Supplemental Digital Content is available in the text
Keywords: Cell salvage, Patient blood management, Transfusion
Abstract
Background:
Cell salvage is commonly used as part of a blood conservation strategy. However concerns among clinicians exist about the efficacy of transfusion of washed cell salvage.
Methods:
We performed a meta-analysis of randomized controlled trials in which patients, scheduled for all types of surgery, were randomized to washed cell salvage or to a control group with no cell salvage. Data were independently extracted, risk ratio (RR), and weighted mean differences (WMD) with 95% confidence intervals (CIs) were calculated. Data were pooled using a random effects model. The primary endpoint was the number of patients exposed to allogeneic red blood cell (RBC) transfusion.
Results:
Out of 1140 search results, a total of 47 trials were included. Overall, the use of washed cell salvage reduced the rate of exposure to allogeneic RBC transfusion by a relative 39% (RR = 0.61; 95% CI 0.57 to 0.65; P < 0.001), resulting in an average saving of 0.20 units of allogeneic RBC per patient (weighted mean differences [WMD] = −0.20; 95% CI −0.22 to −0.18; P < 0.001), reduced risk of infection by 28% (RR = 0.72; 95% CI 0.54 to 0.97; P = 0.03), reduced length of hospital stay by 2.31 days (WMD = −2.31; 95% CI −2.50 to −2.11; P < 0.001), but did not significantly affect risk of mortality (RR = 0.92; 95% CI 0.63 to 1.34; P = 0.66). No statistical difference could be observed in the number of patients exposed to re-operation, plasma, platelets, or rate of myocardial infarction and stroke.
Conclusions:
Washed cell salvage is efficacious in reducing the need for allogeneic RBC transfusion and risk of infection in surgery.
1. Introduction
Allogeneic blood products can be lifesaving in many conditions but limited in supply. However, inappropriate use of allogeneic red blood cell (RBC) transfusion may even be associated with increased risk of adverse outcome.[1–4] Patient blood management (PBM) is a proactive, patient-centered, and multidisciplinary approach to manage anemia, optimize hemostasis, minimize iatrogenic blood loss, and harness tolerance to anemia. Overall the concept aims to improve patient outcome by promoting and preserving body's own blood source. Cell salvage is used as part of a blood conservation strategy, and covers a wide range of techniques that scavenge blood from the operative field and wound drainages and re-infuse washed autologous RBCs back into the patient. With advances in washing and filtration technology, new cell salvage devices now provide a high quality blood product for re-infusion. The latest updated Cochrane meta-analysis was published in 2010 and included 75 trials with washed and unwashed cell salvage suggesting that cell salvage is efficacious in reducing the need for allogeneic RBC transfusion in adult elective cardiac,vascular and orthopedic surgery.[5]
Here, we examined the overall efficacy of washed cell salvage in different fields of surgery by performing an updated systematic review and meta-analysis of recent published randomized controlled trials.
2. Material and methods
2.1. Registration
The presented study has been registered at PROSPERO register (www.crd.york.ac.uk/PROSPERO, registration number: CRD42016035726).
2.2. Population and intervention
Potentially eligible trials had to be prospective and randomized including surgical patients. Patients in the intervention group received intra- and/or postoperatively washed cell salvage (=cell salvage group) whereas control patients received no cell salvage (=control group). There was neither a limit of age nor type of surgery.
2.3. Search methods for identification of studies
We undertook a systematic search of the literature to identify published reports, which investigated surgical patients receiving either cell salvage or no cell salvage. We searched Medline using the search terms “cell salvage” or “auto transfusion” or “autologous blood transfusion” or “blood salvage” or “cell saver” or “reinfusion system” and “blood transfusion” or “hemorrhage” or “anesthesia” or “transfusion” or “bleeding” or “blood loss” or “hemorrhage” and “randomized controlled trial” or “controlled clinical trial” or “randomized controlled trials” or “random allocation” or “double blind method” or “single blind method” and “humans” not including “stem cell”. A total of 954 Medline articles were identified and the abstracts were searched for reference to mortality and relevant topic. Additionally, we searched Cochrane Library using the same search criteria and found 181 articles. We also hand-searched grey literature and reference lists of identified articles and included 5 studies additionally.
Two independent authors screened the abstracts of identified studies (AW, PM). Discrepancies between the two reviewers were resolved through consensus by discussion with a third reviewer (SC). To be eligible for inclusion, studies had to include patients undergoing surgery randomized to cell salvage or to a control group that did not receive cell salvage. Study reports had to provide data either on the number of patients transfused with red cells or the volume of blood transfused. Transfusion data expressed in milliliters were converted to units by dividing by 300. Flow chart and exclusion criteria are displayed in Fig. 1.
Figure 1.
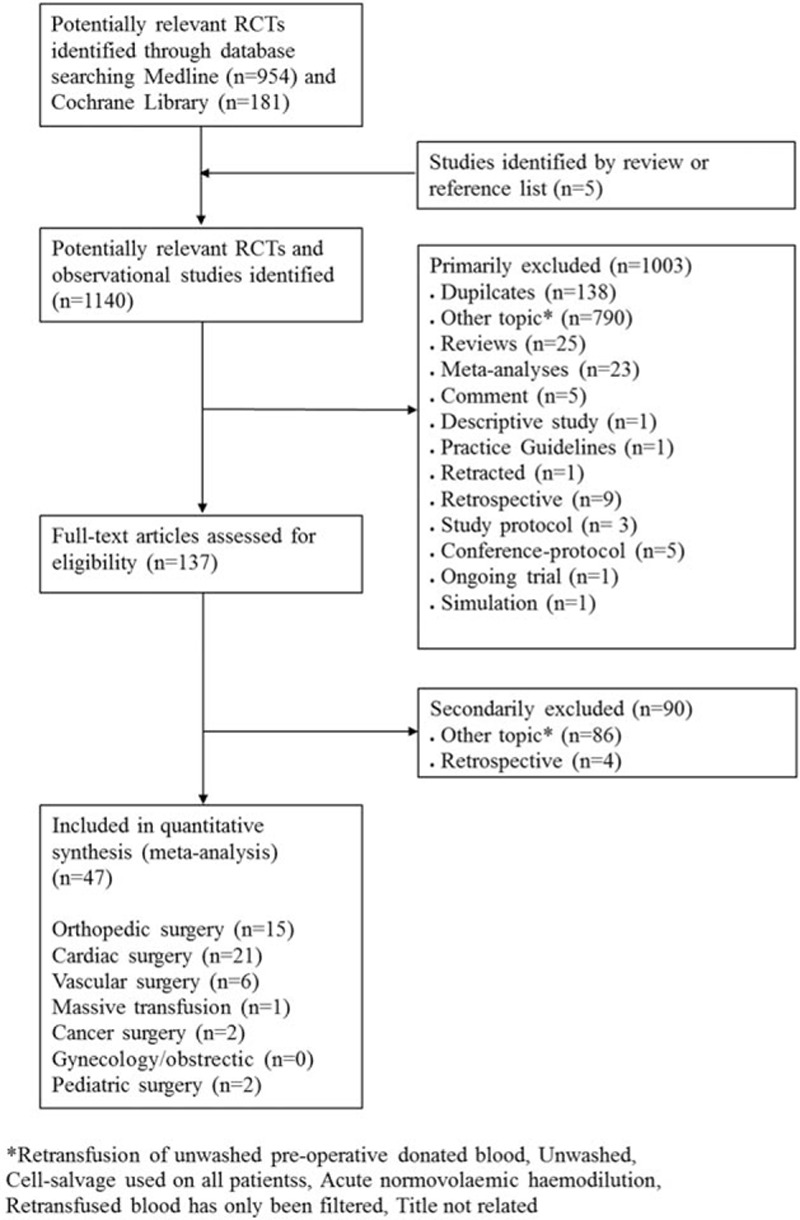
Flow chart of literature search in the systemic review. ∗Studies were excluded if unwashed preoperative donated blood, unwashed cell salvaged blood was retransfused, cell-salvage was used in all patients, acute normovolemic hemodilution was applied, or retransfused blood has only been filtered but not washed.
2.4. Risk of bias assessment
To determine the validity of the included trials, we assessed the risk of bias including the domains of random sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting. If one or more domains were judged as being high or unclear, we classified the trial as having a “high risk of bias.” More details are provided in the Supplemental Digital Content 1–3.
2.5. Endpoints
The primary endpoint of the systematic review was the number of patients exposed to allogeneic RBC transfusion. Secondary endpoints were number of units of allogeneic blood transfused, the number of patients exposed to re-operation for bleeding, number of exposed patients to plasma, number of exposed patients to platelets, infectious complications (pneumonia, wound infection, or/and sepsis), myocardial infarction, stroke, mortality, and length of hospital stay.
2.6. Ethical review
No personal data of patients were used in the present study. We only used published statistical data of meta-analysis and therefore ethical approval was not applicable in the current meta-analysis.
2.7. Statistical analysis
The meta-analysis was done in line with recommendations from the Preferred Reporting Items for Systemic reviews and Meta-Analyses (PRISMA statement).[6] In addition, we used the AMSTAR checklist in terms of the adequacy of conducting this review.[7] All analyses and graphical illustrations were conducted with Review Manager 5.3. for MAC (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012), and with the R (A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria) using package meta of Guido Schwarzer (version 1.6–1) for assessing publication bias. Risk ratio (RR) and 95% confidence intervals (95% CI) were calculated using the fixed effects model (DerSimonian and Laird estimator).[8] RRs were undefined and excluded for studies with no event in either arm.
Dichotomous and continuous data were pooled across trials using a random effects model. The presence of heterogeneity and comparisons of subgroups of trials was tested by Q-test, the χ2 statistic as well as the I2 statistic. To find a possible evidence for publication bias, funnel plots of the RR were generated. We considered P < 0.05 to be statistically significant. Subgroups were prospectively defined according to type of surgery (orthopedic, cardiac, vascular, multiple trauma/massive transfusion, cancer, gynecology/obstetric, and pediatric) and age (pediatrics/adults) to determine whether effect sizes varied according to the type of surgery.
3. Results
3.1. Study selection
Cross searching of electronic databases yielded a total of 1140 reports that met our inclusion criteria. After screening of all abstracts, 137 reports were considered for full-text review. After excluding of 90 additional reports, 47 studies[9–55] were considered for final quality analysis (Fig. 1, Table 1).
Table 1.
Studies included in meta-analysis.
3.2. All types of surgery
Of the 47 trials, 15 included orthopedic surgery,[12,17,18,24–27,30,38,40,42,44,48,54,55] 21 cardiac surgery,[9,14–16,20,22,23,28,29,32,33,35–37,41,43,46,47,50,51,53] 6 vascular surgery,[13,19,31,45,49,52] 1 multiple trauma surgery,[10] 2 cancer surgery,[21,39] and 2 pediatric surgery.[11,34]
Thirty-six trials[9,10,12–16,18–24,26–28,30–34,36,38,41–46,48,50,52–55] reported data on the number of subjects exposed to allogeneic RBC transfusion. These trials included a total of 3433 patients of whom 1783 were randomized to cell salvage. Overall, the use of cell salvage reduced the rate of exposure to allogeneic RBC transfusion by a relative 39% (RR = 0.61; 95% CI 0.57 to 0.65; P < 0.001). Heterogeneity between these trials was statistically significant (P < 0.001, I2 = 87%) (Fig. 2A). Thirty-five studies[9–11,13–25,27,28,30,32–37,39,40,42,46,50–55] including 3027 patients analyzed the number of allogeneic RBC units per patient. Comparison of 1625 patients using cell salvage with 1402 control patients resulted in an average saving of 0.20 units per patient (WMD = −0.20; 95% CI −0.22 to −0.18; P < 0.001) (Fig. 2B). Twenty eight percent less infection were observed in 908 patients with cell salvage compared with 990 control patients (RR = 0.72; 95% CI 0.54 to 0.97; P = 0.03)[10,11,13,16,19,20,22,27,28,31–33,38,42,43,45,48,49] (Fig. 2C). Mortality was assessed in 22 studies[9–11,13,15,16,19,20,22,31–33,35,36,41,45,46,48–52] with a follow-up of 24 hours,[51] hospital stay[9,11,16,31–33,35,36,41,49,52] and 3 years,[48,50] while 8 trials[10,13,15,19,20,22,45,46] did not report time for follow-up. No statistical difference could be observed in mortality (RR = 0.92; 95% CI 0.63 to 1.34; P = 0.66; Fig. 2D), the number of patients exposed to re-operation (RR = 1.13; 95% CI 0.70 to 1.81; P = 0.62), plasma (RR = 1.01; 95% CI 0.78 to 1.29; P = 0.97), platelets (RR = 0.82; 95% CI 0.62 to 1.09; P = 0.16), event rate of myocardial infarction (RR = 0.76; 95% CI 0.47 to 1.24; P = 0.27), and stroke (RR = 0.64; 95% CI 0.30 to 1.37; P = 0.25). However, length of hospital stay was reduced by 2.31 days in the cell salvage group compared with the control group (WMD = −2.31; 95% CI −2.50 to −2.11; P < 0.001) (Supplemental Digital Content 4A-F).
Figure 2.

Forest plot of cell salvage compared with control intervention in all types of surgery. (A) Number of patients exposed to allogeneic RBC, (B) number of units of allogeneic RBC per patients, (C) infections, (D) mortality rate. RBC = red blood cell.
3.3. Subgroups of surgery
3.3.1. Orthopedic surgery
We found 15 studies[12,17,18,24–27,30,38,40,42,44,48,54,55] with a total of 1207 patients undergoing orthopedic surgery. In 12 trials[12,18,24,26,27,30,38,42,44,48,54,55] including 1103 patients, (n = 529 cell salvage vs. n = 574 control group) the use of a cell salvage reduced the need of allogeneic RBC by a relative 57% (RR = 0.43; 95% CI 0.36 to 0.51; P ≤ 0.001; Fig. 3A). Ten studies[17,18,24,25,27,30,40,42,53,54] with 590 patients analyzed the number of allogeneic blood units per patient. Comparison of 293 patients in the cell salvage group and 297 patients in the control group resulted in an average saving of 0.80 units per patient (WDM = −0.80; 95% CI −0.89 to −0.70; P < 0.001) (Fig. 3B). No statistical difference was observed regarding infection (RR = 0.61; 95% CI 0.22 to 1.67; P = 0.33) or mortality rate (RR = 3.03; 95% CI 0.12 to 73.51; P = 0.5) (Fig. 3C, D).
Figure 3.
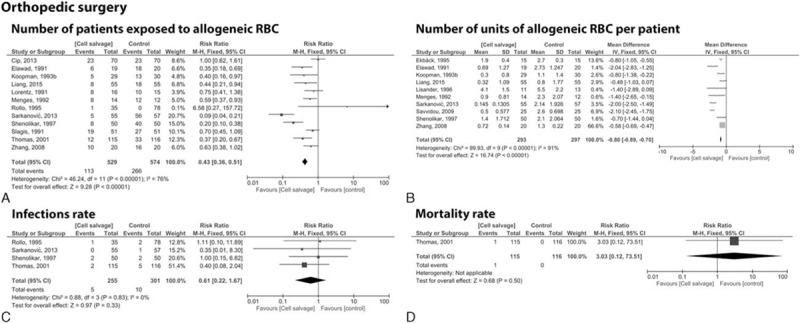
Forest plot of cell saver compared with no cell saver in orthopedic surgery. (A) Number of patients exposed to allogeneic RBC, (B) number of units of allogeneic RBC per patients, (C) infections, (D) mortality rate. RBC = red blood cell.
3.3.2. Cardiac surgery
We found 21 studies[9,14–16,20,22,23,28,29,32,33,35–37,41,43,46,47,50,51,53] with a total of 2249 patients undergoing cardiac surgery. Sixteen trials[14–16,20,22,23,28,32,33,36,41,43,46,50,53,54] including 1056 patients in the cell salvage group and 867 control patients showed an overall reduced exposure to allogeneic blood cells by a relative 29% (RR = 0.71; 95% CI 0.62 to 0.81; P < 0.001) (Fig. 4A). Analysis of 17 trials[9,14–16,20,22,23,28,32,35–37,41,46,50,51,53] involving 1109 patients assigned to the cell salvage group and 908 control patients revealed an average saving of 0.65 units per patient (RR = −0.65; 95% CI −0.69 to −0.62; P < 0.001) (Fig. 4B). The use of cell salvage did neither affect infection (RR = 0.94; 95% CI 0.61 to 1.46; P = 0.79) nor mortality rate (RR = 0.72; 95% CI 0.37 to 1.39; P = 0.32) (Fig. 4C, D).
Figure 4.
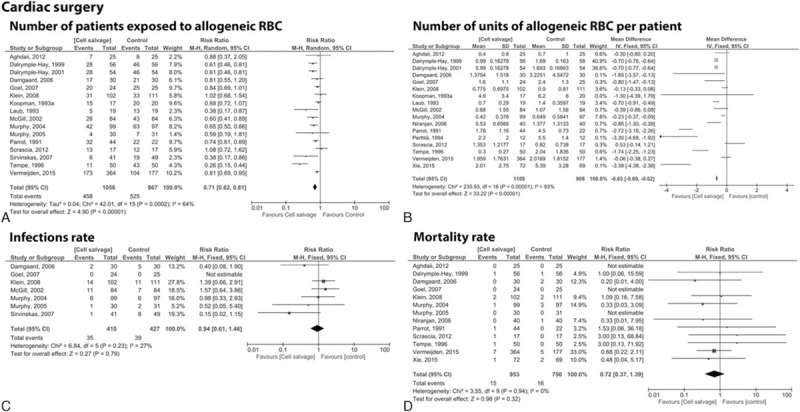
Forest plot of cell saver compared with no cell saver in cardiac surgery. (A) Number of patients exposed to allogeneic RBC, (B) number of units of allogeneic RBC per patient, (C) infections, (D) mortality rate. RBC = red blood cell.
3.3.3. Vascular surgery
We found 6 studies[13,19,31,45,49,52] with a total of 384 patients undergoing vascular surgery, of which 5 trials[13,19,31,45,52] with 316 patients provided data on the number of patients (n = 154 cell salvage vs. n = 162 control group). The exposure to allogeneic RBC transfusion was reduced by a relative 45% in the cell salvage group (RR = 0.55; 95% CI 0.44 to 0.68; P < 0.001; Fig. 5A). Three studies[13,19,52] including 186 patients (n = 91 cell salvage vs. n = 95 control group) provided data for the usage of RBC units that did not differ significantly (RR = 0.04; 95% CI −0.11 to 0.19; P = 0.59; Fig. 5B). Infection rate was significantly reduced in the cell salvage group (n = 169) compared with control group (n = 179) by relative 62% (RR = 0.38; 95% CI 0.22 to 0.67; P < 0.001; Fig. 5C). The use of a cell saver did not show statistically significant difference in mortality (RR = 0.79; 95% CI 0.20 to 3.13; P = 0.74; Fig. 5D).
Figure 5.
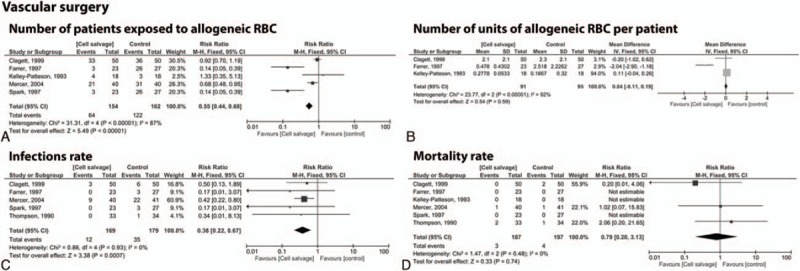
Forest plot of cell saver compared with no cell saver in vascular surgery. (A) Number of patients exposed to allogeneic RBC, (B) number of units of allogeneic RBC per patient, (C) infections, (D) mortality rate. RBC = red blood cell.
3.3.4. Multiple trauma/massive transfusion
We found only one trial[10] including 44 patients (n = 21 cell salvage vs. n = 23 control group) with multiple trauma. The use of cell salvage resulted in an overall saving of allogeneic RBC by a relative 99% (RR = 1.00; 95% CI 0.92 to 1.09; P = 1.00; Supplemental Digital Content 5A). In average 4.70 units (RR = −4.70; 95% CI −8.01 to −1.39; P = 0.005) of RBCs per patient could be saved in the cell salvage group (Supplemental Digital Content 5B). Infection and mortality rate did not vary between the two groups (infection: RR = 0.78; 95% CI 0.29 to 2.09; P = 0.62; mortality: RR = 1.02; 95% CI 0.67 to 1.56; P = 0.92; Supplemental Digital Content 5C, D).
3.3.5. Cancer surgery
We found 2 studies[21,39] that used cell salvage including 65 participants undergoing cancer surgery. One trial[21] addressed the number of patients exposed to allogeneic blood transfusion (n = 12 cell saver group vs. n = 12 control group), and revealed a reduced exposure by a relative of 25% (RR = 0.75; 95% CI 0.38 to 1.50; P = 0.42; Supplemental Digital Content 6A). In average 1.21 units of RBCs (RR = −1.21; 95% CI −2.27 to −0.16; P = 0.02) could be saved (Supplemental Digital Content 6B). None of the two studies reported infection or mortality for either the cell salvage or control group.
3.3.6. Pediatric surgery
Two studies[11,34] used cell salvage of which one study[34] assessed the number of patients (11 patients per group) exposed to allogeneic RBC transfusion that was reduced by a relative 78% (RR = 0.22; 95% CI 0.07 to 0.66; P = 0.007; Supplemental Digital Content 7A). Both studies examined the number of blood units applied per patient (n = 56 cell salvage vs. n = 56 control group). In average, a reduction of 0.87 RBC units per patient could be observed upon cell salvage usage (RR = −0.87; 95% CI −1.16 to −0.57; P < 0.001; Supplemental Digital Content 7B). One trial[11] reported the number of infections and mortality in which 106 patients were equally randomly distributed into the cell salvage or control group. The use of cell salvage did not reduce infection (RR = 1.13; 95% CI 0.47–2.69; P = 0.79) or mortality rate (RR = 3.00; 95% CI 0.32–27.93; P = 0.33; Supplemental Digital Content 7C, D).
3.3.7. Transfusion “triggers” / thresholds
Of the 47 included trials 22 studies[9,13–15,18,20–22,24,26–28,31,33,35,37,39,40,42,48,49,51] reported the use of a transfusion protocol for allogeneic RBC transfusion. Transfusion threshold did not vary between cell salvage and control group. Cell salvaged blood was retransfused at the end of surgery. The intra- and postoperative transfusion trigger for hemoglobin (Hb) ranged from 7 to 10 g/dL. Sixteen trials reported an intraoperative transfusion threshold, 2 trials[13,37] reported an Hb transfusion threshold of 10.0 g/dL, 4 trials[20,26,28,48] between 9.0 and 9.5 g/dL, 4 trials[21,27,31,51] between 8.0 and 8.9 g/dL, 6 trials[9,15,22,24,32,40] of 7.0 g/dL. Eighteen trials reported a postoperative transfusion threshold, 5 trials[13–15,26,49] reported a Hb transfusion threshold of 10.0 g/dL, 3 trials[28,42,48] between 9.0 and 9.5 g/dL, 7 trials[18,21,22,27,35,39,51] between 8.0 and 8.9 g/dL, 3 trials[24,33,40] of 7.0 g/dL, and 10 trials[9,13,16,19,20,28,33,36,40,49] transfused patients when the hematocrit value was less than 30%.
3.3.8. Quality management and cost analysis
All included studies did not provide sufficient comparable data to either estimate the quality of salved blood or to perform cost analysis (see Supplemental Digital Content 8–9).
4. Discussion
Patient blood management encompasses multiple blood-sparing techniques that minimize the risk for blood loss and the need for allogeneic RBC transfusion.[56] In this respect, cell salvage is an important tool of blood conservation but is inconsistently used in daily practice due to uncertainties about its efficiency.
The latest Cochrane meta-analysis of prospective trials was published in 2010 and included 75 studies focusing on the field of cardiac, vascular, and orthopedic surgery that used both washed and unwashed cell salvage reporting an overall reduction by a relative 38%.[5] Recent studies, however, demonstrated that the use of unwashed cell salvage negatively affect recovery after surgery. Unwashed salvaged blood may be enriched with inflammatory mediators,[57–59] fibrin degradation products[60,61] and interleukins[62,63] that exceed body's own circulating levels leading to enhanced coagulopathy, systemic inflammation, or acute respiratory distress syndrome. Additionally, some concerns emerged during the last years upon the observation that total drain output was shown to be significantly greater after unwashed salvaged blood retransfusion during total knee arthroplasty compared with patients receiving allogeneic transfusion only.[64–66] In this study, we performed an up-dated meta-analysis of randomized controlled trials focusing on washed salvage in which patients of all ages were randomized either to washed cell salvaged or to a control group scheduled for all types of surgery with a broad spectrum of relevant clinical endpoints. Our search resulted in 8 additional trials published beyond 2010 and 10 trials with a total of 1630 patients that were not included in the latest Cochrane Analysis. We showed that the overall use of washed cell salvaged blood reduces perioperative allogeneic RBC transfusion exposure by a relative 39% with the most significant result in orthopedic surgery where the use of cell salvage reduced the exposure by 57% in 15 trials. Thus, the overall consumption decreased by 0.80 RBC units per patient. A recent observational study including approximately 1.6 million patients revealed that transfusion of a single unit allogeneic RBC already double the risk for myocardial infarction and/or ischemic stroke.[4] Notable, Rohde et al performed a systematic review and meta-analysis including 7456 patients assigned either to a restrictive or liberal transfusion practice. A restrictive transfusion strategy with less RBC transfusion was associated with a reduced risk of serious infections.[67] In accordance, our meta-analysis demonstrated a reduced infection rate upon usage of cell salvage reduced allogeneic RBC transfusion rate.
In cancer surgery, however, the use of cell salvage is controversially debated and much reservation exists among surgeons. Retransfusion may increase the risk of cancer recurrence, while allogeneic RBC transfusion may also be associated with increased risk of cancer recurrence. However, a recent systematic review emphasizes that the use of a leucocyte depletion filter enables the elimination of tumor cells from blood–tumor cells mixture, whereas a standard filter did not. Overall the study showed that the use of cell salvage in patients having cancer surgery was not associated with a greater risk of tumor dissemination or metastasis. However, caution must be given when tumor rupture occurs during surgery as washed blood remained positive for tumor cells.[68] A statistically powered trial is essentially needed to finally elucidate whether cell salvaged blood or allogeneic RBC transfusion is superior in terms of cancer recurrence.
To our knowledge no results of prospective randomized controlled trials have been published in the last decade describing the use of cell salvage within multiple trauma surgery. The technique has not been widely accepted for trauma patients undergoing urgent surgery because of various reasons. Firstly, logistic issues exist for implementing an immediate auto-transfusion protocol. In addition, trauma induced injuries persist a high potential for infections and bacterial contamination. However, our analysis of one trial including multiple traumata surgeries confirmed the beneficial use of cell sever in that 4.70 units of blood could be saved per patient, thereby strongly supporting it's use within trauma patients. Cholette et al[11] showed impressively, that cell saver blood can safely be stored at the bedside for immediate transfusion for 24 hours after collection disclosing an additional opportunity to retransfuse autologous blood. It would be of interest to conduct a randomized controlled study that is statistically powered to confirm these results.
Till now, cell salvage is not widely used during cancer or trauma surgery, however current evidence justify further evaluation of its use in clinical studies.
Technical limitations generally restrict the use of cell salvage for infants during surgery. Yet advancements and increasing knowledge may allow volume-independent collection and retransfusion of salvaged blood.[69] Our search revealed two trials that support the use of cell salvage technique during pediatric surgery. Analysis of 106 infants undergoing cardiac surgery with cardiopulmonary bypass weighing less than or equal to 20 kg and 22 patients between 6 and 17 years undergoing acetabulosplasty revealed a 5-fold less exposure to allogeneic RBCs and 0.87 less RBC units per patient in the cell salvage group compared with the control group. Thus, our analysis supports and recommends the use of cell salvage during pediatric surgery.
Inappropriate allogeneic RBC transfusion may be associated with increased morbidity and mortality. From the urgent need to formulate common transfusion threshold the World Health Assembly has endorsed PBM, requesting the World Health Organization to provide its member states with training on the safe, rational use of allogenic RBC transfusion and transfusion alternatives (WHA63.12) in 2010.[70] In accordance with this release we observed a change in transfusion practice in the trials outlined in this study. Initially, we detected a broad transfusion range between 7 and 10 g/dL in studies published until 2005 that became more restricted in the last decade with a transfusion trigger ranging between 7 and 8 g/dL. In most studies, transfusion thresholds did not vary between the cell salvage and control group. Due to the different benefit-risk-ratio, we rather suggest that salvaged blood should be routinely retransfused irrespective of the Hb level. It is noteworthy, that in addition to a restrictive transfusion policy an improvement in patient outcome (reduced RBC transfusion rate, infection rate and length of hospital stay) could be observed upon utilization of cell salvage within the surgical setting. Our meta-analysis could not clarify whether the use of cell salvage is associated with increased or decreased costs. Three trials[10,16,22] observed no cost differences; one[48] reported an increased cost investment for the use of cell salvage usage and one[40] estimated reduced costs of 225 € per patient upon retransfusion of salvaged blood. Thus, estimation of cost is challenging as different parameters were defined for cost calculation.
With regard to the pooled analysis, some limitations must be taken into account. In our meta-analysis we found that most of the studies were of limited methodological quality and risk of bias could not be fully judged in any of the included trials. The majority of the included trials did not provide detailed information about procedure or blinding. Analyses of individual trials suggest that some effect sizes were overestimated, for example participants with long hospitalization were excluded from the analysis.[54] Investigation of publication bias by generating funnel plots showed no obvious deviations from symmetry excluding the possibility of potential publication bias. However, a tendency may exist to publish more likely trials in favor of the studied intervention and may challenge the significance of the meta-analysis presented in this study.[71] The times of re-infusion of autologous RBCs differed widely from the time point of surgery, to skin closure up to 6 hours post-surgery or were not described at all and might affect study outcome.
5. Conclusion
On the basis of this meta-analysis, washed cell salvage is efficacious in reducing the need for allogeneic RBC transfusion in surgery and even in decreasing the risk of infection. Thus, we recommend the use of cell salvage for surgery in which blood loss is a concern. However, the methodical qualities of the presented study are poor in quality, and further large randomized prospective trials are still needed.
Acknowledgments
The authors would like to thank Simone Lindau for technical support.
Supplementary Material
Footnotes
Abbreviations: CIs = confidence intervals, Hb = hemoglobin, PBM = patient blood management, RBC = red blood cell, RR = risk ratio, WMD = weighted mean differences.
PM and SC both first authors equally contributed to this work.
KZ and DRS are shared last author.
No pharmaceutical company has funded the presented study.
Disclosure of Conflict: PM and KZ received honoraria from the following companies for conducting a large clinical cohort trial in the field of Patient Blood Management: Vifor Pharma Deutschland GmbH, Muenchen, Germany; B. Braun Melsungen AG, Melsungen, Germany; CSL Behring, Marburg, Germany; and Fresenius Kabi, Bad Homburg, Germany. DRS: Dr Spahn's academic department is/has been receiving grant support from the Swiss National Science Foundation, Berne, Switzerland, the Ministry of Health (Gesundheitsdirektion) of the Canton of Zurich, Switzerland for Highly Specialized Medicine, the Swiss Society of Anesthesiology and Reanimation (SGAR), Berne, Switzerland, the Swiss Foundation for Anesthesia Research, Zurich, Switzerland, Bundesprogramm Chancengleichheit, Berne, Switzerland, CSL Behring, Berne, Switzerland, Vifor SA, Villars-sur-Glâne, Switzerland. DRS was the chairman of the ABC Faculty and is the co-chairman of the ABC-Trauma Faculty, which both are managed by Physicians World Europe GmbH, Mannheim, Germany and sponsored by unrestricted educational grants from Novo Nordisk Health Care AG, Zurich, Switzerland, CSL Behring GmbH, Marburg, Germany and LFB Biomédicaments, Courtaboeuf Cedex, France. In the past 5 years, DRS has received honoraria or travel support for consulting or lecturing from the following companies: Abbott AG, Baar, Switzerland, AMGEN GmbH, Munich, Germany, AstraZeneca AG, Zug, Switzerland, Baxter AG, Volketswil, Switzerland, Baxter S.p.A., Roma, Italy, Bayer (Schweiz) AG, Zürich, Switzerland, Bayer Pharma AG, Berlin, Germany, B. Braun Melsungen AG, Melsungen, Germany, Boehringer Ingelheim (Schweiz) GmbH, Basel, Switzerland, Bristol-Myers-Squibb, Rueil-Malmaison Cedex, France and Baar, Switzerland, CSL Behring GmbH, Hattersheim am Main, Germany and Berne, Switzerland, Curacyte AG, Munich, Germany, Daiichi Sankyo (Schweiz) AG, Thalwil, Switzerland, Ethicon Biosurgery, Sommerville, New Jersey, USA, Fresenius SE, Bad Homburg v.d.H., Germany, Galenica AG, Bern, Switzerland (including Vifor SA, Villars-sur-Glâne, Switzerland), GlaxoSmithKline GmbH & Co. KG, Hamburg, Germany, Janssen-Cilag AG, Baar, Switzerland, Janssen-Cilag EMEA, Beerse, Belgium, LFB Biomédicaments, Courtaboeuf Cedex, France, Merck Sharp & Dohme AG, Luzern, Switzerland, Novo Nordisk A/S, Bagsvärd, Denmark, Octapharma AG, Lachen, Switzerland, Organon AG, Pfäffikon/SZ, Switzerland, Oxygen Biotherapeutics, Costa Mesa, CA, PAION Deutschland GmbH, Aachen, Germany, Pharmacosmos A/S, Holbaek, Denmark, Photonics Healthcare B.V., Utrecht, Netherlands, ratiopharm Arzneimittel Vertriebs-GmbH, Vienna, Austria, Roche Diagnostics International Ltd, Reinach, Switzerland, Roche Pharma (Schweiz) AG, Reinach, Switzerland, Schering-Plough International, Inc., Kenilworth, New Jersey, USA, Tem International GmbH, Munich, Germany, Verum Diagnostica GmbH, Munich, Germany, Vifor Pharma Deutschland GmbH, Munich, Germany, Vifor Pharma Österreich GmbH, Vienna, Austria, Vifor (International) AG, St. Gallen, Switzerland. The remaining author has no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- 1.Glance LG, Dick AW, Mukamel DB, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology 2011; 114:283–292. [DOI] [PubMed] [Google Scholar]
- 2.Hopewell S, Omar O, Hyde C, et al. A systematic review of the effect of red blood cell transfusion on mortality: evidence from large-scale observational studies published between 2006 and 2010. BMJ open 2013; 3: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shander A, Fink A, Javidroozi M, et al. Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes. Transfus Med Rev 2011; 25:232.e53–246.e53. [DOI] [PubMed] [Google Scholar]
- 4.Whitlock EL, Kim H, Auerbach AD. Harms associated with single unit perioperative transfusion: retrospective population based analysis. BMJ 2015; 350:h3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carless PA, Henry DA, Moxey AJ, et al. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 2010; Cd001888. [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Int Med 2009; 151:264–269.w64. [DOI] [PubMed] [Google Scholar]
- 7.Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007; 7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 9.Aghdaii NKM, Yazdanian F, Ghaffarinejad MH. Effect of retransfusion of heparin remaining in the salvaged blood on postoperative blood loss in coronary artery bypass grafting: comparison with homologous blood transfusion. Iran Heart J 2012; 13:10. [Google Scholar]
- 10.Bowley DM, Barker P, Boffard KD. Intraoperative blood salvage in penetrating abdominal trauma: a randomised, controlled trial. World J Surg 2006; 30:1074–1080. [DOI] [PubMed] [Google Scholar]
- 11.Cholette JM, Powers KS, Alfieris GM, et al. Transfusion of cell saver salvaged blood in neonates and infants undergoing open heart surgery significantly reduces RBC and coagulant product transfusions and donor exposures: results of a prospective, randomized, clinical trial. Pediatr Crit Care Med 2013; 14:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cip J, Widemschek M, Benesch T, et al. Does single use of an autologous transfusion system in TKA reduce the need for allogenic blood? A prospective randomized trial. Clin Orthop Relat Res 2013; 471:1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clagett GP, Valentine RJ, Jackson MR, et al. A randomized trial of intraoperative autotransfusion during aortic surgery. J Vasc Surg 1999; 29:22–30.discussion -1. [DOI] [PubMed] [Google Scholar]
- 14.Dalrymple-Hay MJ, Dawkins S, Pack L, et al. Autotransfusion decreases blood usage following cardiac surgery—a prospective randomized trial. Cardiovasc Surg 2001; 9:184–187. [DOI] [PubMed] [Google Scholar]
- 15.Dalrymple-Hay MJ, Pack L, Deakin CD, et al. Autotransfusion of washed shed mediastinal fluid decreases the requirement for autologous blood transfusion following cardiac surgery: a prospective randomized trial. Eur J Cardiothorac Surg 1999; 15:830–834. [DOI] [PubMed] [Google Scholar]
- 16.Damgaard S, Steinbruchel DA. Autotransfusion with cell saver for off-pump coronary artery bypass surgery: a randomized trial. Scand Cardiovasc J 2006; 40:194–198. [DOI] [PubMed] [Google Scholar]
- 17.Ekbäck GSK, Axelsson K, Carlberg M. Perioperative autotransfusion and functional coagulation analysis in total hip replacement. Acta Anaesthesiol Scand 1995; 39:390–395. [DOI] [PubMed] [Google Scholar]
- 18.Elawad AA, Ohlin AK, Berntorp E, et al. Intraoperative autotransfusion in primary hip arthroplasty. A randomized comparison with homologous blood. Acta Orthop Scand 1991; 62:557–562. [DOI] [PubMed] [Google Scholar]
- 19.Farrer A, Spark JI, Scott DJ. Autologous blood transfusion: the benefits to the patient undergoing abdominal aortic aneurysm repair. J Vasc Nurs 1997; 15:111–115. [DOI] [PubMed] [Google Scholar]
- 20.Goel P, Pannu H, Mohan D, et al. Efficacy of cell saver in reducing homologous blood transfusions during OPCAB surgery: a prospective randomized trial. Transfus Med 2007; 17:285–289. [DOI] [PubMed] [Google Scholar]
- 21.Jacobi K, Walther A, Kuhn R, et al. Advantages and limitations of intraoperative mechanical autotransfusion in al prostatectomies. Anaesthesist 1997; 46:101–107. [DOI] [PubMed] [Google Scholar]
- 22.Klein AA, Nashef SA, Sharples L, et al. A randomized controlled trial of cell salvage in routine cardiac surgery. Anesth Analg 2008; 107:1487–1495. [DOI] [PubMed] [Google Scholar]
- 23.Laub GW, Dharan M, Riebman JB, et al. The impact of intraoperative autotransfusion on cardiac surgery. A prospective randomized double-blind study. Chest 1993; 104:686–689. [DOI] [PubMed] [Google Scholar]
- 24.Liang J, Shen J, Chua S, et al. Does intraoperative cell salvage system effectively decrease the need for allogeneic transfusions in scoliotic patients undergoing posterior spinal fusion? A prospective randomized study. Eur Spine J 2015; 24:270–275. [DOI] [PubMed] [Google Scholar]
- 25.Lisander BJR, Nordwall A. Combination of blood-saving methods decreases homologous blood requierments in scoliosis surgery. Anaesth Intens Care 1996; 24:555–558. [DOI] [PubMed] [Google Scholar]
- 26.Lorentz A, Osswald PM, Schilling M, et al. A comparison of autologous transfusion procedures in hip surgery. Anaesthesist 1991; 40:205–213. [PubMed] [Google Scholar]
- 27.Lukic-Sarkanovic M, Gvozdenovic L, Savic D, et al. Autologous blood transfusion in total knee replacement surgery. Vojnosanit Pregl 2013; 70:274–278. [DOI] [PubMed] [Google Scholar]
- 28.McGill N, O'Shaughnessy D, Pickering R, et al. Mechanical methods of reducing blood transfusion in cardiac surgery: randomised controlled trial. BMJ 2002; 324:1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McShane AJ, Power C, Jackson JF, et al. Autotransfusion: quality of blood prepared with a red cell processing device. Br J Anaesth 1987; 59:1035–1039. [DOI] [PubMed] [Google Scholar]
- 30.Menges T, Rupp D, van Lessen A, et al. Measures for reducing the use of homologous blood. Effects on blood coagulation during total endoprosthesis. Anaesthesist 1992; 41:27–33. [PubMed] [Google Scholar]
- 31.Mercer KG, Spark JI, Berridge DC, et al. Randomized clinical trial of intraoperative autotransfusion in surgery for abdominal aortic aneurysm. Br J Surg 2004; 91:1443–1448. [DOI] [PubMed] [Google Scholar]
- 32.Murphy GJ, Allen SM, Unsworth-White J, et al. Safety and efficacy of perioperative cell salvage and autotransfusion after coronary artery bypass grafting: a randomized trial. Ann Thorac Surg 2004; 77:1553–1559. [DOI] [PubMed] [Google Scholar]
- 33.Murphy GJ, Rogers CS, Lansdowne WB, et al. Safety, efficacy, and cost of intraoperative cell salvage and autotransfusion after off-pump coronary artery bypass surgery: a randomized trial. J Thorac Cardiovasc Surg 2005; 130:20–28. [DOI] [PubMed] [Google Scholar]
- 34.Nicolai P, Leggetter PP, Glithero PR, et al. Autologous transfusion in acetabuloplasty in children. J Bone Joint Surg 2004; 86:110–112. [PubMed] [Google Scholar]
- 35.Niranjan G, Asimakopoulos G, Karagounis A, et al. Effects of cell saver autologous blood transfusion on blood loss and homologous blood transfusion requirements in patients undergoing cardiac surgery on- versus off-cardiopulmonary bypass: a randomised trial. Eur J Cardiothorac Surg 2006; 30:271–277. [DOI] [PubMed] [Google Scholar]
- 36.Parrot D, Lancon JP, Merle JP, et al. Blood salvage in cardiac surgery. J Cardiothorac Vasc Anesth 1991; 5:454–456. [DOI] [PubMed] [Google Scholar]
- 37.Perttila JT, Salo MS, Jalonen JR, et al. Blood transfusion with autologous and leukocyte-depleted or standard allogeneic red blood cells and the immune response to open heart surgery. Anesth Analg 1994; 79:654–660. [PubMed] [Google Scholar]
- 38.Rollo VJ, Hozack WJ, Rothman RH, et al. Prospective randomized evaluation of blood salvage techniques for primary total hip arthroplasty. J Arthroplasty 1995; 10:532–539. [DOI] [PubMed] [Google Scholar]
- 39.Sankarankutty AK, Teixeira AC, Souza FF, et al. Impact of blood salvage during liver transplantation on reduction in transfusion requirements. Acta Cir Bras 2006; 21 suppl 1:44–47. [DOI] [PubMed] [Google Scholar]
- 40.Savvidou C, Chatziioannou SN, Pilichou A, et al. Efficacy and cost-effectiveness of cell saving blood autotransfusion in adult lumbar fusion. Transfus Med 2009; 19:202–206. [DOI] [PubMed] [Google Scholar]
- 41.Scrascia G, Rotunno C, Nanna D, et al. Pump blood processing, salvage and re-transfusion improves hemoglobin levels after coronary artery bypass grafting, but affects coagulative and fibrinolytic systems. Perfusion 2012; 27:270–277. [DOI] [PubMed] [Google Scholar]
- 42.Shenolikar A, Wareham K, Newington D, et al. Cell salvage auto transfusion in total knee replacement surgery. Transfus Med 1997; 7:277–280. [DOI] [PubMed] [Google Scholar]
- 43.Sirvinskas E, Veikutiene A, Benetis R, et al. Influence of early re-infusion of autologous shed mediastinal blood on clinical outcome after cardiac surgery. Perfusion 2007; 22:345–352. [DOI] [PubMed] [Google Scholar]
- 44.Slagis SV, Benjamin JB, Volz RG, et al. Postoperative blood salvage in total hip and knee arthroplasty. A randomised controlled trial. J Bone Joint Surg 1991; 73:591–594. [DOI] [PubMed] [Google Scholar]
- 45.Spark JI, Chetter IC, Kester RC, et al. Allogeneic versus autologous blood during abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg 1997; 14:482–486. [DOI] [PubMed] [Google Scholar]
- 46.Tempe D, Bajwa R, Cooper A, et al. Blood conservation in small adults undergoing valve surgery. J Cardiothorac Vasc Anesth 1996; 10:502–506. [DOI] [PubMed] [Google Scholar]
- 47.Tempe DK, Banerjee A, Virmani S, et al. Comparison of the effects of a cell saver and low-dose aprotinin on blood loss and homologous blood usage in patients undergoing valve surgery. J Cardiothorac Vasc Anesth 2001; 15:326–330. [DOI] [PubMed] [Google Scholar]
- 48.Thomas D, Wareham K, Cohen D, et al. Autologous blood transfusion in total knee replacement surgery. Br J Anaesth 2001; 86:669–673. [DOI] [PubMed] [Google Scholar]
- 49.Thompson JF, Webster JH, Chant AD. Prospective randomised evaluation of a new cell saving device in elective aortic reconstruction. Eur J Vasc Surg 1990; 4:507–512. [DOI] [PubMed] [Google Scholar]
- 50.Vermeijden WJ, van Klarenbosch J, Gu YJ, et al. Effects of cell-saving devices and filters on transfusion in cardiac surgery: a multicenter randomized study. Ann Thorac Surg 2015; 99:26–32. [DOI] [PubMed] [Google Scholar]
- 51.Xie Y, Shen S, Zhang J, et al. The efficacy, safety and cost-effectiveness of intra-operative cell salvage in high-bleeding-risk cardiac surgery with cardiopulmonary bypass: a prospective randomized and controlled trial. Int J Med Sci 2015; 12:322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelley-Patteson C, Ammar AD, Kelley H. Should the cell saver autotransfusion device be used routinely in all infrarenal abdominal aortic bypass operations? J Vasc Surg 1993; 18:261–265. [PubMed] [Google Scholar]
- 53.Koopman G. Peri-operative Autotransfusion by Means of a Blood Cell Separator. Processed Autotransfusion and Homologous Red Cell Requirement in Elective Cardiac and Orthopaedic Surgery: a Randomised Prospective Study. 1993; Den Haag: Cip-Data Koninklijke Bibliotheek, 1993a(chapter 5): 96–112. [Google Scholar]
- 54.Koopman G. Peri-operative Autotransfusion by Means of a Blood Cell Separator. Processed Autotransfusion and Homologous Red Cell Requirement in Elective Cardiac and Orthopaedic Surgery: A Randomised Prospective Study. 1993; DenHaag: Cip-Data Koninklijke Bibliotheek, 1993b; chapter 5: 96–112. [Google Scholar]
- 55.Zhang XF, Dong JM, Gong ML, et al. Effectiveness of preoperative autologous plateletpheresis combined with intraoperative autotransfusion on the blood coagulation in orthopaedic patients. Zhonghua wai ke za zhi 2008; 46:118–121. [PubMed] [Google Scholar]
- 56.Spahn DR, Goodnough LT. Alternatives to blood transfusion. Lancet 2013; 381:1855–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andersson I, Tylman M, Bengtson JP, et al. Complement split products and pro-inflammatory cytokines in salvaged blood after hip and knee arthroplasty. Can J Anaesth 2001; 48:251–255. [DOI] [PubMed] [Google Scholar]
- 58.Bengtsson A, Avall A, Hyllner M, et al. Formation of complement split products and proinflammatory cytokines by reinfusion of shed autologous blood. Toxicol Lett 1998; 100–101:129–133. [DOI] [PubMed] [Google Scholar]
- 59.Sinardi D, Marino A, Chillemi S, et al. Composition of the blood sampled from surgical drainage after joint arthroplasty: quality of return. Transfusion 2005; 45:202–207. [DOI] [PubMed] [Google Scholar]
- 60.Krohn CD, Reikeras O, Bjornsen S, et al. Fibrinolytic activity and postoperative salvaged untreated blood for autologous transfusion in major orthopaedic surgery. Eur J Surg 2001; 167:168–172. [DOI] [PubMed] [Google Scholar]
- 61.Duchow J, Ames M, Hess T, et al. Activation of plasma coagulation by retransfusion of unwashed drainage blood after hip joint arthroplasty: a prospective study. J Arthroplasty 2001; 16:844–849. [DOI] [PubMed] [Google Scholar]
- 62.Tylman M, Bengtson JP, Avall A, et al. Release of interleukin-10 by reinfusion of salvaged blood after knee arthroplasty. Intensive Care Med 2001; 27:1379–1384. [DOI] [PubMed] [Google Scholar]
- 63.Handel M, Winkler J, Hornlein RF, et al. Increased interleukin-6 in collected drainage blood after total knee arthroplasty: an association with febrile reactions during retransfusion. Acta Orthop Scand 2001; 72:270–272. [DOI] [PubMed] [Google Scholar]
- 64.Matsuda K, Nozawa M, Katsube S, et al. Reinfusion of unwashed salvaged blood after total knee arthroplasty in patients with rheumatoid arthritis. Int Orthop 2009; 33:1615–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsuda K, Nozawa M, Katsube S, et al. Activation of fibrinolysis by reinfusion of unwashed salvaged blood after total knee arthroplasty. Transfus Apher Sci 2010; 42:33–37. [DOI] [PubMed] [Google Scholar]
- 66.Singh VK, Singh PK, Javed S, et al. Autologous transfusion of drain contents in elective primary knee arthroplasty: its value and relevance. Blood Trans 2011; 9:281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rohde JM, Dimcheff DE, Blumberg N, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA 2014; 311:1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar N, Chen Y, Zaw AS, et al. Use of intraoperative cell-salvage for autologous blood transfusions in metastatic spine tumour surgery: a systematic review. Lancet Oncol 2014; 15:e33–e41. [DOI] [PubMed] [Google Scholar]
- 69.Booke M, Hagemann O, Van Aken H, et al. Intraoperative autotransfusion in small children: an in vitro investigation to study its feasibility. Anesth Analg 1999; 88:763–765. [DOI] [PubMed] [Google Scholar]
- 70.Organization WH. Global Forum for Blood Safety: Patient Blood Management. 2010 [Google Scholar]
- 71.Dickersin K, Chan S, Chalmers TC, et al. Publication bias and clinical trials. Controll Clin Trials 1987; 8:343–353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


