarify this association. In addition, the predictors of ICU mortality by multiple logistic regression analysis should be considered as the association with mortality but not the biological causes of death. Finally, despite several reports[32–34] showed the association between different medications, such as N-acetyl cysteine, factor VII, diuretic, and fluid administration, and the outcome of dengue patients, we did not collect any information about this association.
In conclusion, the mortality of severe dengue patients admitted to the ICU remains high, and the mortality was associated with lower GCS scores, lower platelet count before being discharged from the ICU discharge, and more organ failures. In addition, a significant portion of patients with severe dengue who die in the ICU have bacterial infections.
Footnotes
Abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II, CRRT = continuous renal replacement therapy, ICU = intensive care unit, TISS = Therapeutic Intervention Scoring System.
CCL and CMC designed the study, interpreted the data, and drafted and revised the article. KSC, WLY, KCC, HCC, and CYY contributed to data collection.
The authors have no conflicts of interest to disclose.
References
- 1.Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol 2013; 5:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guzman MG, Harris E. Dengue. Lancet 2015; 385:453–465. [DOI] [PubMed] [Google Scholar]
- 3.Halstead SB. Dengue. Lancet 2007; 370:1644–1652. [DOI] [PubMed] [Google Scholar]
- 4.Stanaway JD, Shepard DS, Undurraga EA, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis 2016; 16:712–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature 2013; 496:504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbons RV, Vaughn DW. Dengue: an escalating problem. BMJ 2002; 324:1563–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang SF, Yang CF, Hsu TC, et al. Laboratory-based surveillance and molecular characterization of dengue viruses in Taiwan, 2014. Am J Trop Med Hyg 2016; 94:804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin CH, Schioler KL, Jepsen MR, et al. Dengue outbreaks in high-income area, Kaohsiung City, Taiwan, 2003–2009. Emerg Infect Dis 2012; 18:1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang SF, Chang K, Lu RW, et al. Large Dengue virus type 1 outbreak in Taiwan. Emerg Microbes Infect 2015; 4:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang SF, Wang WH, Chang K, et al. Severe dengue fever outbreak in Taiwan. Am J Trop Med Hyg 2016; 94:193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amancio FF, Heringer TP, de Oliveira Cda C, et al. Clinical profiles and factors associated with death in adults with dengue admitted to intensive care units, Minas Gerais, Brazil. PLoS One 2015; 10:e0129046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandralekha, Gupta P, Trikha A. The north Indian dengue outbreak 2006: a retrospective analysis of intensive care unit admissions in a tertiary care hospital. Trans R Soc Trop Med Hyg 2008; 102:143–147. [DOI] [PubMed] [Google Scholar]
- 13.Juneja D, Nasa P, Singh O, et al. Clinical profile, intensive care unit course, and outcome of patients admitted in intensive care unit with dengue. J Crit Care 2011; 26:449–452. [DOI] [PubMed] [Google Scholar]
- 14.Pang J, Thein TL, Leo YS, et al. Early clinical and laboratory risk factors of intensive care unit requirement during 2004–2008 dengue epidemics in Singapore: a matched case–control study. BMC Infect Dis 2014; 14:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soni A, Chugh K, Sachdev A, et al. Management of dengue fever in ICU. Indian J Pediatr 2001; 68:1051–1055. [DOI] [PubMed] [Google Scholar]
- 16.Thein TL, Leo YS, Fisher DA, et al. Risk factors for fatality among confirmed adult dengue inpatients in Singapore: a matched case–control study. PLoS One 2013; 8:e81060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitz L, Prayag S, Varghese S, et al. Nonhematological organ dysfunction and positive fluid balance are important determinants of outcome in adults with severe dengue infection: a multicenter study from India. J Crit Care 2011; 26:441–448. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva, Switzerland: WHO; 2009. [PubMed] [Google Scholar]
- 19.Hadinegoro SR. The revised WHO dengue case classification: does the system need to be modified? Paediatr Int Child Health 2012; 32 (s1):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khilnani P, Sarma D, Zimmerman J. Epidemiology and peculiarities of pediatric multiple organ dysfunction syndrome in New Delhi, India. Intensive Care Med 2006; 32:1856–1862. [DOI] [PubMed] [Google Scholar]
- 21.Laoprasopwattana K, Chaimongkol W, Pruekprasert P, et al. Acute respiratory failure and active bleeding are the important fatality predictive factors for severe dengue viral infection. PLoS One 2014; 9:e114499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas L, Brouste Y, Najioullah F, et al. Predictors of severe manifestations in a cohort of adult dengue patients. J Clin Virol 2010; 48:96–99. [DOI] [PubMed] [Google Scholar]
- 23.Miyata N, Yoshimura Y, Tachikawa N, et al. Cavity forming pneumonia due to Staphylococcus aureus following dengue fever. Am J Trop Med Hyg 2015; 93:1055–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagassar RP, Bridgelal-Nagassar RJ, McMorris N, et al. Staphylococcus aureus pneumonia and dengue virus co-infection and review of implications of coinfection. BMJ Case Rep 2012; 2012: pii: bcr0220125804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Araújo SA, Moreira DR, Veloso JM, et al. Fatal staphylococcal infection following classic dengue fever. Am J Trop Med Hyg 2010; 83:679–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan NA, Azhar EI, El-Fiky S, et al. Clinical profile and outcome of hospitalized patients during first outbreak of dengue in Makkah, Saudi Arabia. Acta Trop 2008; 105:39–44. [DOI] [PubMed] [Google Scholar]
- 27.Chen CC, Lee IK, Liu JW, et al. Utility of C-reactive protein levels for early prediction of dengue severity in adults. Biomed Res Int 2015; 2015:936062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jog S, Prayag S, Rajhans P, et al. Dengue infection with multiorgan dysfunction: SOFA score, arterial lactate and serum albumin levels are predictors of outcome. Intensive Care Med 2015; 41:2029–2030. [DOI] [PubMed] [Google Scholar]
- 29.Teelucksingh S, Mangray AS, Barrow S, et al. Dengue haemorrhagic fever/dengue shock syndrome. An unwelcome arrival in Trinidad. West Indian Med J 1997; 46:38–42. [PubMed] [Google Scholar]
- 30.Fox A, Le NM, Simmons CP, et al. Immunological and viral determinants of dengue severity in hospitalized adults in Ha Noi, Viet Nam. PLoS Negl Trop Dis 2011; 5:e967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yung CF, Lee KS, Thein TL, et al. Dengue serotype-specific differences in clinical manifestation, laboratory parameters and risk of severe disease in adults, Singapore. Am J Trop Med Hyg 2015; 92:999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranjit S, Kissoon N, Jayakumar I. Aggressive management of dengue shock syndrome may decrease mortality rate: a suggested protocol. Pediatr Crit Care Med 2005; 6:412–419. [DOI] [PubMed] [Google Scholar]
- 33.Manoj EM, Ranasinghe G, Ragunathan MK. Successful use of N-acetyl cysteine and activated recombinant factor VII in fulminant hepatic failure and massive bleeding secondary to dengue hemorrhagic fever. J Emerg Trauma Shock 2014; 7:313–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddy KR, Basavaraja GV, Shivananda Furosemide infusion in children with dengue fever and hypoxemia. Indian Pediatr 2014; 51:303–305. [DOI] [PMC free article] [PubMed] [Google Scholar]


