Supplemental Digital Content is available in the text
Keywords: diabetes mellitus, fatty liver index, integrated discrimination index, net reclassification improvement, prospective study
Abstract
The fatty liver index (FLI), calculated from serum triglyceride, body mass index, waist circumference, and gamma-glutamyltransferase, is considered a surrogate marker of nonalcoholic fatty liver disease (NAFLD). We investigated whether FLI predicts the development of diabetes mellitus (DM) and assessed the predictive ability of FLI for new onset of DM in a prospective population-based cohort study.
We analyzed a total of 2784 adults (944 men and 1840 women) aged 40 to 70 years without DM at baseline. Participants were classified according to FLI values into 3 groups: FLI < 30, no NAFLD; 30 ≤ FLI ≤ 59, intermediate NAFLD; and FLI ≥ 60, participants with NAFLD. The area under the receiver-operating characteristic curve (AUC), net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were calculated to determine whether FLI improved DM risk prediction.
During a mean of 2.6 years follow-up, 88 (3.16%) participants developed DM. The odds ratio analyzed from multivariable-adjusted models (95% confidence interval [CI]) for new onset of DM increased in a continuous manner with increased FLI (<30 vs 30–59 vs ≥60 = 1 vs 1.87 [95% CI 1.05–3.33] vs 2.84 [95% CI 1.4–5.75], respectively). The AUC significantly increased when FLI was added to the conventional DM prediction model (0.835, 95% CI: 0.789–0.881, P = 0.0289 vs traditional DM prediction model). The category-free NRI was 0.417 (95% CI: 0.199–0.635) and the IDI was 0.015 (95% CI: 0.003–0.026) for overall study participants.
We found that FLI, a surrogate marker of hepatic steatosis, resulted in significant improvement in DM risk prediction. Our finding suggests that FLI may have clinical and prognostic information for incident DM among the Korean adult population.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD), characterized by increased fat storage in the liver in the absence of excessive alcohol consumption, is the most prevalent form of liver disease in developed countries, including Korea.[1] High prevalence of NAFLD is due to the adherence of rapidly growing Westernized lifestyle and has been estimated to range from 10% to 25% in Korea.[2] NAFLD is known to be closely associated with obesity, insulin resistance, dyslipidemia, and diabetes mellitus (DM) and is now regarded as the hepatic manifestation of metabolic syndrome.[3,4]
Because NAFLD and type 2 DM share similar pathogenesis such as insulin resistance, oxidative stress, and inflammation, it has been hypothesized that NAFLD may associate with increased risk of DM. Moreover, altered secretion of hepatokines such as fetuin-A, fibroblast growth factor 21, and selenoprotein P in the inflamed liver is directly associated with impairment of glucose metabolism.[5] Accordingly, many studies have evaluated the association between NAFLD and DM.[6,7] In fact, Cusi et al[8] demonstrated that 80% of patients with DM had liver fat that might be accompanied by a more violent course of inflammation and fibrosis (i.e., nonalcoholic steatohepatitis) in the later stage of disease. Further, some prospective studies have also reported that NAFLD was associated with the development of DM in community-based cohort studies.[9,10] Given that DM is a growing public health problem globally and it is paramount to identify subjects who are at higher risk of new onset of DM, we hypothesized that NAFLD might be applied as a predictor of incident DM.
Although the gold standard for estimating NAFLD is liver biopsy, this method is quite invasive and often only advised in cases of severe liver disease.[11] Noninvasive techniques such as 1H-magnetic resonance spectroscopy, ultrasound, and computed tomography are commonly used for diagnosis of hepatic steatosis in epidemiologic studies.[12] However, these procedures are time consuming and generally pricey, therefore, may not be reasonable in a large population-based screening of NAFLD. Therefore, more simple tests based on biochemical analysis and anthropometrical parameters that could overcome these issues are advocated. A decade ago, Bedogni et al[13] reported a simple index called the fatty liver index (FLI) for predicting NAFLD in the general population in Italy. The FLI is composed of body mass index (BMI), triglyceride (TG), gamma-glutamyltransferase (GGT), and waist circumference (WC). Earlier, 1 study in Korean general population has reported that NAFLD determined by FLI was well correlated with hepatic steatosis diagnosed by abdominal ultrasonography.[14] To date, although 3 previous studies have analyzed the association between FLI and new onset of DM in French, Korean, and German populations,[9,10,15] there is no information on its role and clinical utility as a predictor of incident DM.
Therefore, the aim of the study was to evaluate the predictive role of FLI for incident DM in a Korean rural cohort. In addition, we also used the improvement reclassified model net reclassification improvement (NRI) and integrated discrimination index (IDI) to examine the incremental predictive value of FLI in diagnosing individuals who will develop new-onset diabetes over 2.6 years of follow-up.
2. Methods
2.1. Study population
The study participants were taken from the Korean Genome and Epidemiology Study on Atherosclerosis Risk of Rural Areas in the Korean General Population (KoGES-ARIRANG). This longitudinal cohort study was designed with a focus to determine the prevalence, incidence, and risk factors for metabolic disorders such as hypertension, diabetes, obesity, and cardiovascular disease.[16–18] All participants were aged between 40 and 70 years belonging to the rural area of Wonju and Pyeongchang in South Korea. The baseline study was executed from November 2005 to January 2008, encompassed 5178 adults (2127 men and 3051 women). Study participants were invited to join the first follow-up visit (2008–2011) and 3862 (74.6%) attended. Participants with unavailable data for FLI (N = 12) and DM (N = 575) at baseline were excluded. We excluded participants with a history of cardiovascular disease (N = 48) at baseline and excessive alcohol consumption (alcohol consumption >140 g/wk for men and 70 g/wk for women) (N = 442) and 1 subject with missing information on DM at follow-up. Finally, 2784 participants (944 men and 1840 women) were included in the present analysis (Fig. 1). All participants were given a written informed consent to participate in this survey, and the protocol was approved by the institutional review board of Wonju Severance Christian Hospital, and this study was carried out in accordance with the ethical standards of the Helsinki Declaration.
Figure 1.
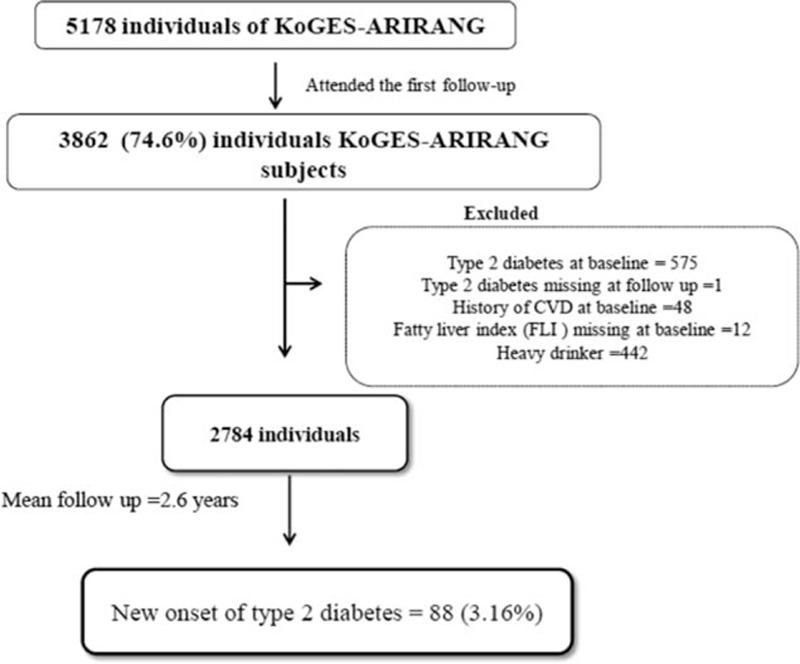
Study population.
2.2. Data collection and measurements
At study entry and follow-up survey, each participant completed both medical history and lifestyle questionnaire according to the standardized procedure. For anthropometrical measurements, body weight, height, and WC were measured whilst participants wore light clothing. BMI was calculated by the formula: (weight in kg)/height2 in meter. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured with a standard mercury sphygmomanometer twice on the right arm of participants (Baumanometer, Copiague, NY). The mean of the 2 readings was utilized in the data analysis. A self-reported questionnaire (yes/no) was used for the information on smoking and current alcohol intake. Subjects answering to the question “do you perform physical exercise regularly enough to make you sweat?” were assigned to the regular exercise group.
Venous blood samples were collected from all participants after an overnight fast. Fasting glucose, fasting insulin, HbA1c, high-sensitivity C-reactive protein (hs-CRP), and homeostasis model assessment of insulin resistance (HOMA-IR) were measured by the standardized protocol described elsewhere.[17,19] Lipid profiles and liver enzymes were determined by enzymatic methods (Advia 1650; Siemens, Tarrytown, NY). Serum concentrations of adiponectin and leptin were measured by radioimmunoassay (RIA) (LINCO Research, Inc., Saint Charles, MO).
2.3. Definition of incident diabetes
The study endpoint was development of DM at the follow-up visit, defined by criteria of the American Diabetes Association as follows: 8-hour fasting blood glucose (FBG) ≥126 mg/dL, or HbA1c level ≥6.5%, or 2-hour plasma glucose level ≥200 mg/dL during a 75-g oral glucose tolerance test.[20] In addition, participants who reported currently taking antidiabetic medicine during the follow-up were considered to have DM.
2.4. Definition of fatty liver (NAFLD) according to fatty liver index
The FLI, a surrogate marker of NAFLD, was analyzed on the basis of the report by Bedogni et al[13] as follows: FLI = (e0.953 × loge [TG] + 0.139 × BMI + 0.718 × loge [GGT] + 0.053 × WC − 15.745)/(1 + e0.953 × loge [TG] + 0.139 × BMI + 0.718 × loge [GGT] + 0.053 × WC − 15.745) × 100. The units of TG, GGT, and WC were mmol/L, U/L, and cm, respectively. According to the report by Bedogni et al, we categorized the study participants into 3 groups (group 1, group 2, and group 3) based on the value of FLI which varies from 0 to 100. Group 1, FLI < 30, described as not having NAFLD; group 2, FLI 30 to 59, defined as intermediate FLI; and group 3, FLI ≥60, defined as having NAFLD.
2.5. Statistical analysis
Data are expressed as frequencies with percentage or means with standard deviation. The association between new onset of diabetes and FLI categories was analyzed by the two-sample t test, one-way analysis of variance (ANOVA), and chi-square test, as applicable. Pearson correlation analysis was used to evaluate the association between FLI and baseline metabolic parameters. Multivariate analysis was performed to assess the independent association of baseline FLI with new onset of diabetes. Three models were used for the adjustment. First, the age- and sex-adjustment analyses were used in the first model. Second, we further adjusted for family history of diabetes, smoking, alcohol intake, and regular exercise. Finally, in the third model, we adjusted for baseline levels of SBP, fasting glucose, HOMA-IR (log-transformed), high-density lipoprotein cholesterol (HDL-C), and total cholesterol. The odds ratios and 95% confidence intervals (CIs) were analyzed with reference to the increase in FLI indices. We also calculated the additional effect of FLI using area under the receiver-operating characteristic (AUC) curve to demonstrate the improvement in the diagnostic accuracy. Furthermore, we used NRI and IDI calculations to quantify the improvement in actual reclassification and sensitivity based on the addition of FLI in the traditional existing model.[21]P values <0.05 were considered statistically significant, and all statistical analyses were performed using SAS 9.2 Ver. (SAS Institute Inc., Cary, NC).
3. Results
3.1. Baseline characteristics of the population according to the development of DM and FLI
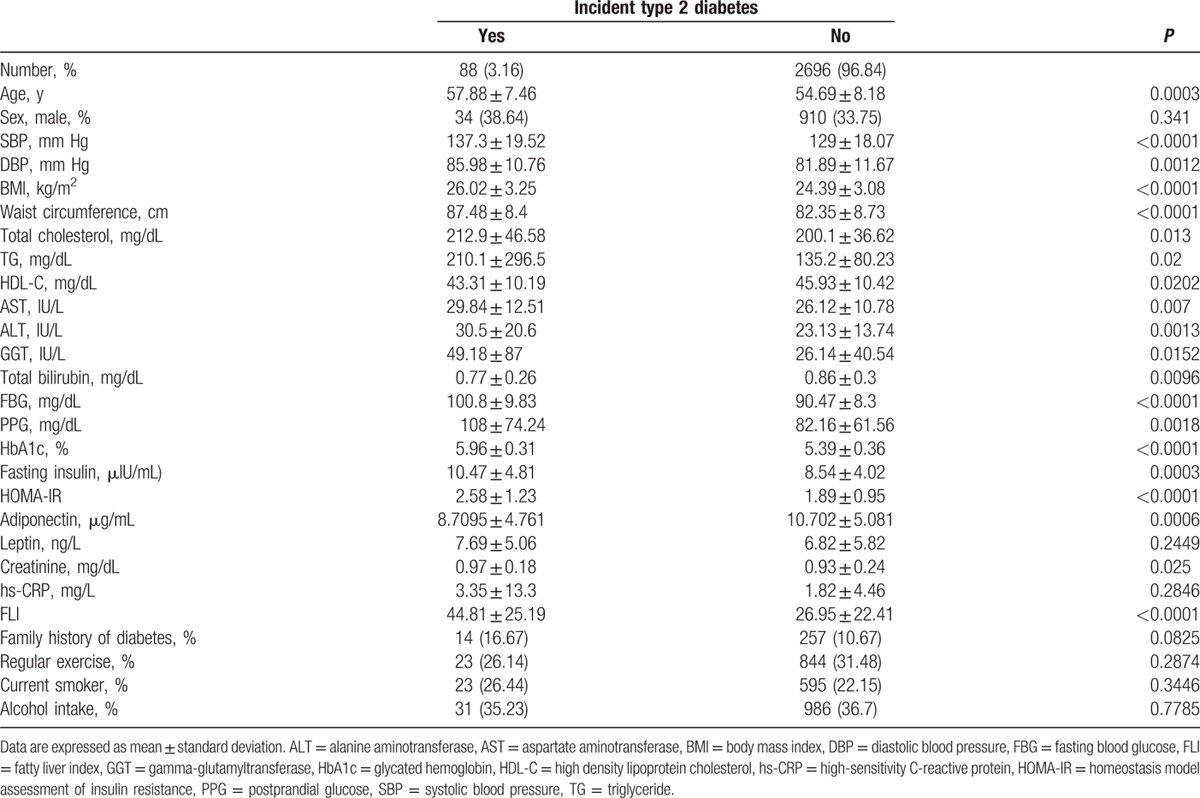
During an average follow-up of 2.6 years, 88 (3.16%) participants developed DM. Baseline characteristics of the incident DM group and nonincident DM group are shown in Table 1. Baseline blood pressure, BMI, WC, total cholesterol, TG, aspartate aminotransferase (AST), and alanine aminotransferase (ALT), GGT, total bilirubin, FBG, postprandial blood glucose (PPG), HbA1c, fasting insulin, HOMA-IR, creatinine, and FLI were significantly higher in individuals who developed DM than in those who did not. HDL-C and adiponectin levels were significantly lower in participants who developed DM compared with those who did not. There were no significant differences in hs-CRP, leptin, exercise, current smoker, and alcohol intake between the 2 groups.
Table 1.
Baseline characteristics of study participants by incident type 2 diabetes.
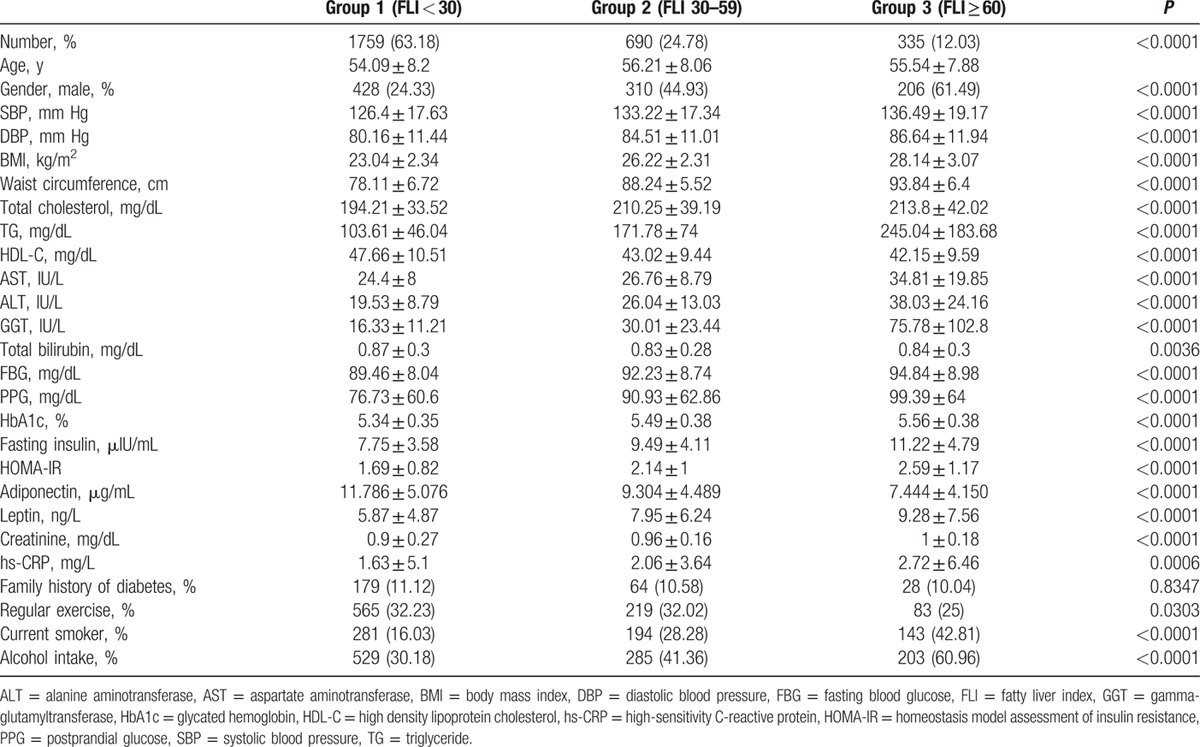
The characteristics of study participants categorized into 3 groups according to the baseline FLI are shown in Table 2. A total of 335 (12.03%) participants had NAFLD as assessed by FLI. Considering age, participants in group 2 (intermediate) were significantly older than those in group 1 (no NAFLD) or group 3 (NAFLD). As the FLI elevated, participants were more likely to be drinkers, current smokers, and insulin-resistant. Moreover, we found a positive association between FLI groups and SBP, DBP, BMI, WC, total cholesterol, TG, AST, ALT, GGT, FBG, PPG, HbA1c, fasting insulin, HOMA-IR, creatinine, leptin, and hs-CRP levels, whereas a negative association was found with HDL-C, total bilirubin, and adiponectin levels.
Table 2.
Baseline characteristics of study participants according to the FLI group.
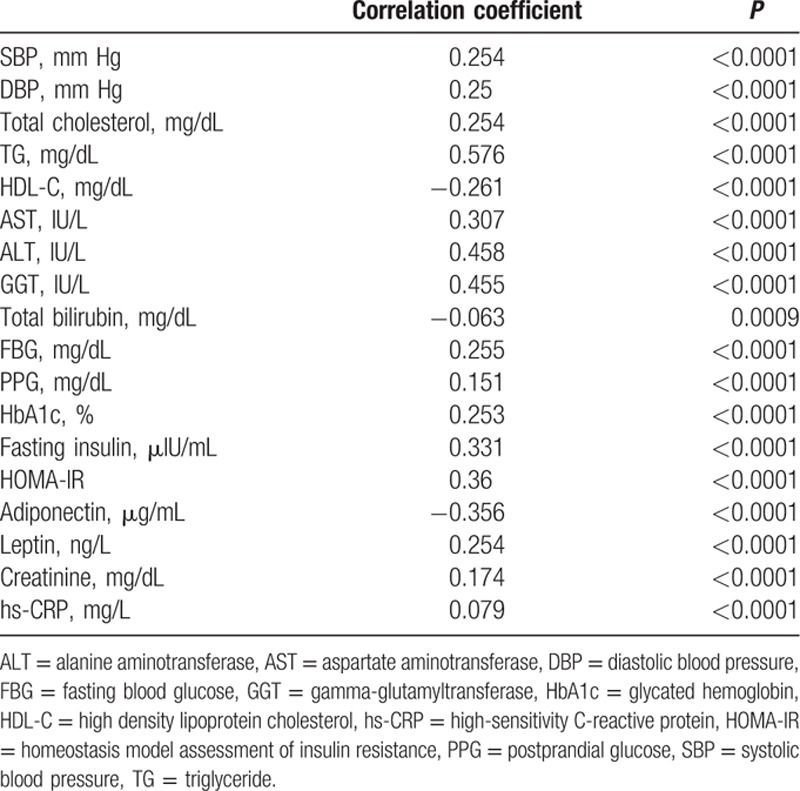
3.2. Correlation of FLI and metabolic parameters
Table 3 shows a correlation between FLI and baseline metabolic parameters. FLI score was positively correlated with SBP, DBP, total cholesterol, TG, AST, ALT, GGT, FBG, PPG, HbA1c, fasting insulin levels, HOMA-IR, leptin, creatinine, and hsCRP. There was a significant negative association between FLI and HDL-C, total bilirubin, and adiponectin levels.
Table 3.
Simple correlation between FLI and metabolic parameters.
3.3. Assessment of FLI for the prediction of new onset of DM over 2.6 years
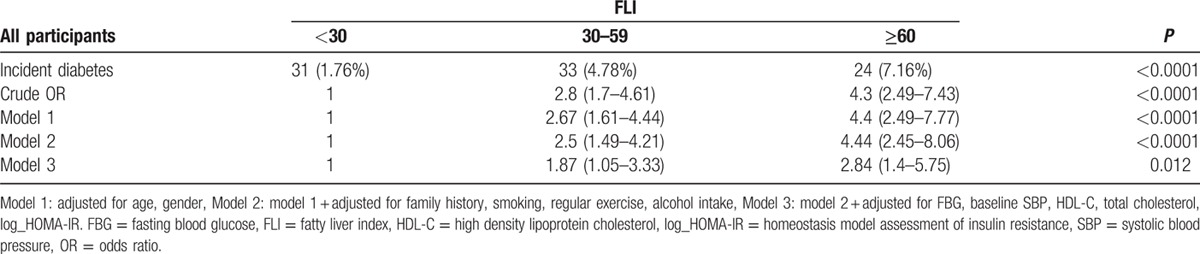
Table 4 shows the multiple logistic regression models for risk of incident DM according to the categories of FLI. The odds ratio for incident DM increased across FLI groups following adjustment for age and gender. This trend remained significant even after further adjustment of smoking, regular exercise, family history of DM, and alcohol intake. In fully adjusted model including baseline FBG, baseline SBP, HDL-C, and HOMA-IR, the odds ratios (95% CI) for new-onset DM in group 2 (FLI: 30–59) and group 3 (FLI: ≥60) were 1.87 (95% CI: 1.05–3.33) and 2.84 (95% CI: 1.40–5.75), respectively, compared to those in group 1 (FLI: <30; P for trend ≤0.012). We also analyzed the odds ratios of each 4 component of FLI (TG, BMI, WC, and GGT) for new-onset DM. As a result, we found that TG, BMI, and WC were independently associated with incident DM even after adjustment for confounding factors. However, GGT did not significantly increase the odds ratios for incident DM in a fully adjusted model (Supplemental Table 1).
Table 4.
Odds ratios and 95% confidence intervals for diabetes according to FLI categories.
3.4. Additional clinical information for prediction of incident DM
The addition of FLI to traditional risk models including conventional risk factors for the prediction of incident DM is shown in Fig. 2. The AUC for predicting future incidence of DM using age, gender, family history, smoking, regular exercise, alcohol intake, FBG, baseline SBP, HDL-C, total cholesterol, and log-transformed HOMA-IR was 0.818 (95% CI: 0.769–0.867). The AUC significantly increased when FLI was added to the conventional DM prediction model (0.835, 95% CI: 0.789–0.881, P = 0.0289 versus traditional DM prediction model). However, the addition of BMI and/or WC to conventional DM prediction model did not significantly improve the AUC values (Supplemental Table 2). We also assessed whether the addition of FLI to the conventional DM prediction model can improve the predictive ability for new-onset DM using NRI and IDI. We found that the category-free NRI was 0.417 (95% CI: 0.199–0.635, P = 0.0002) and the IDI was 0.015 (95% CI: 0.003–0.026, P = 0.0121) for the overall study participants. Thus, the addition of FLI to the basic DM risk model correctly reclassified 40% more cases in the overall study population.
Figure 2.
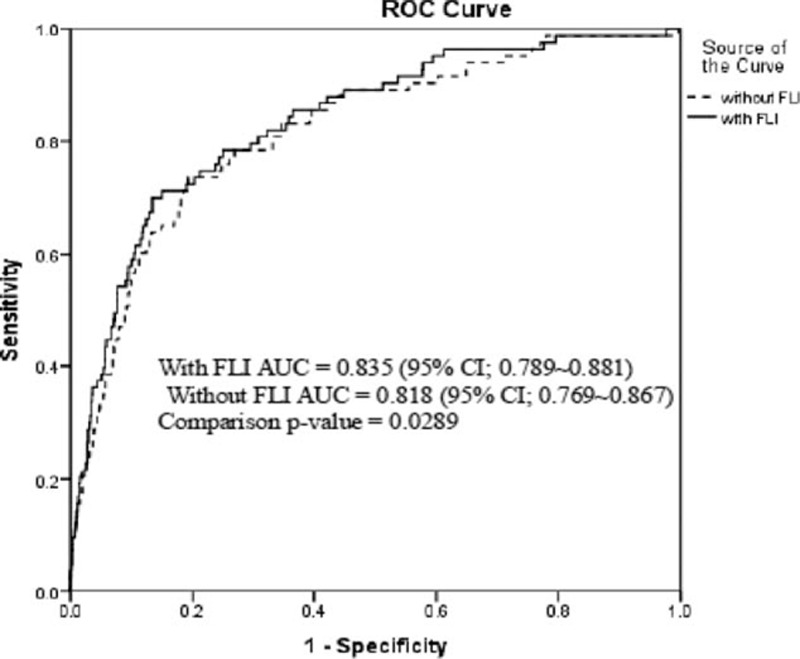
The comparison of area under the receiver-operating characteristic curve for incident diabetes mellitus (DM) according to adding or not fatty liver index to the conventional DM prediction risk model∗. ∗Age, gender, family history of DM, smoking, regular exercise, alcohol intake, fasting blood glucose, baseline systolic blood pressure, high density lipoprotein-cholesterol, total cholesterol, and log-transformed homeostatic model assessment.
4. Discussion
In this prospective cohort study, we found that FLI, a surrogate marker of NAFLD, well predicts future development of DM over 2.6 years of follow-up in Korean adults. This association was independent of age, gender, family history of diabetes, smoking, exercise, alcohol intake, FBG, baseline SBP, HDL-C, total cholesterol, and HOMA-IR. We also demonstrated that the FLI significantly improved the AUC, NRI, and IDI. These results suggest that FLI might be helpful for DM risk stratification in Korean adults.
As the prevalence of DM increases, there is an increasing need to identify individuals at risk early so that they may benefit from early interventions to prevent DM later in life. Indeed, a number of risk prediction tools for DM have been developed that could be used for screening in clinical practice. However, to date, there is no widely accepted risk prediction score that has been validated. Developing a simple index that identifies those at high risk of DM is important because DM can be mostly preventable through early interventions.
The pathogeneses of NAFLD and DM are known to be closely related with regard to insulin resistance and chronic inflammation.[22,23] Consequently, several studies designed to investigate whether fatty liver disease is a predictor for incident DM have demonstrated a strong relationship between NAFLD and DM.[7,24–27] However, almost all of these studies assessed fatty liver using ultrasonography, which requires a well trained radiologist and is probably not practicable for application in epidemiologic studies. In contrast, FLI is a noninvasive and simple marker of NAFLD that predicts the presence of hepatic steatosis. In addition, it was shown to be a validated index against magnetic resonance spectroscopy in a population study, with moderate diagnostic accuracy.[28] Therefore, we hypothesized that FLI, a surrogate marker of NAFLD, might be applied as an index for prediction of incident DM. In the present study, we evaluated whether FLI, a simple and validated index for NAFLD, is associated with incident DM and assessed its predictive value as a risk marker for DM development in a population-based cohort.
We noted a significant association between NAFLD as evaluated through FLI (FLI ≥ 60) and the development of DM. These findings are steady with results of other cohort studies on NAFLD and incident DM.[15,27,29] However, previous studies did not discuss the possibility of FLI as a prognostic tool of incident DM in a clinical setting. Our study demonstrated that the incorporation of FLI into traditional DM risk prediction models significantly improved the prediction of DM after 2.6 years of follow-up. Specifically, as shown in Fig. 2, there was a statistically significant improvement in the AUC with the addition of FLI (P = 0.0289). To further explore the added value of FLI as a predictor, we applied specific statistical techniques including 2 metrics, IDI, and NRI. The NRI can provide clinical information by presenting the quantified improvement resulting from the addition of a new biomarker to the logistic previous model and the IDI can provide clinical information on increased sensitivity through the addition of new markers without sacrificing specificity.
The present study showed that FLI considerably improved the NRI and IDI, which is known to be more sensitive than the AUC for determination of improvement in the predictive value.[30] These results indicate that FLI might act as an additional contributor to predicting the risk of incident DM when applied with conventional risk factors. Thus, FLI could increase the predictive ability for identification of participants at future risk for developing DM and therefore has a clinical role in screening for type 2 diabetes. Our leading strength of the study is the data that were obtained from a large rural cohort with rare demographic shift. Furthermore, this is the first population-based prospective cohort study showing the potential role of FLI in the prediction of DM in adults.
There are some limitations to our study. First, because each parameter of FLI—WC, BMI, TG, and GGT—is a risk factor for DM by itself, thus NAFLD may not be an independent predictor for the new onset of diabetes. Second, the follow-up period of this cohort was only 2.6 years, and approximately 25% of the participants did not complete the second visit; therefore, we could not analyze a large-sized population. Third, alcohol consumption is an influential factor distinguishing NAFLD and alcoholic fatty liver disease. Although our study excluded subjects with higher alcohol drinkers and also adjusted for alcohol consumption, we could not procure histories of alcohol intake in a quantitative manner; therefore, it was difficult to discriminate alcoholic fatty liver disease and NAFLD. Lastly, our study participants were restricted to Korean rural adults and hence it is uncertain whether our outcome could be applicable to other ethnic and regional populations.
Our study demonstrated an independent association between FLI and new onset of DM in a relatively healthy rural Korean population. Our results also indicate that the FLI, a simple surrogate measure of hepatic steatosis, is a useful and easily accessible tool for identifying individuals at high risk for DM. In addition, we demonstrated that FLI could provide additional information for the prediction of future DM beyond the conventional risk factors. A further long-term follow-up study with larger sample size is prerequisite to generalize the value of this risk-scoring tool for predicting incident DM.
Supplementary Material
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, AUC curve = area under the receiver-operating characteristic curve, BMI = body mass index, DBP = diastolic blood pressure, DM = diabetes mellitus, FBG = fasting blood glucose, FLI = fatty liver index, GGT = gamma-glutamyltransferase, HDL-C = high-density lipoprotein cholesterol, HOMA-IR = homeostasis model assessment of insulin resistance, hs-CRP = high-sensitivity C-reactive protein, IDI = integrated discrimination index, NAFLD = nonalcoholic fatty liver disease, NRI = net reclassification improvement, SBP = systolic blood pressure, TG = triglyceride, WC = waist circumference.
This study was supported in part by a grant from the Korea Centers for Disease Control and Prevention (2005-E71013-00, 2006-E71002-00, 2007-E71013-00, 2008-E71004-00, 2009-E71006-00, and 2010-E71003-00).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Jeong EH, Jun DW, Cho YK, et al. Regional prevalence of non-alcoholic fatty liver disease in Seoul and Gyeonggi-do, Korea. Clin Mol Hepatol 2013; 19:266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JY, Kim KM, Lee SG, et al. Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol 2007; 47:239–244. [DOI] [PubMed] [Google Scholar]
- 3.Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2014; 2:901–910. [DOI] [PubMed] [Google Scholar]
- 4.Souza MR, Diniz Mde F, Medeiros-Filho JE, et al. Metabolic syndrome and risk factors for non-alcoholic fatty liver disease. Arq Gastroenterol 2012; 49:89–96. [DOI] [PubMed] [Google Scholar]
- 5.Stefan N, Haring HU. The role of hepatokines in metabolism. Nat Rev Endocrinol 2013; 9:144–152. [DOI] [PubMed] [Google Scholar]
- 6.Sung KC, Kim SH. Interrelationship between fatty liver and insulin resistance in the development of type 2 diabetes. J Clin Endocrinol Metab 2011; 96:1093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim CH, Park JY, Lee KU, et al. Fatty liver is an independent risk factor for the development of type 2 diabetes in Korean adults. Diabet Med 2008; 25:476–481. [DOI] [PubMed] [Google Scholar]
- 8.Cusi K. Nonalcoholic fatty liver disease in type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes 2009; 16:141–149. [DOI] [PubMed] [Google Scholar]
- 9.Jager S, Jacobs S, Kroger J, et al. Association between the fatty liver index and risk of type 2 diabetes in the EPIC-Potsdam Study. PLoS One 2015; 10:e0124749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung CH, Lee WJ, Hwang JY, et al. Assessment of the fatty liver index as an indicator of hepatic steatosis for predicting incident diabetes independently of insulin resistance in a Korean population. Diabet Med 2013; 30:428–435. [DOI] [PubMed] [Google Scholar]
- 11.Barsic N, Lerotic I, Smircic-Duvnjak L, et al. Overview and developments in noninvasive diagnosis of nonalcoholic fatty liver disease. World J Gastroenterol 2012; 18:3945–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahani DV, Kalva SP. Imaging the liver. Oncologist 2004; 9:385–397. [DOI] [PubMed] [Google Scholar]
- 13.Bedogni G, Bellentani S, Miglioli L, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006; 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JH, Kwon SY, Lee SW, et al. Validation of fatty liver index and lipid accumulation product for predicting fatty liver in Korean population. Liver Int 2011; 31:1600–1601. [DOI] [PubMed] [Google Scholar]
- 15.Balkau B, Lange C, Vol S, et al. Nine-year incident diabetes is predicted by fatty liver indices: the French D.E.S.I.R. study. BMC Gastroenterol 2010; 10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh SB, Yoon J, Kim JY, et al. Relationships between serum adiponectin with metabolic syndrome and components of metabolic syndrome in non-diabetic Koreans: ARIRANG study. Yonsei Med J 2011; 52:234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JY, Ahn SV, Yoon JH, et al. Prospective study of serum adiponectin and incident metabolic syndrome: the ARIRANG study. Diabetes Care 2013; 36:1547–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yadav D, Lee ES, Kim HM, et al. Prospective study of serum uric acid levels and incident metabolic syndrome in a Korean rural cohort. Atherosclerosis 2015; 241:271–277. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28:412–419. [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care 2012; 35 suppl 1:S11–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008; 27:157–172.discussion 207–212. [DOI] [PubMed] [Google Scholar]
- 22.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006; 116:1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444:860–867. [DOI] [PubMed] [Google Scholar]
- 24.Park SK, Seo MH, Shin HC, et al. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology 2013; 57:1378–1383. [DOI] [PubMed] [Google Scholar]
- 25.Sung KC, Wild SH, Byrne CD. Resolution of fatty liver and risk of incident diabetes. J Clin Endocrinol Metab 2013; 98:3637–3643. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto M, Takeda Y, Yoda Y, et al. The association of fatty liver and diabetes risk. J Epidemiol 2003; 13:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibata M, Kihara Y, Taguchi M, et al. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care 2007; 30:2940–2944. [DOI] [PubMed] [Google Scholar]
- 28.Kahl S, Strassburger K, Nowotny B, et al. Comparison of liver fat indices for the diagnosis of hepatic steatosis and insulin resistance. PLoS One 2014; 9:e94059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang Y, Jung HS, Yun KE, et al. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am J Gastroenterol 2013; 108:1861–1868. [DOI] [PubMed] [Google Scholar]
- 30.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 2011; 30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


