Abstract
Introduction:
Chronic active Epstein–Barr virus (EBV) infection (CAEBV) is characterized as chronic or recurrent mononucleosis-like symptoms and elevated EBV deoxyribonucleic acid (EBV-DNA) copies. Cardiovascular complications have high morbidity and mortality. The treatment regimen for CAEBV has not been established yet, resulting in poor prognoses. Herein, we present a case of cardiovascular magnetic resonance imaging (CMRI) evaluation with a series of sequences for CAEBV-associated cardiovascular involvement, which has never been reported.
Case presentation:
A 16-year-old female (body weight, 55 kg) developed a persistent fever and a positive EBV-DNA level of 28,000 copies/mL. Computed tomography angiography (CTA) showed aneurysms involving the aorta and its major branches, as well as multiple aneurysms and stenoses of the coronary arteries. CMRI of the coronary arteries depicted the dilution and stenosis of the arterial lumen as well as the thickening of the arterial wall. Late gadolinium enhancement (LGE) showed subendocardial and transmural delayed enhancement of the left ventricle, suggesting myocardial infarction.
CAEBV and associated cardiovascular complications were diagnosed. After treatment with Medrol and Leflunomide, the clinical manifestation and serological parameters reversed to normal. However, the EBV-DNA level increased again to 13,900 copies/mL 2 months later. A follow-up with aorta CTA showed that the arterial walls of the bilateral common iliac artery aneurysms were thicker with new-onset mural thrombi. The aorta CTA also showed new-onset occlusion of the right coronary artery, but a follow-up of CMRI at the same day did not find new-onset delayed enhancement lesion.
Conclusion:
This case reminds clinicians of the vital importance of early diagnosis and close follow-up of CAEBV-associated cardiovascular complications. With cine imaging, coronary artery imaging, LGE imaging, and other novel techniques, CMRI can effectively and comprehensively reveal the early and dynamic changes, and act as an important tool in the field of cardiovascular diseases.
Keywords: cardiovascular magnetic resonance imaging, chronic active Epstein–Barr virus infection, computed tomography angiography
1. Introduction
Chronic active Epstein–Barr virus (EBV) infection (CAEBV) is characterized as chronic or recurrent mononucleosis-like symptoms and elevated EBV deoxyribonucleic acid (EBV-DNA) copies or EBV antibody titer. The clonal expansion of EBV-infected T or natural killer cells is associated with serious complications, such as interstitial pneumonia, encephalitis, lymphoma, hemophagocytic syndrome, and cardiovascular diseases.[1] Coronary artery aneurysm, myocarditis, large-vessel arteritis, pulmonary arterial hypertension, and other cardiovascular complications have been reported based on laboratory tests, ultrasound, computed tomography, and autopsy.[2–6] Here, a case of cardiovascular magnetic resonance imaging (CMRI) evaluation with a series of sequences for CAEBV-associated cardiovascular involvement is presented, which has never been reported.
2. Consent
Informed consent was signed by the patient for the publication of this report and related images. This study was approved by the institutional review board of Peking Union Medical College Hospital.
3. Case presentation
A 16-year-old girl (body weight, 55 kg; Chinese; student) developed a persistent fever for 18 months, with a peak temperature of approximately 39 °C. Laboratory tests showed a low white blood cell level, normal hepatic and renal functions, elevated erythrocyte sedimentation rate (ESR) and high-sensitivity C-reactive protein (hsCRP), and negative antinuclear antibodies, anti-double stranded deoxyribonucleic acid (dsDNA) and anti–extractable nuclear antigen. Tests for infectious diseases were all negative, except for a positive EBV-DNA of 28,000 copies/mL in peripheral blood. Positron emission tomography showed hepatosplenomegaly and extensive lymphadenopathy. The bone marrow aspirate and lymph node biopsy showed no presence of malignant cells. Computed tomography angiography (CTA) showed aneurysms involving the aorta and its major branches, as well as multiple aneurysms and stenoses of the coronary arteries (Fig. 1A–H).
Figure 1.
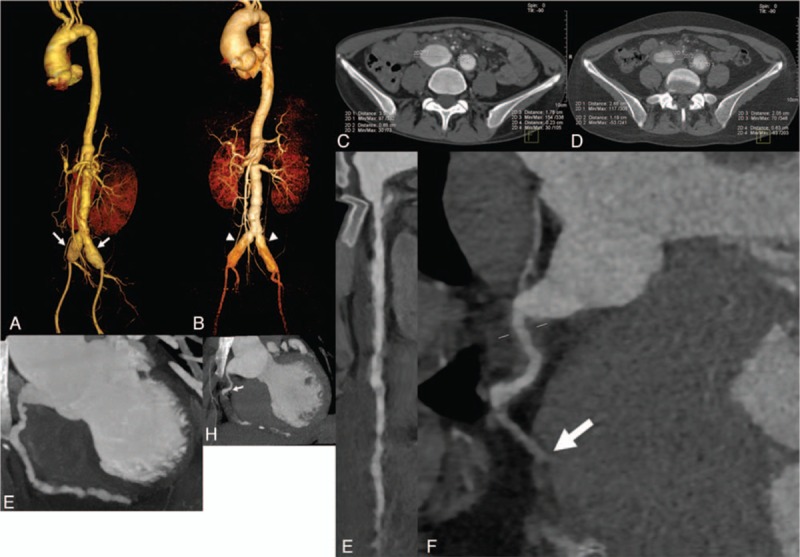
(A) VRT reconstruction image of aortic computed tomography angiography (CTA) on admission shows aneurysms involving bilateral common iliac arteries (arrow). (B) VRT reconstruction image of aortic CTA 12 months later shows aneurysms at the same site with less-dilated arterial lumen (arrowhead). (C) Axial image of aortic CTA on admission shows aneurysmal dilation and arterial wall thickening involving bilateral common iliac arteries. (D) Axial image of aortic CTA 12 months later shows that the arterial walls were thicker with new-onset mural thrombi. (E) Curved planar reformation (CPR) reconstruction image of coronary CTA on admission shows multiple stenoses and dilations involving the right coronary artery (RCA). (F) CPR reconstruction image of aorta CTA 12 months later shows new-onset occlusion involving middle segment of the RCA (arrow). (G) Maximum intensity projection (MIP) reconstruction image of coronary CTA on admission shows multiple stenoses and dilations involving the RCA. (H) MIP reconstruction image of coronary CTA 12 months later shows new-onset occlusion involving middle segment of the RCA (arrow). CPR = curved planar reformation, CTA = computed tomography angiography, MIP = maximum intensity projection, RCA = right coronary artery, VRT = volume rendering technique.
CMRI was performed using a 3.0-T whole-body scanner (Magnetom Skyra, Siemens, Erlangen, Germany). The MRI sequences and parameters were as follows: first, cine imaging was performed using a two-dimensional breath-hold balanced steady-state free precession sequence. Two-, three-, and four-chamber long-axis planes and 10 to 12 short-axis slices covering the entire left ventricle were scanned to evaluate cardiac movement and function. The parameters for scanning were as follows: repetition time (TR)/echo time (TE) = 3.3/1.4 ms, temporal resolution = 45 ms, in-plane spatial resolution = 1.6 × 1.6 mm2, and slice thickness = 8 mm. Second, coronary vessel wall images were acquired using a two-dimensional black blood gradient echo sequence with the following parameters: TR/TE = 5.2/2.6 ms, flip angle = 12°, and voxel size = 0.8 × 0.8 × 4 mm3. Third, contrast-enhanced whole-heart coronary magnetic resonance angiography (CE WH-CMRA) was acquired using a navigator-gated, electrocardiogram (ECG) triggered, fat saturated, and segmented three-dimensional gradient echo sequence. Gadopentetate dimeglumine (Gd-DTPA, Magnevist, Bayer HealthCare Pharmaceuticals, Wayne, NJ) was injected at a dose of 0.2 mmol/kg and a rate of 0.3 mL/s. Forty seconds after the injection began, the CE WH-CMRA data collection was started. The parameters used included the following: TR/TE = 2.8/1.2 ms, flip angle = 20°, readout bandwidth = 610 Hz/pixel, acquired voxel size = 1.25 × 1.25 × 1.8 mm3, and interpolated voxel size = 0.63 × 0.63 × 0.9 mm3. A generalized autocalibrating partially parallel acquisition with an acceleration factor of 3 was applied. The total acquisition time was 6 minutes and 45 seconds. Fourth, late gadolinium enhancement (LGE) imaging was performed 15 minutes after the injection using a two-dimensional phase-sensitive inversion-recovery gradient echo pulse sequence and the following parameters: TR/TE = 5.2/2.0 ms, flip angle = 25°, field of view = 219 × 350 mm2, and matrix = 120 × 256. The inversion time (TI) was set as the null normal myocardium and was obtained using a TI scout sequence.
On cine images, the enlargement of the left ventricle and right atrium was observed, in addition to aortic valve regurgitation and mild pericardial effusion (Fig. 2A). A visual assessment of the segmented cine images showed a moderate increase of left ventricle volume at the end-diastolic stage and a slightly restricted movement of the right ventricular wall. The right ventricular ejection fraction was slightly decreased to 41%. On the coronary vessel wall images, the dilutions and stenoses of the lumen were depicted (Fig. 2B), and thickening of arterial walls involving the left anterior descending artery and diagonal branch artery was also clearly identified (Fig. 2C). Three-dimensional volume-rendered reconstruction (Fig. 2D and E) presented multiple aneurysms and stenoses of the coronary arteries as well as an aneurysm at the root of the aorta. On the LGE images, subendocardial delayed enhancement of the mid anterior and mid inferolateral left ventricular wall was shown, as well as transmural delayed enhancement of the basal inferior and mid inferior left ventricular wall (Fig. 2F), suggesting myocardial infarction.
Figure 2.
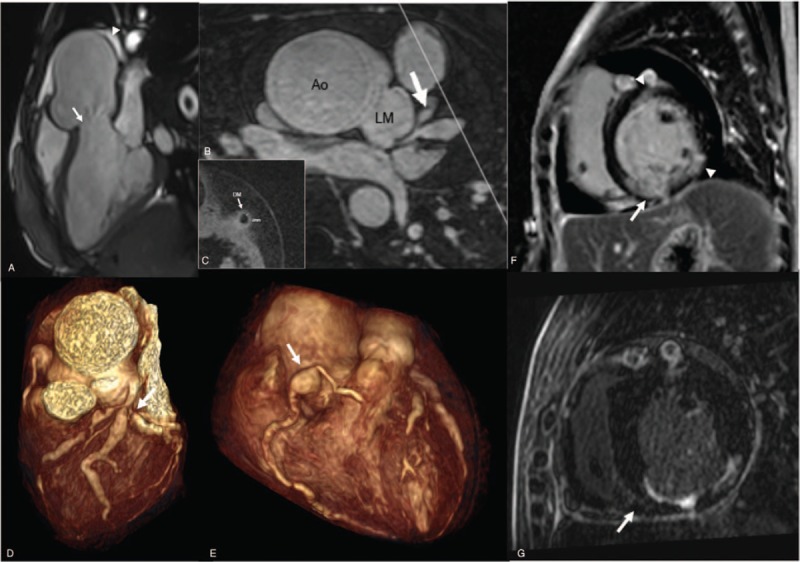
(A) Three-chamber cine image of cardiovascular magnetic resonance (CMR) on admission shows aortic valve regurgitation (arrow) at the end-diastolic stage and pericardial effusion (arrowhead). (B) Multiplane reconstruction image of contrast-enhanced whole-heart coronary magnetic resonance angiography shows significant stenoses and dilutions involving the left anterior descending coronary artery and diagonal branch coronary artery (DM) (arrow). (C) Two-dimensional black blood coronary vessel wall image shows arterial wall thickening of the DM (2 mm). The white line in (B) defined the slice position where (C) was acquired. (D) VRT reconstruction image of CMR on admission shows severe left main coronary artery stenosis (arrow). (E) VRT reconstruction image of CMR on admission shows proximal right coronary artery (RCA) aneurysm (arrow). (F) Late gadolinum enhancement (LGE) image of CMR on admission shows subendocardial delayed enhancement of the mid anterior and mid inferolateral left ventricular wall (arrowhead), as well as transmural delayed enhancement of the basal inferior and mid inferior left ventricular wall (arrow). (G) LGE image of CMR 12 months later does not find new-onset delayed enhancement lesion. Ao = aorta, CMR = cardiac magnetic resonance, DM = diagonal branch coronary artery, LAD = left anterior descending coronary artery, LGE = late gadolinum enhancement, LM = left main coronary artery, MPR = multiplane reconstruction, RCA = right coronary artery, VRT = volume rendered reconstruction, WH-CMRA = whole-heart coronary magnetic resonance angiography.
Given the prolonged clinical manifestation, persistent EBV-DNA level, and CTA and CMRI results, CAEBV was diagnosed. The differential diagnosis for the large artery lesions included Kawasaki disease, Takayasu arteritis, Behcet's disease, and Marfan syndrome, but none was completely compatible with the patient's condition. After a 10-day treatment with Leflunomide, the clinical manifestation and serological parameters, such as temperature, ESR and hsCRP, reversed to normal, and the EBV-DNA level decreased to 5900 copies/mL.
After discharge, the patient received regular Medrol (40 mg, 5 weeks; then 36 mg, 1 week; then 32 mg, 1 week; then 28 mg, 1 week; then 24 mg till follow-up) and Leflunomide (30 mg, 3 months; 20 mg till follow-up) treatment as well as laboratory follow-up. Six months later, the EBV-DNA level decreased to 500 copies/mL. Twelve months later, the EBV-DNA level increased again to 13,900 copies/mL, without a predisposing cause or clinical manifestation. Follow-up with aorta CTA and CMRI at the same day were performed. The aorta CTA showed aneurysmal dilation of the bilateral common iliac artery at the same site, whereas the arterial walls were thicker with new-onset mural thrombi (Fig. 1B and D). The aorta CTA also showed new-onset occlusion involving middle segment of the right coronary artery (Fig. 1F and H), but CMRI did not find new-onset delayed enhancement lesion (Fig. 2G).
4. Discussion
CAEBV has high mortality with life-threatening complications. Cardiovascular complications are not rare in patients with CAEBV. Muneuchi reported that 60% of patients with CAEBV developed cardiovascular diseases including coronary artery lesions, pericardial effusion, decreased cardiac function, and complete atrioventricular block.[7] The pathological results suggested that the infiltration of EBV-infected T or natural killer cells and associated inflammation might contribute to cardiovasculopathy.[4,7,8] Kimura et al[9] reported that coronary artery aneurysm and myocarditis are the major complications resulting in poor prognoses. A treatment regimen for CAEBV has not been established. For this patient, although received active treatment, she had relapsed EBV-DNA level and progressive radiographic findings. Therefore, early diagnosis, intervention, close follow-up of CAEBV, and associated complications are of vital importance.
Various cardiovascular imaging techniques have been applied to evaluate the anatomy, metabolism, and function of the heart and large vessels. Ultrasound is convenient and economical for the preliminary evaluation of morphologic and hemodynamic changes, but it does not adequately evaluate the coronary arteries. Coronary artery angiography provides diagnostic value and interventional approach but involves radiation dose and invasive risk. In addition, angiography only shows the vessel lumen and dynamic blood flow inside; it does not provide the condition of the vessel wall and does not portray the entire picture of the heart or the aorta. CTA is commonly used and is effective for a broad spectrum of cardiovascular diseases. However, hemodynamic data are not concluded, and the spatial resolution is not ideal to observe the vessel walls of the coronary arteries. Moreover, for this young girl, the relatively high radiation dose of CTA is worth consideration. On admission, aorta CTA was performed using an ECG-gated second-generation dual-source CT with tube voltage 100 kV, tube current time 278 mAs, and the total effective radiation dose was 9.95 mSv. Coronary CTA was performed with tube voltage 80 kV, tube current time 114 mAs, and the total effective radiation dose was 3.26 mSv. The follow-up aorta CTA was performed using an ECG-gated third-generation dual-source CT with tube voltage 80 kV, tube current time 377 mAs, and the total effective radiation dose was 5.46 mSv.
In contrast, a one-stop examination of CMRI provides a series of scanning sequences that provide substantial information.[10,11] Cine imaging reveals anatomical abnormalities of the heart and surrounding structures, together with hemodynamic dysfunction. LGE imaging catches myocardial infarction that might be missed by ECG, echocardiography, or CTA.[12] Contrast-enhanced MRA figuratively presents the shape and running of the coronary arteries. Interestingly, coronary vessel wall imaging can visualize the dilation or stenosis of vessel lumen as well as the thickness of the vessel wall.[13] In particular, a black blood sequence is sensitive for vessel wall edema and thickening, which might be the earliest finding of inflammatory changes before clinical manifestations appear. However, CMRI is relatively expensive and much more time consuming. Besides, the scanning protocol and reporting system is much more complex. It is quite true that CMRI is not as widely used as CTA, and its advantage is not adequately recognized and appreciated by clinicians. So, more cases are needed to show and popularize its clinical practice.
In conclusion, CMRI including cine imaging, coronary vessel wall imaging, CE WH-CMRA, and LGE imaging can effectively and comprehensively reveal early and dynamic changes, thus playing a vital role in early diagnosis, intervention, and close follow-up. With its rapid advancement, CMRI is playing a substantial role in the field of cardiovascular diseases.
Footnotes
Abbreviations: CAEBV = chronic active Epstein–Barr virus infection, CE WH-CMRA = contrast-enhanced whole-heart coronary magnetic resonance angiography, CMRI = cardiovascular magnetic resonance imaging, CTA = computed tomography angiography, EBV = Epstein–Barr virus, EBV-DNA = EBV deoxyribonucleic acid, ECG = electrocardiogram, ESR = erythrocyte sedimentation rate, hsCRP = high-sensitivity C-reactive protein, LGE = late gadolinium enhancement, TE = echo time, TI = inversion time, TR = repetition time.
SJ and XL have contributed equally to the article.
Funding/support: This work is supported by the National Natural Science Foundation of China (2015, Grant NO. 81471725), the Beijing Municipal Natural Science Foundation (2014, Grant NO. 7142133), and the Health Industry Special Scientific Research Project (201402019).
The authors have no conflicts of interest to disclose.
References
- 1.Fujiwara S, Kimura H, Imadome K, et al. Current research on chronic active Epstein–Barr virus infection in Japan. Pediatr Int 2014; 56:159–166. [DOI] [PubMed] [Google Scholar]
- 2.Kikuta H, Taguchi Y, Tomizawa K, et al. Epstein–Barr virus genome-positive T lymphocytes in a boy with chronic active EBV infection associated with Kawasaki-like disease. Nature 1988; 333:455–457. [DOI] [PubMed] [Google Scholar]
- 3.Toubo T, Ohga S, Takada H, et al. Rheumatic fever-mimicking carditis as a first presentation of chronic active Epstein–Barr virus infection. Acta Paediatr 2006; 95:614–618. [DOI] [PubMed] [Google Scholar]
- 4.Murakami K, Ohsawa M, Hu SX, et al. Large-vessel arteritis associated with chronic active Epstein–Barr virus infection. Arthritis Rheum 1998; 41:369–373. [DOI] [PubMed] [Google Scholar]
- 5.Giardina A, Rizzo A, Ferrante A, et al. Giant cell arteritis associated with chronic active Epstein–Barr virus infection. Reumatismo 2013; 65:36–39. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda Y, Momoi N, Akaihata M, et al. Pulmonary arterial hypertension associated with chronic active Epstein–Barr virus infection. Pediatr Int 2015; 57:731–734. [DOI] [PubMed] [Google Scholar]
- 7.Muneuchi J, Ohga S, Ishimura M, et al. Cardiovascular complications associated with chronic active Epstein–Barr virus infection. Pediatr Cardiol 2009; 30:274–281. [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara M, Shimozono H, Ono H, et al. Polyclonal proliferation of lymphocytes containing the Epstein–Barr virus genome in a patient dying of myocarditis in chronic active Epstein–Barr virus infection. J Pediatr Hematol Oncol 2003; 25:85–88. [DOI] [PubMed] [Google Scholar]
- 9.Kimura H, Morishima T, Kanegane H, et al. Prognostic factors for chronic active Epstein–Barr virus infection. J Infect Dis 2003; 187:527–533. [DOI] [PubMed] [Google Scholar]
- 10.Jiang L, Li D, Yan F, et al. Evaluation of Takayasu arteritis activity by delayed contrast-enhanced magnetic resonance imaging. Int J Cardiol 2012; 155:262–267. [DOI] [PubMed] [Google Scholar]
- 11.Aluquin VP, Albano SA, Chan F, et al. Magnetic resonance imaging in the diagnosis and follow up of Takayasu's arteritis in children. Ann Rheum Dis 2002; 61:526–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishimura S, Ehara S, Hanatani A, et al. Chronic active Epstein–Barr virus infection complicated with multiple artery aneurysms. Eur Heart J Cardiovasc Imaging 2014; 15:1255. [DOI] [PubMed] [Google Scholar]
- 13.Yun H, Jin H, Yang S, et al. Coronary artery angiography and myocardial viability imaging: a 3.0-T contrast-enhanced magnetic resonance coronary artery angiography with Gd-BOPTA. Int J Cardiovasc Imaging 2014; 30:99–108. [DOI] [PubMed] [Google Scholar]


