Abstract
Background:
The aims of this study were to compare the efficacy and safety of the addition of adefovir dipivoxil (ADV) (started at different time points) to pegylated interferon alpha-2a (PEG-INF-α2a) and PEG-INF-α2a monotherapy. This prospective, randomized study sought to evaluate the safety and efficacy of the combination of PEG-INF-α2a and ADV at different time points.120 patients were randomized into groups that received PEG-INF-α2a as monotherapy (group A) or in combination with ADV started at week 0 (group B), 12 (group C), or 24 (group D). All patients were followed for 48 weeks. Efficacy and safety analyses were performed.
Methods:
Patients in group a received 135 μg of PEG-INF-α2a by subcutaneous injection once weekly for 48 weeks. Patients in the ADV add-on group received 135 μg of PEG-INF-α2a subcutaneously once weekly and received 10 mg of ADV administered once daily for 48 weeks. HBV DNA, HBsAg, HBeAg, and hepatitis B e antibody levels were determined. Responses were determined at week 12 (ADV add-on), the end of treatment for PEG-INF-α2a (48weeks) and ADV (EOT) and at the end of 96 weeks of follow-up (EOF).
Results:
The rate of HBV DNA loss were higher in the combination groups than group A at the week 12, week 48, the EOT and EOF (P < 0.05). The rates of HBeAg seroconversion and HBsAg loss were similar among the treatment groups (P>0.05). The alanineaminotransferase (ALT) normalization rate was higher in the combination group than group A only at the EOT (P = 0.007). By the EOF, the patients with ADV added at week 12 achieved higher rates of HBV DNA loss (71.9%), HBeAg seroconversion (50.0%), HBsAg loss (15.6%), and ALT normalization (78.1%).
Conclusions:
PEG-INF-α2a plus ADV combination therapy is safe and superior to PEG-INF-α2amonotherapyfor decreasing serum HBV DNA and normalizing the ALT level but has no significant impact on the rate of HBeAg seroconversion and HBsAg loss. Adding ADV at week 12 may be an optimal combination strategy.
Keywords: adefovir dipivoxil, chronic hepatitis B, combination therapy, pegylated interferon alfa-2a
1. Introduction
Hepatitis B virus (HBV) infection is a major public health problem. More than 350 million people are chronically infected with HBV throughout the world.[1] Patients with HBV infection are at risk of progressing to long-term complications, including cirrhosis of the liver, decompensated liver disease, and/or hepatocellular carcinoma (HCC). Approximately 93 million people are chronic HBV (CHB) carriers in China, and 300,000 individuals die from chronic disease every year.[2]
Interferon (IFN) and several nucleoside/nucleotide analogs (NAs) are currently approved for the treatment of CHB in several countries. Moreover, pegylated interferon (peg-IFN) alfa-2a (40 kD) and NAs are recommended by treatment guidelines as a first-line therapy for CHB. However, a 48-week course of peg-IFN alfa-2a treatment only results in sustained immune control in 36% of HBeAg-positive patients.[3,4] After the completion of peg-IFN treatment, the response rates among HBeAg-positive patients were 13%, 25%, 14%, 21%, and 21% at 1, 2, 3, 4, and 5 years, respectively.[5] Thus, the HBeAg seroconversion rate and the HBsAg loss rate of peg-IFN remain low. In contrast, NAs have high rates of suppression of viral replication in HBeAg-positive CHB patients but rarely lead to sustained HBeAg or HBsAg loss or seroconversion.[6,7] Although ADV was not recommended as a first-line treatment, ADV is widely used in most Asian countries for nucleos (t)ide-naïve Asian CHB patients owing to low cost and easy availability. Entecavir (ETV) is superior to adefovir (ADV) for decreasing serum HBV-DNA and normalizing the alanine aminotransferase (ALT) level but is similar to ADV for clearing HBeAgs and favoring HBeAg seroconversion for HBeAg-positive NA-naïve Asian patients with CHB.[8] Tenofovir disoproxil fumarate (TDF) demonstrated superiority over ADV with respect to the primary endpoint of viral suppression in both HBeAg-positive and HBeAg-negative patients. However, the proportions of HBeAg loss and HBeAg seroconversion at week 48 were similar. In a word, combination therapy of PEG-INF-α2a and ADV may be a good plan for the CHB patients in the countries with limited health resources, which can improve overall response rates.
In our previous peg-IFN alfa-2a and ADV combination studies, the addition of ADV to PEG-INF-α2a at week 24 improved the inhibition of HBV DNA in CHB patients who were HBeAg-positive, and the level of HBsAg at l2 and 24 weeks was a predictor used to evaluate the efficacy of combination therapy. However, the rates of HBeAg loss and HBeAg seroconversion were not improved compared to PEG-INF-α2a monotherapy.[9,10] Takkenberg et al[11] reported the results of combination therapy with peg-IFN and ADV for 48 weeks and showed a high rate of HBeAg loss (41%) and HBsAg loss (11%) in HBeAg-positive patients after the end of treatment (EOT). However, in these studies, combination therapy was stared at week 0 and compared to peg-IFNα-2a monotherapy. To date, no reports have described the efficacy and safety of PEG-INF-α2a therapy with ADV started at different time points (week 0, 12, and 24).
To explore the optimal time to begin combination treatment with PEG-INF-α2a and ADV in HBeAg-positive, treatment-naïve CHB patients, we compared the efficacy and safety of 48 weeks of treatment with PEG-INF-α2a monotherapy to treatment with PEG-INF-α2a combined with ADV therapy added at weeks 0, 12, and 24.
2. Methods
2.1. Study population
This was an open-labeled pilot study performed in accordance with the Declaration of Helsinki. The Ethics Committee of the Third Affiliated Hospital of Sun Yat-Sen University approved this study.
Informed consent was obtained from all patients. The inclusion criteria consisted of patients with a documented HBV infection lasting at least 6 months who were 18 to 60 years of age, were HBeAg-positive, had serum HBV DNA levels ≥100,000 copies/mL, had experienced 2 episodes of elevated serum ALT levels (>2 but ≤10 times the upper limit of normal), had no history of antiviral treatment for HBV in the previous 6 months, and did not have cirrhosis (confirmed by liver biopsy or ultrasound). The exclusion criteria consisted of antiviral, antineoplastic, or immunomodulatory treatment within the previous 6 months; co-infection with hepatitis A, C, or D virus or human immune deficiency virus; evidence of nonviral chronic liver disease or liver decompensation; or a history of severe psychiatric disease, particularly depression.[12,13]
2.2. Study design
This investigator-initiated, prospective, randomized, open-label study (ClinicalTrials. Govregistration no: 09000612) was conducted in the Department of Infectious Diseases of the Third Affiliated Hospital of Sun Yat-Sen University between October 2009 and December 2014. Eligible patients were randomized into 4 treatment groups. Randomization was centralized, and a patient identification number assigned by the randomization system was used to allocate the patients to treatment groups. Patients in the PEG-INF-α2a monotherapy group received 135 μg of peg-IFN alfa-2a (Pegasys,F. Hoffmann-La Roche Ltd., Basel, Switzerland) by subcutaneous injection once weekly for 48 weeks (group A). Patients in the ADV add-on group received 135 μg of PEG-INF-α2a subcutaneously once weekly for 48 weeks and were divided into 3 groups according to the ADV add-on time (group B, ADV add-on at week 0; group C, ADV add-on at week 12; and group D, ADV add-on at week 24). These patients received 10 mg of ADV (GelanSushike [Tianjin] Co., Ltd, China) administered once daily (Fig. 1). All patients were followed for 48 weeks. Routine blood tests were performed each month. HBV DNA, HBsAg, HBeAg, and hepatitis B e antibody levels were determined every 4 to 8 weeks. The efficacy parameters were the EOT viral and biochemical responses. Responses were determined at week 12 (ADV add-on), the EOT for PEG-INF-α2a (48 weeks) and ADV, and at the end of 96 weeks of follow-up (EOF). The biochemical response was defined as the normalization of ALT levels. Viral responses included the HBV DNA loss rate, HBeAg seroconversion rate, and HBsAg loss rate. Response definitions were previously described in detail.[11]
Figure 1.
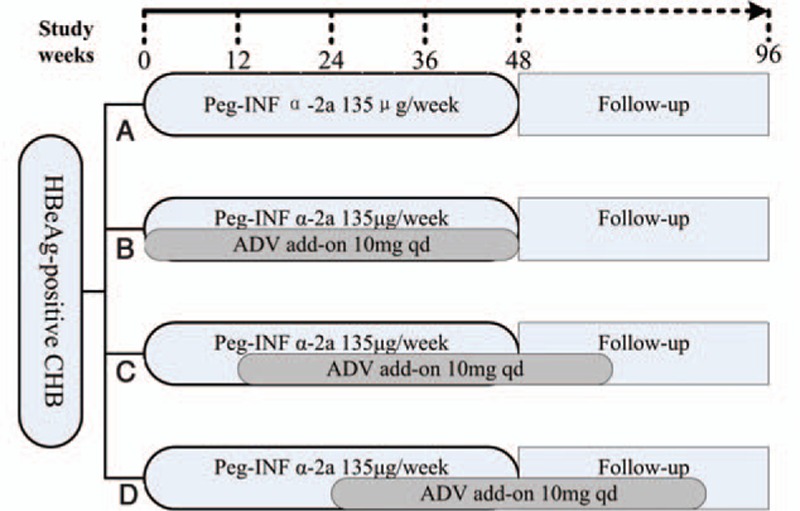
Study design. ADV = adefovirdipivoxil, CHB = chronic hepatitis B, HBeAg = hepatitis B e antigen, Peg-INF-α2a = pegylated interferon alfa-2a.
2.3. Laboratory assessments
HBeAg, HBsAg, and hepatitis B e antibody level testing were performed using Roche chemiluminescent assays (Roche Elecsys 2010 analyzer; Roche Diagnostics, Indianapolis, IN). Serum HBV DNA levels were measured using in-house real-time PCR with a sensitivity limit of 500 copies/mL. Testing was performed at baseline and at weeks 4, 8, 12, 24, 36,48, 72, and 96 at a central laboratory of the Third Affiliated Hospital of Sun Yat-Sen University. All patients were assessed for thyroid function, autoimmune antibodies, blood sugar, and routine blood tests before and during treatment.
2.4. Sample size calculation
A previous study demonstrated that about 27 subjects in each group can reveal the different effects of peg-IFN alfa on HBeAg-positive patients. Therefore, we decided to include 30 patients in each group, which allowed for an expected 15% dropout rate.
2.5. Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile) depending on the result of normality test. The Shapiro–Wilk test of normality was performed. Normally distributed variables were analyzed with ANOVA test, otherwise with nonparametric test. Categorical variables were expressed as the number of patients (percentage) and tested with the χ2 test or Fisher exact test, as appropriate. All analyses were performed as 2-sided tests with a 0.05 level of significance. The Bonferroni correction was used for multiple testing. In addition, the logistic regression analysis techniques were also used to access the difference of efficacy for 4 different treatment schemes, adjusting for the possibly influencing covariates. Patients with missing data were counted as nonresponders. SPSS software (version 22.0; SPSS Inc, Chicago, IL) was used to perform the statistical analyses.
3. Results
3.1. Patient characteristics
A total of 147 patients signed informed consent forms. Six patients did not meet the inclusion criteria because of a viral load <100,000 copies/mL. One patient developed cirrhosis, which was confirmed by an ultrasound evaluation. Ultimately,140 patients were randomized into the 4 treatment groups (group A, n = 35; group B, n = 35; group C, n = 35; and group D, n = 35). Of these patients,11 patients discontinued treatment because of loss to follow-up (1 in group A, 1 in group B, 2 in group C, and 3 in group D), patient request (1 in group A and 1 in group B), and side effects (1 in group A and 1 in group C). In total, 129 patients completed at least 96 weeks of treatment follow-up.
3.2. Patient baseline clinical characteristics
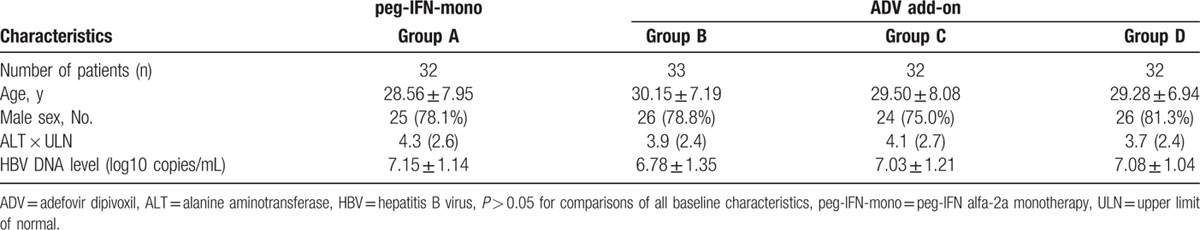
Table 1 shows the age, sex, and baseline clinical and viral characteristics. These were generally similar among the 4 groups, and no significant differences were observed in the baseline characteristics between the groups.
Table 1.
Demographics and baseline characteristics of the patients.
3.3. Response to treatment at week 12 (ADV add-on)
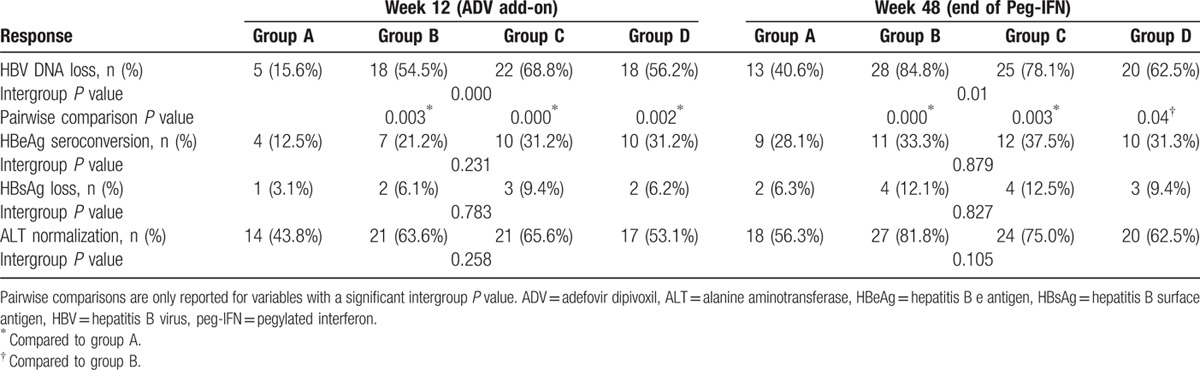
At week 12 (ADV add-on for 12 weeks), patients in the ADV add-on groups (groups B, C, and D; ADV add-on at week 0, 12, 24) achieved higher rates of HBV DNA loss than those in the PEG-INF-α2a monotherapy group (group A) (P = 0.000; Table 2). However, similar rates of combined response (HBeAg seroconversion, HBsAg loss, and ALT normalization) were achieved in all treatment groups at week 12 (Table 2).
Table 2.
Outcome measures at week 12 and 48.
3.4. Response to treatment at week 48
In total, 13 of 32 (40.6%) patients in the PEG-INF-α2a monotherapy group (group A), 28 of 33 (84.8%) patients in group B (ADV add-on at week 0), 25 of 32 (78.1%) patients in group C (ADV add-on at week 12), and 20 of 32 (62.5%) patients in group D (ADV add-on at week 24) achieved HBV DNA loss, and the difference across treatment groups was statistically significant (P = 0.01; Table 2). A greater proportion of patients in group B and group C achieved HBV DNA loss at week 48 (end of peg-IFN) than in group A (84.8% and 78.1% vs. 40.6%). However, no difference was found in the HBV DNA loss rate at week 48 between group A and group D (40.6% vs. 62.5%). In the ADV add-on groups, patients who received ADV add-on at week 0 (group B) demonstrated a higher rate of HBV DNA loss at week 48 than group D (P = 0.04; Table 2). Similar rates of combined response (HBeAg seroconversion, HBsAg loss, and ALT normalization) were achieved in all treatment groups at week 48 (Table 2).
3.5. Response to treatment at the EOT
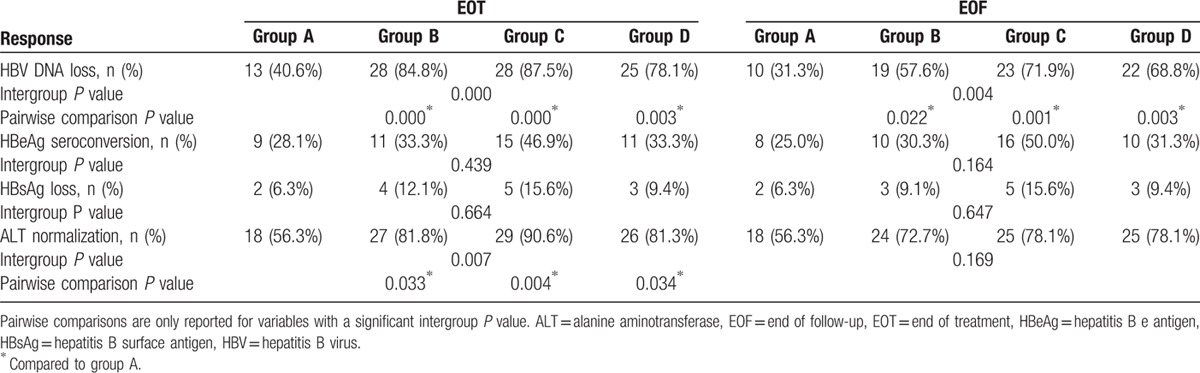
At the EOT, patients in the ADV add-on groups (groups B, C, and D) achieved higher rates of HBV DNA loss than those in group A (P = 0.000; Table 3). The rates of HBV DNA loss increased from week 48 to the EOT in group C and group D (78.1%–87.5% and62.5%-78.1%, respectively). Similarly, the rates of ALT normalizationachieved in all ADV add-on groups (groups B, C, and D) at the EOT were higher than those of group A (P = 0.007; Table3). However, the rates of HBsAg loss and HBeAg seroconversion were similar in all groups at the EOT (Table 3).
Table 3.
Outcome measures at the EOT and EOF.
3.6. Response to treatment at the EOF (week 96)
By the EOF (week 96), viral rebound occurred in all groups. However, the groups showed comparable levels at the EOF (Table 3). Patients in the ADV add-on groups (groups B, C, and D) continued to have higher rates of HBV DNA loss than patients in group A (P = 0.004; Table 3). No difference was observed for the HBV DNA loss rate at the EOF for the 3 ADV add-on groups. Patients in group C had a higher rate of HBeAg seroconversion at the EOF than the other 3 groups (P = 0.164; Table 3). Comparable rates of HBsAg loss and ALT normalization were achieved in all groups by the EOF (P = 0.647 and 0.169; Table 3).
3.7. Safety and tolerability
Combined peg-IFN and ADV therapy was generally well tolerated. No side effects related to the combination of peg-IFN and ADV therapy were reported. The most common side effects in the add-on arm were those known to occur with peg-IFN therapy. The most frequent treatment-related side effects consisted of abnormal laboratory results, such as decreased white blood cell and neutrophil counts, which are known to occur with PEG-INF-α2a treatment. No unexpected side effects were reported. Six patients in the combination therapy group required symptomatic treatment for persistent fever before week 24, but they all completed the treatment. One patient assigned to group B experienced an ALT flare after stopping ADV. One patient in group A experienced thyroid dysfunction.
4. Discussion
ADV and peg-IFN are 2 antiviral drugs with different targets and pharmacokinetics. Peg-IFN produces viral suppression and immunemodulatory activity simultaneously. The favorable meta-analysis outcomes of peg-IFN include the sustained suppression of viral replication and HBeAg seroconversion.[14] However, the rate of HBeAg seroconversion remains low. Combining ADV and peg-IFN may be a logical approach because they activate different mechanisms to control HBeAg-positive CHB. A series of previous studies with thorough viral analyses showed that the combination of ADV and peg-IFN induced a higher viral response but with differences in the rate of HBeAg seroconversion and HBsAg loss.[11,15,16] Interestingly, these studies selected the beginning of treatment as the time of combination of PEG-INF-α2a and ADV. Based on current knowledge and previous studies, this study compared the efficacy and safety of 48 weeks of combination treatment with PEG-INF-α2a and ADV (with ADV started at different time points) with PEG-INF-α2a monotherapy.
In our study, a greater proportion of patients in the combination therapy group than in the monotherapy group achieved HBV DNA loss at week 48, the EOT, and the EOF. Despite the more pronounced on-treatment suppression of HBV DNA in the combination therapy group compared to PEG-INF-α2a monotherapy, the rates of HBV DNA loss were similar in all 3 combination therapy groups at the different endpoints. This finding is consistent with previous reports of HBV DNA kinetics in combination studies of lamivudine, ADV, or ETV with PEG-INF-α2a monotherapy.[13,17,18] Recently, Gu etal[19] observed that combination therapy of adefovir dipivoxil and PEG-INF-α2b also achieved higher HBV DNA uncertainty rate than the group treated with adefovir dipivoxil only at the 12th, 24th, 36th, and 48th week. Because these 2 drugs have different function mechanisms and combination of them can produce coordination or superimposed effect, the higher rate of HBV DNA loss in the combination therapy group is not difficult to understand. Interestingly, we found that patients treated with peg-IFN and ADV add-on at week 12 (group C) achieved a significantly higher rate of HBV DNA loss than the other combination treatment groups (ADV add-on at week 0 or 24). At the EOT and EOF, the HBV DNA loss rates were 87.5% and 71.9%, respectively, in the patients assigned to the group with ADV add-on at week 12. Our result suggests that ADV add-on at week 12 may lead to a higher proportion of HBV DNA loss than the other ADV add-on times. Zheng et al's[20] study observed that total intrahepatic HBV-DNA and cccDNA had significantly decreased after 12 weeks of ADV treatment; meanwhile, HBV-specific IFN-γ-producing CD4+ T cells were greatly enhanced and reached the highest level at week 12 with viral load reduction and the level was further increased between week 36 and week 48. So, patients treated with peg-IFN and ADV add-on at week 12 achieved a significantly higher rate of HBV DNA loss because of strengthening and improving of immune response.
Previous study[21] confirmed that ADV was attributed not only to their direct effect on HBV suppression but also to their immune regulatory capabilities. ADV treatment can increase IFN-γ production by CD4+T cells, which was related to the reduction of HBeAg,levels.[22] Some clinical studies also suggested that combination therapy with peg-IFN and ADV for 48 weeks led to a marked decrease in both serum HBV DNA and the rate of HBeAg seroconversion (53%) compared to treatment with peg-IFN alone. Moreover, after 96 weeks of ADV monotherapy, the HBeAg loss was 80%.[15,16,23,24] However, the rates of HBeAg seroconversion and HBsAg loss at week 48, theEOT, and the EOF were not superior to the addition of ADV compared to PEG-INF-α2a monotherapy in the present study. These findings support previous data, which show that combining PEG-INF-α2a with lamivudine[5] or ETV[13] does not increase the post-treatment efficacy of PEG-INF-α2a. In our study, 46.9% of patients in the ADV 12 week add-on combination therapy group and 28.1% of patients in the monotherapy group showed HBeAg seroconversion at the EOT. We found that patients with ADV add-on at week 12 (group C)achieved a higher rate of HBeAg seroconversion (50%), but no significant differences were found. This discrepancy may be related to the relatively small size of our studied population. In our study, the rates of HBsAg loss in the combination therapy group were 12.1%, 15.6%, and 9.4% for ADV add-on at weeks0, 12, and 24, respectively, whereas a rate of 6.3% was observed in the monotherapy group. This result is consistent with previous reports. However, we found a significant trend for the group that received ADV add-on at week 12 at the EOF.
In our study, compared to the monotherapy group, the ALT normalization rate was similar in the combination groups at 48 weeks of peg-IFN treatment and at the EOF. Some studies[25] have reported that the ALT normalization rate after treatment was higher in the combination group. A meta-analysis also indicated that peg-IFN and ADV combination therapy is superior to IFN-a monotherapy for normalizing the ALT level.[24] Our results only demonstrated a difference between the combination groups and the PEG-INF-α2a monotherapy group at the EOT. This discrepancy may be related to the relatively small size of our studied population or biochemical changes after the EOT.
Regarding safety, ADV add-on was generally well tolerated without unexpected adverse effects. The safety profiles of PEG-INF-α2a monotherapy and combination therapy with PEG-INF-α2a and ADV were similar to those reported previously in other PEG-INF-α2a and NA combination therapy studies. No unexpected adverse effects were reported.[3,13,18,26]
The limitations of this study are that the study period was only 96 weeks; a longer time could provide additional insights. In addition, the number of patients evaluated for treatment efficacy was relatively small. Furthermore, HBV genotype data were not collected. Previous studies suggest that HBsAg kinetics and rates of HBeAg seroconversion vary by HBV genotype.[27,28]
In conclusion, PEG-INF-α2a and ADV combination therapy was safe and superior to PEG-INF-α2a monotherapy for decreasing serum HBV DNA and normalizing the ALT level. However, patients in all treatment groups had similar rates of HBeAg seroconversion and HBsAg loss at the EOF. The addition of ADV at week 12 produced a significantly better response and may be the optimal combination time.
Acknowledgments
The authors would like to thank Lubiao Chen, Xingfei Pan, Li Mai, Ying Yan, and Hong Deng for their contributions tothis work.
Footnotes
Abbreviations: ADV = adefovir dipivoxil, ALT = alanineaminotransferase, CHB = chronic hepatitis B, EOF = the end of follow-up, EOT = the end of treatment, ETV = entecavir, HBeAg = hepatitis B virus e antigen, HBsAg = hepatitis B virus surface antigen, HBV = hepatitis B virus, HBV = hepatitis Bvirus, PEG-IFN = pegylated interferon, TDF = tenofovir disoproxil fumarate.
KZ and HC contributed equally to this work.
Financial Support: This work was supported by research grants from a project supported by the National Natural Science Foundation of China (Grant No. 81500462) and the Medical Research Fund of Guangdong Pharmaceutical Association (Grant Nos. 2012GRS01 and 2013GRS06). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors report no conflicts of interest.
References
- 1.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539. [DOI] [PubMed] [Google Scholar]
- 2.Lu FM, Zhuang H. Prevention of hepatitis B in China: achievements and challenges. Chin Med J (Engl) 2009; 122:2925–2927. [PubMed] [Google Scholar]
- 3.Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005; 352:2682–2695. [DOI] [PubMed] [Google Scholar]
- 4.Liaw YF, Jia JD, Chan HL, et al. Shorter durations and lower doses of peginterferon alfa-2a are associated with inferior hepatitis B e antigen seroconversion rates in hepatitis B virus genotypes B or C. Hepatology 2011; 54:1591–1599. [DOI] [PubMed] [Google Scholar]
- 5.Masaki K, Suzuki F, Hara T, et al. Long-term effects of peginterferon alfa-2a therapy in Japanese patients with chronic hepatitis B virus infection. Virol J 2015; 12:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reijnders JG, Perquin MJ, Zhang N, et al. Nucleos (t) ide analogues only induce temporary hepatitis B e antigen seroconversion in most patients with chronic hepatitis B. Gastroenterology 2010; 139:491–498. [DOI] [PubMed] [Google Scholar]
- 7.Heathcote EJ, Marcellin P, Buti M, et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology 2011; 140:132–143. [DOI] [PubMed] [Google Scholar]
- 8.Zhao P, Liu W, Zhao J, et al. Comparison of the 48-week efficacy between entecavir and adefovir in HBeAg-positive nucleos (t) ide-naïve Asian patients with chronic hepatitis B: a meta-analysis. Virol J 2011; 8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Shu X, Jie Y, et al. [Short-term efficacy of adefovir dipivoxil as an add-on therapy in the pegylated IFNalpha-2a treatment for HBeAg positive chronic hepatitis B patients]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2010; 24:39–41. [PubMed] [Google Scholar]
- 10.Sun H, Cao H, Zhang K, et al. [The levels of HBsAg can predict the efficacy of peglated interferon-alpha 2a combined with adefovir dipivoxil, in HBeAg-positive chronic hepatitis B patients]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2011; 25:132–134. [PubMed] [Google Scholar]
- 11.Takkenberg RB, Jansen L, de Niet A, et al. Baseline hepatitis B surface antigen (HBsAg) as predictor of sustained HBsAg loss in chronic hepatitis B patients treated with peginterferon alfa-2a and adefovir. Antivir Ther 2013; 18:895–904. [DOI] [PubMed] [Google Scholar]
- 12.Huang Z, Deng H, Zhao Q, et al. Peginterferon-alpha2a combined with response-guided short-term lamivudine improves response rate in hepatitis B e antigen-positive hepatitis B patients: a pilot study. Eur J Gastroenterol Hepatol 2013; 25:1165–1169. [DOI] [PubMed] [Google Scholar]
- 13.Xie Q, Zhou H, Bai X, et al. A randomized, open-label clinical study of combined pegylated interferon Alfa-2a (40KD) and entecavir treatment for hepatitis B “e” antigen-positive chronic hepatitis B. Clin Infect Dis 2014; 59:1714–1723. [DOI] [PubMed] [Google Scholar]
- 14.Li W-c, Wang M-r, Kong L-b, et al. Peginterferon alpha-based therapy for chronic hepatitis B focusing on HBsAg clearance or seroconversion: a meta-analysis of controlled clinical trials. BMC Infect Dis 2011; 11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wursthorn K, Lutgehetmann M, Dandri M, et al. Peginterferon alpha - 2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology 2006; 44:675–684. [DOI] [PubMed] [Google Scholar]
- 16.Lutgehetmann M, Volzt T, Quaas A, et al. Sequential combination therapy leads to biochemical and histological improvement despite low ongoing intrahepatic hepatitis B virus replication. Antiviral Ther 2008; 13:57. [PubMed] [Google Scholar]
- 17.Huang R, Hao Y, Zhang J, et al. Interferon - alpha plus adefovir combination therapy versus interferon - alpha monotherapy for chronic hepatitis B treatment: A meta - analysis. Hepatol Res 2013; 43:1040–1051. [DOI] [PubMed] [Google Scholar]
- 18.Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005; 352:2682–2695. [DOI] [PubMed] [Google Scholar]
- 19.Gu J, Sun R, Shen S, et al. The curative effect of adefovir dipivoxil treating HBeAg negative chronic hepatitis B and treating HBeAg positive chronic hepatitis B combining interferon α-2b. Pak J Pharm Sci 2015; 28:1493–1497. [PubMed] [Google Scholar]
- 20.Zheng Q, Zhu Y, Chen J, et al. Decline in intrahepatic cccDNA and increase in immune cell reactivity after 12 weeks of antiviral treatment were associated with HBeAg loss. J Viral Hepat 2014; 21:909–916. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y, Li W, Yu L, et al. Enhancing the antihepatitis B virus immune response by adefovir dipivoxil and entecavir therapies. Cell Mol Immunol 2011; 8:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooksley H, Chokshi S, Maayan Y, et al. Hepatitis B virus e antigen loss during adefovir dipivoxil therapy is associated with enhanced virus-specific CD4+ T-cell reactivity. Antimicrob Agents Chemother 2008; 52:312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou P, Yang F, Wang J, et al. Improved efficacy of a pegylated interferon-(-2a stepwise optimization treatment strategy in the treatment of hepatitis B e antigen-positive chronic hepatitis B patients. Medicine 2015; 94:e730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim V, Abreu R, Nakagawa D, et al. Pegylated interferon alfa for chronic hepatitis B: systematic review and meta - analysis. J Viral Hepat 2016; 23:154–169. [DOI] [PubMed] [Google Scholar]
- 25.Wang YD, Zhao CY, Wang W, et al. Improved efficacy by individualized combination therapy with Peg IFN-a 2a and ADV in HBeAg positive chronic hepatitis B patients. Hepatogastroenterology 2012; 59:680–686. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y-h, Wu T, Sun N, et al. Combination therapy with pegylated interferon alpha-2b and adefovir dipivoxil in HBeAg-positive chronic hepatitis B versus interferon alone: a prospective, randomized study. J Huazhong Univ Sci Technol Med Sci 2014; 34:542–547. [DOI] [PubMed] [Google Scholar]
- 27.Brunetto MR, Marcellin P, Cherubini B, et al. Response to peginterferon alfa-2a (40KD) in HBeAg-negative CHB: on-treatment kinetics of HBsAg serum levels vary by HBV genotype. J Hepatol 2013; 59:1153–1159. [DOI] [PubMed] [Google Scholar]
- 28.Sonneveld MJ, Hansen BE, Piratvisuth T, et al. Response - guided peginterferon therapy in hepatitis B e antigen - positive chronic hepatitis B using serum hepatitis B surface antigen levels. Hepatology 2013; 58:872–880. [DOI] [PubMed] [Google Scholar]


