Abstract
Background:
Previous randomized controlled trials investigating exercise training programs in facioscapulohumeral muscular dystrophy (FSHD) patients are scarce and of short duration only. This study assessed the safety and efficacy of a 6-month home-based exercise training program on fitness, muscle, and motor function in FSHD patients.
Methods:
Sixteen FSHD patients were randomly assigned to training (TG) and control (CG) groups (both n = 8) in a home-based exercise intervention. Training consisted of cycling 3 times weekly for 35 minutes (combination of strength, high-intensity interval, and low-intensity aerobic) at home for 24 weeks. Patients in CG also performed an identical training program (CTG) after 24 weeks. The primary outcome was change in peak oxygen uptake (VO2 peak) measured every 6 weeks. The principal secondary outcomes were maximal quadriceps strength (MVC) and local quadriceps endurance every 12 weeks. Other outcome measures included maximal aerobic power (MAP) and experienced fatigue every 6 weeks, 6-minute walking distance every 12 weeks, and muscle characteristics from vastus lateralis biopsies taken pre- and postintervention.
Results:
The compliance rate was 91% in TG. Significant improvements with training were observed in the VO2 peak (+19%, P = 0.002) and MAP by week 6 and further to week 24. Muscle endurance, MVC, and 6-minute walking distance increased and experienced fatigue decreased. Muscle fiber cross-sectional area and citrate synthase activity increased by 34% (P = 0.008) and 46% (P = 0.003), respectively. Dystrophic pathophysiologic patterns were not exacerbated. Similar improvements were experienced by TG and CTG.
Conclusions:
A combined strength and interval cycling exercise-training program compatible with patients’ daily professional and social activities leads to significant functional benefits without compromising muscle tissue.
Keywords: combined strength and endurance training, exercise training, facioscapulohumeral muscular dystrophy, home-based, myopathy, randomized controlled trial
1. Introduction
Facioscapulohumeral muscular dystrophy (FSHD) is an incurable[1] progressive autosomal-dominant neuromuscular disease initially affecting face, shoulder girdle, and upper arm muscles. The muscle weakness that develops is typically asymmetric, progressively worsening with increasing muscle wasting,[2] associated with fatigue, reduced endurance, and increased ventilatory needs during normal daily activities.[3] Reports indicate peak oxygen uptake (VO2 peak) is lower in patients with neuromuscular diseases compared to nondiseased sedentary controls.[4] Muscle weakness and fatigue, mobility impairment, and reduced physical activity contribute to progressive general health deterioration including physical, mental, and social dimensions.[5]
There is a great potential role for exercise in neuromuscular disease populations, especially dystrophic myopathies,[6,7] for the prevention of excessive fatigue and to limit muscle atrophy and cardiorespiratory and metabolic deconditioning. Only 3 aerobic-training[6,8,9] and 2 strength-training[10,11] studies have been performed on FSHD patients. Aerobic training increased aerobic capacity and quality of life.[8,9] Conversely, maximal strength gains via resistance training remain equivocal. Combined aerobic and strength training, however, may be more effective than aerobic training alone[5,7] yet no study has investigated combined aerobic and strength training in FSHD patients. There is also a lack of long-term studies on FSHD and exercise, specifically long-term exercise effects on muscle and patient autonomy and whether regular exercise can realistically be integrated into patients’ daily lives.
Therefore, a randomized controlled trial was conducted to investigate the effects of a 24-week adapted home-based exercise training program designed to be compatible with FSHD patients’ daily professional, social, and familial activities. We hypothesized that the program, combining high-intensity, low-intensity aerobic and strength exercises, would improve aerobic capacity, muscle characteristics (i.e., histological, chemical) and function (i.e., strength, fatigue, endurance), and quality of life.
2. Methods
2.1. Study design
A 24-week multicenter randomized controlled trial with outcome assessments at 0 (T0), 6 (T6), 12 (T12), 18 (T18), and 24 (T24) weeks for training (TG) and control (CG) groups was performed (Fig. 1). A second 24-week nonrandomized trial from T24 to 48 (T48) weeks after the start of the randomized controlled trial was then performed by CG patients wishing to perform the same supervised home-based training program (CTG).
Figure 1.
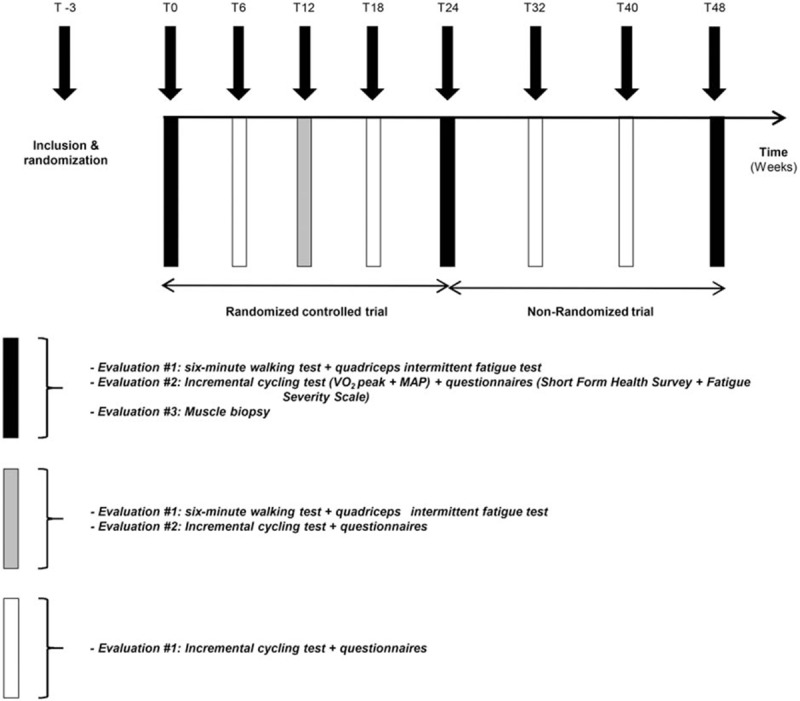
Global view of the experimental protocol. MAP = maximal aerobic power; T = time (in weeks from baseline measures, T0); VO2 peak = peak oxygen uptake.
2.2. Participants
Nineteen FSHD patients were enrolled (Fig. 2) in this multicenter study conducted at Centre Hospitalier Universitaire de Saint-Etienne and Centre Hospitalier Universitaire de Grenoble, France. Eligible participants were identified through the Rhône-Alpes Referent Center for Rare Neuromuscular Diseases, France. Inclusion criteria were (i) molecular FSHD Type 1 diagnosis, (ii) age ≥ 18 years, and (iii) the ability to perform the cycling program in this study. Exclusion criteria were a history of cardiovascular disease including cardiac arrhythmias and clinical cardiovascular anomaly, evidence of inflammatory syndrome or diabetes, abnormal coagulation, or body mass index ≥ 35 kg m−2. All procedures were performed in accordance with the Declaration of Helsinki and approved by the local ethics committee (Comité de Protection des Personnes Sud-Est 1, France). All participants gave written informed consent. This trial is registered with ClinicalTrials.gov, number NCT01116570.
Figure 2.
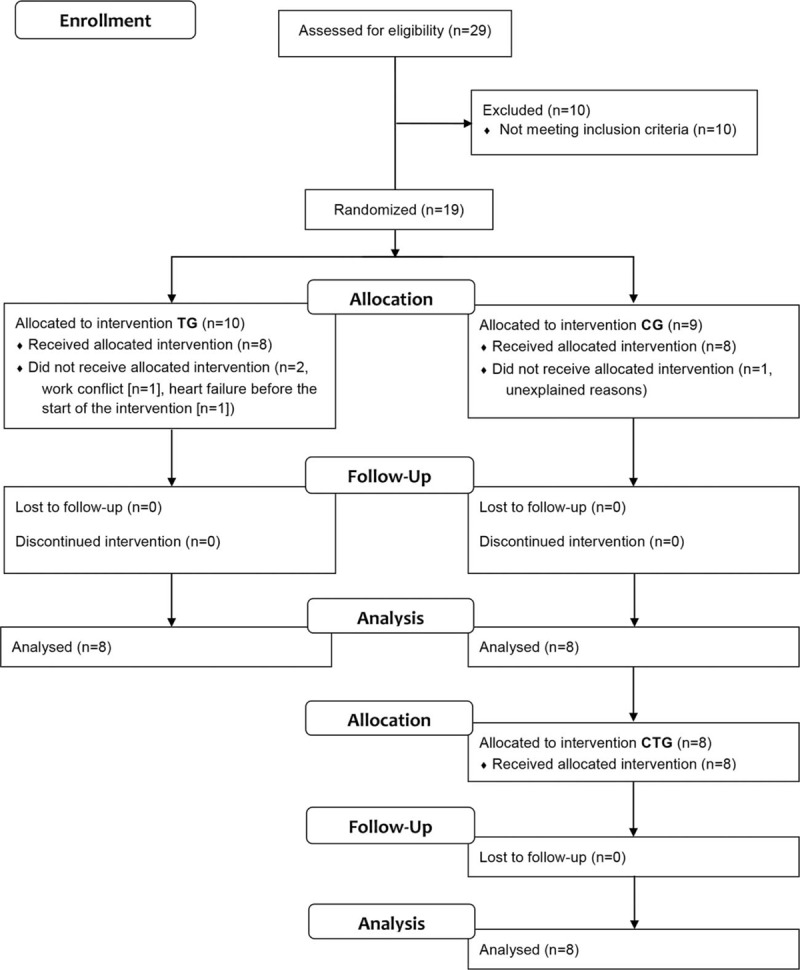
Study flow diagram. CG = control group; CTG = control-training group; TG = training group.
None of the patients had previously participated in a study including a physical exercise program. Patients were randomly allocated to TG (6 men, 4 women) or CG (8 men, 1 woman). The study administrative coordinator performed a computer-generated pairwise randomization by enrolment order, ensuring similar numbers of patients allocated to each group. Patients were informed of group allocation group when a sealed envelope was opened in their presence. Due to the nature of the training study, investigators involved in training and testing patients were unable to be blinded. All histological analyses were conducted by individuals blinded to training status.
2.3. Procedures
At least 3 weeks before the start of the experimental period, patients underwent baseline testing (T0) comprising 3 visits separated by ≥ 72 hours at the closest partner laboratory (Saint-Etienne or Grenoble). Visit 1 consisted of quadriceps neuromuscular function (i.e., strength, fatigability, endurance) assessment (QIF-test)[12,13] and 6-minute walking test. During visit 2, patients performed an incremental cycling test to task failure and completed questionnaires about perceived fatigue and quality of life. The same investigator performed muscle biopsies at both centers during visit 3 using the Weil–Blakesley percutaneous conchotome technique.[14] Creatine kinase concentrations were measured before training at baseline and 24 hours after each functional evaluation session (see Fig. 1). In the nonrandomized trial, CG values at T24 were considered baseline for CTG. The randomized controlled trial interassessment period was 6 weeks and increased to 8 weeks in the nonrandomized trial to facilitate compliance over the additional 24 weeks.
Aside from the training program, patients in all groups were instructed not to change normal daily activities or habits. Training consisted of three 35-minute cycling sessions per week at home performed entirely on a stationary ergocycle (Fig. 3). Two combined sessions consisted of aerobic exercise at constant moderate intensity (60% of maximal aerobic power, MAP) followed by sets of near-maximal revolutions. Session 3 consisted of interval training (see Fig. 3). The first 5 to 10 training sessions were supervised by an exercise physiologist experienced in training clinical populations at the patients’ homes. Every week, the exercise physiologist provided telephone support for 2 sessions and attended the third session to supervise it, assess heart rate recorded from each training session, verify training log completion, check equipment, and adjust the individualized exercise intensity based on either observed heart rate reduction with training or the new MAP from incremental cycling assessments.
Figure 3.
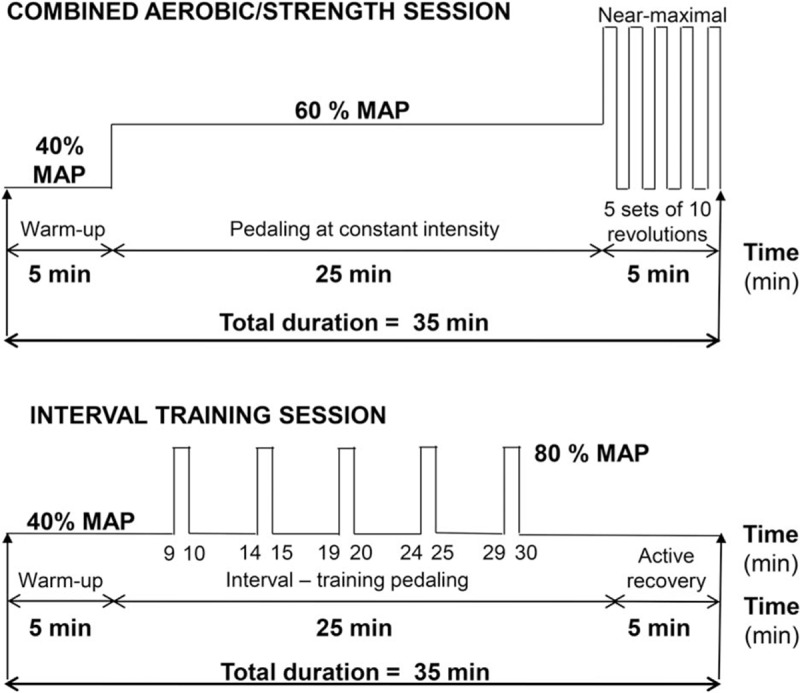
Training sessions. Content of (A) the combined aerobic/strength and (B) interval training sessions. MAP = maximal aerobic power.
2.4. Outcomes
Peak oxygen uptake was the primary outcome measure of the study. Both VO2 peak and MAP were assessed using an incremental cycling test performed on a stationary bicycle (Monark Ergomedic, 839E, Vansbro, Sweden) with breath-by-breath gas analysis and electrocardiogram (Ergocard, Medisoft, Sorinnes, Belgium). Power output was increased every 2 minutes (10–30 W increment) until task failure. VO2 peak was considered as the oxygen consumption during the last 30 s before task failure and MAP as the power output of the last completed stage plus 25% of per stage increment for each additional 30 s completed. Before and 3 minutes after exercise termination, a 20-μL capillary blood sample was collected for lactate concentration determination.[15]
Quadriceps neuromuscular function was assessed by the QIF test as previously developed and validated in FSHD patients by our group (typical error coefficient of variation of voluntary activation and evoked force responses < 8 and 10%, respectively; intraclass correlation coefficient > 0.85).[12] Patients were seated in a custom chair with hips at 130° and knees at 90° and noncompliant straps securing the hips and chest. Force was measured via force transducer (SBB 200 kg Tempo Technologies, Taipei, Taiwan) connected by noncompliant strap to the ankle, sampled at 2000 Hz and analyzed using Labchart 8 software (ADInstruments, Bella Vista, Australia). Torque was calculated as force measured by the force transducer multiplied by the length of the lever arm. The test consisted of stages of 10 intermittent submaximal isometric contractions (5-s on/5-s off) with neuromuscular evaluations before the QIF-test, immediately after each stage and immediately after task failure. The neuromuscular evaluation was composed of isometric maximal voluntary contractions (MVCs) with femoral nerve magnetic stimuli delivered during MVCs and at rest. Magnetic stimulation (FNMS) was delivered via 2 Magstim 200 stimulators (Magstim, Whitland, UK) connected by Bistim module and a 45-mm figure-eight coil. Submaximal target torques started at 10% MVC for the first stage and increased by 10% MVC per stage until task failure. Target torque levels were identical for a given patient throughout the intervention (i.e., as a percentage of T0 MVC). Peak MVC and evoked torques in relaxed muscle and maximal voluntary activation were also calculated. In addition to pre-post changes for each test, data from the 10% to 40% MVC stages were analyzed because they were completed by all patients for all tests.
Participant adherence is reported as the percentage of scheduled training sessions completed.
Muscle damage 24 hours after neuromuscular and physiological assessments (incremental cycling, fatigue, and strength tests) was assessed from serum creatine kinase concentrations. All patients had serum creatine kinase analysis performed at their same local laboratory.
The 6-minute walking test to assess motor function consisted of walking as far as possible in 6 minutes and was conducted as previously described.[16]
Quality of life and perceived fatigue were respectively evaluated by 2 questionnaires validated in French: the Short Form Health Survey (SF-36) and Fatigue Severity Scale. Subjective ratings of daily levels of soreness and tiredness were also evaluated. Scoring of these questionnaires has been previously described.[17–19]
Before and 2 to 7 days after the last training session, muscle biopsies were obtained from the vastus lateralis in TG and CG. The post-training biopsy was taken 2 cm vertical and lateral to the pre-training biopsy site. The procedure used for removing[14] and conserving muscle samples has been extensively used by our research group.[20] Fiber type composition and muscle fiber cross-sectional area were analyzed as previously described.[21,22] Hemalum eosin safran staining enabled evaluation of common muscle remodeling signs such as necrotic fibers, fibrosis, and internalized nuclei.[22] Enzyme activities of creatine kinase, lactate dehydrogenase, and citrate synthase were determined fluorimetrically from muscle biopsies.[23]
2.5. Statistical analysis
Power assessment for the primary outcome (VO2 peak) was based on results from Olsen et al.[8] To detect a minimum of 20% difference from T0 to T24 between groups (CG and TG), assuming α of 5% and power of 90%, 12 patients were required per group.
Statistical analyses were performed with Statistica (version 8; StatSoft, Tulsa). Assumptions of normal data distribution and variance homogeneity were verified by Shapiro–Wilk and Levene's tests, respectively. The assumption of sphericity was assessed with Mauchly's test, and in the case of significant violations, the Greenhouse–Geisser correction was applied. Two-way (Group × Time) repeated-measures ANOVAs were used to analyze changes between TG and CG at each time point. Post-hoc analyses were performed by the Bonferroni multiple comparison procedure. Pearson's correlation coefficient was used to determine relationships between selected variables. For CTG, 1-way repeated-measures ANOVAs were used to assess the intervention effect. All analyses were performed with 8 patients per group. Two-sided statistical significance was set at P < 0.05.
3. Results
Between October 2010 and August 2012, 19 FSHD patients were enrolled in the study (Fig. 2). Ten patients were allocated to TG (6 men) and 9 patients to CG (8 men). Two patients dropped out in the TG group before starting the training program, one because of heart failure and the other for personal reasons. Another patient, included in CG, withdrew for unknown reasons before starting the training program. In total 16 patients completed the study, 8 in TG (5 men) and 8 in CG (7 men) (Table 1). Primary outcome analysis from 16 patients (8 per group) at the scheduled study end date showed larger than expected improvements in TG compared to CG; thus, the study was concluded despite a smaller than calculated sample size.
Table 1.
Baseline characteristics of participants.
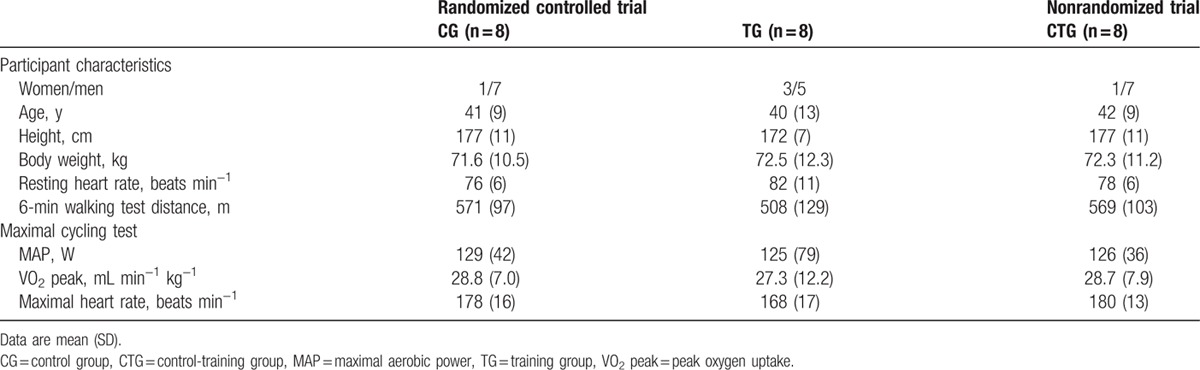
Participant characteristics were similar for CG and TG (all P > 0.05, Table 1). Patients in TG and CTG completed 91 [mean (SD), 66 (7)] and 83% [60 (10)] of 72 prescribed training sessions, respectively. No training complications were reported.
3.1. Oxygen uptake and aerobic power
Peak oxygen uptake and MAP increased from baseline in TG by T6 (mean difference in change, 20%, P = 0.002 and 27%, P = 0.010, respectively, Table 2, Fig. 4A and B) and continued through T24. Similarly, CTG VO2 peak and MAP were significantly greater at T48 than T24 (by 17%, P = 0.001 and 30%, P = 0.001, respectively, Fig. 4A and B). No changes in either VO2 peak or MAP were observed for CG over the 24 weeks. Maximal lactatemia did not change for any group (data not shown).
Table 2.
Changes in the main cardio-respiratory, quadriceps function, and fatigue and functional parameters during the randomized controlled trial.
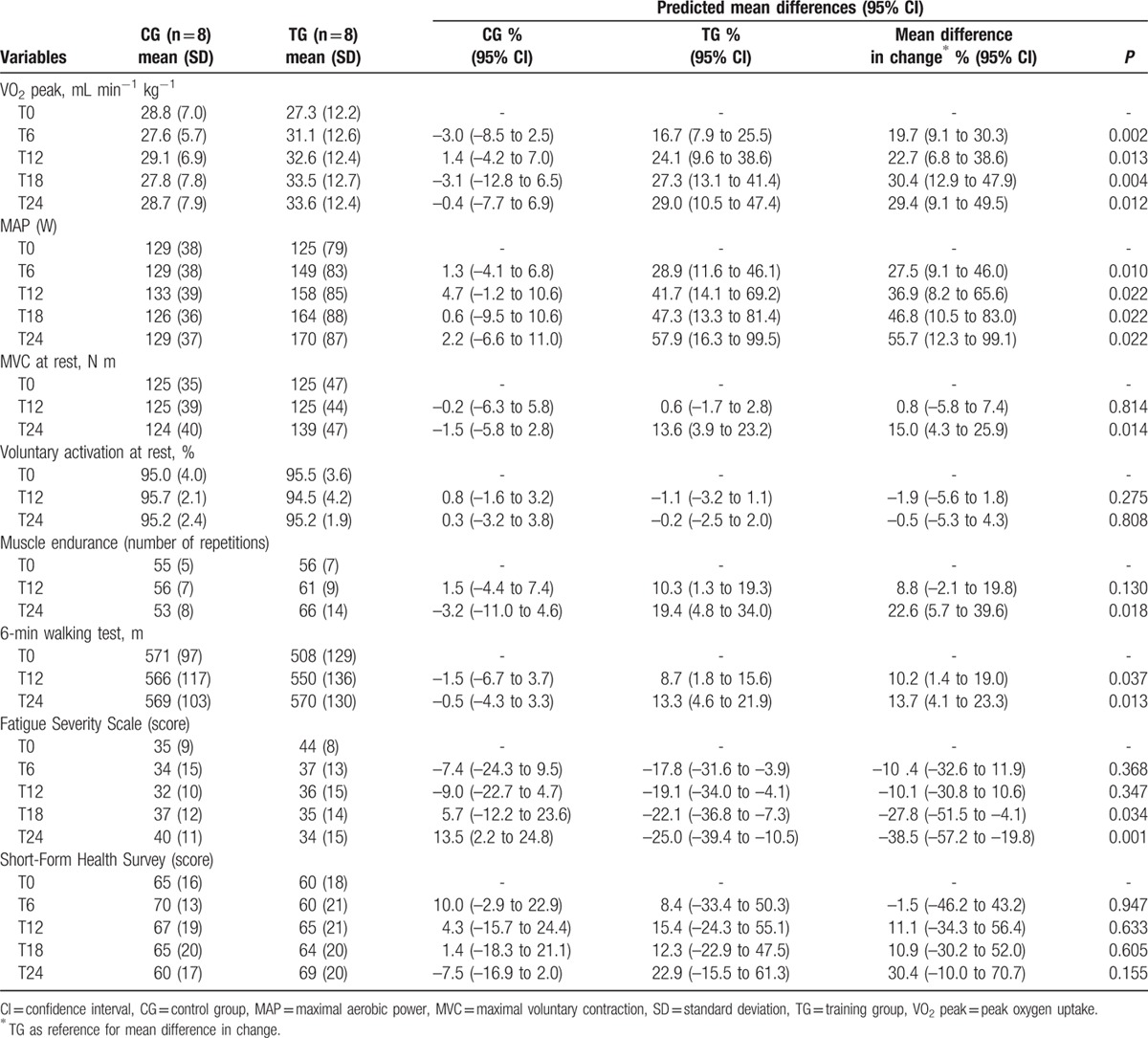
Figure 4.
Training effects on cycling parameters. Mean changes in (A) peak oxygen uptake, (B) maximal aerobic power in the training group versus control group in the randomized controlled trial and in the control-training group in the nonrandomized trial. ∗P < 0.05, ∗∗∗P < 0.001, for within-group differences in the randomized controlled trial compared to T0 (2-way repeated measures ANOVA); ††P < 0.01, †††P < 0.001, for differences compared to T24 in the nonrandomized trial (1-way repeated measures ANOVA). CG = control group; CTG = control-training group; MAP = maximal aerobic power; T = time (in weeks from baseline measures, T0); TG = training group; VO2 peak = peak oxygen uptake.
3.2. Quadriceps intermittent fatigue test
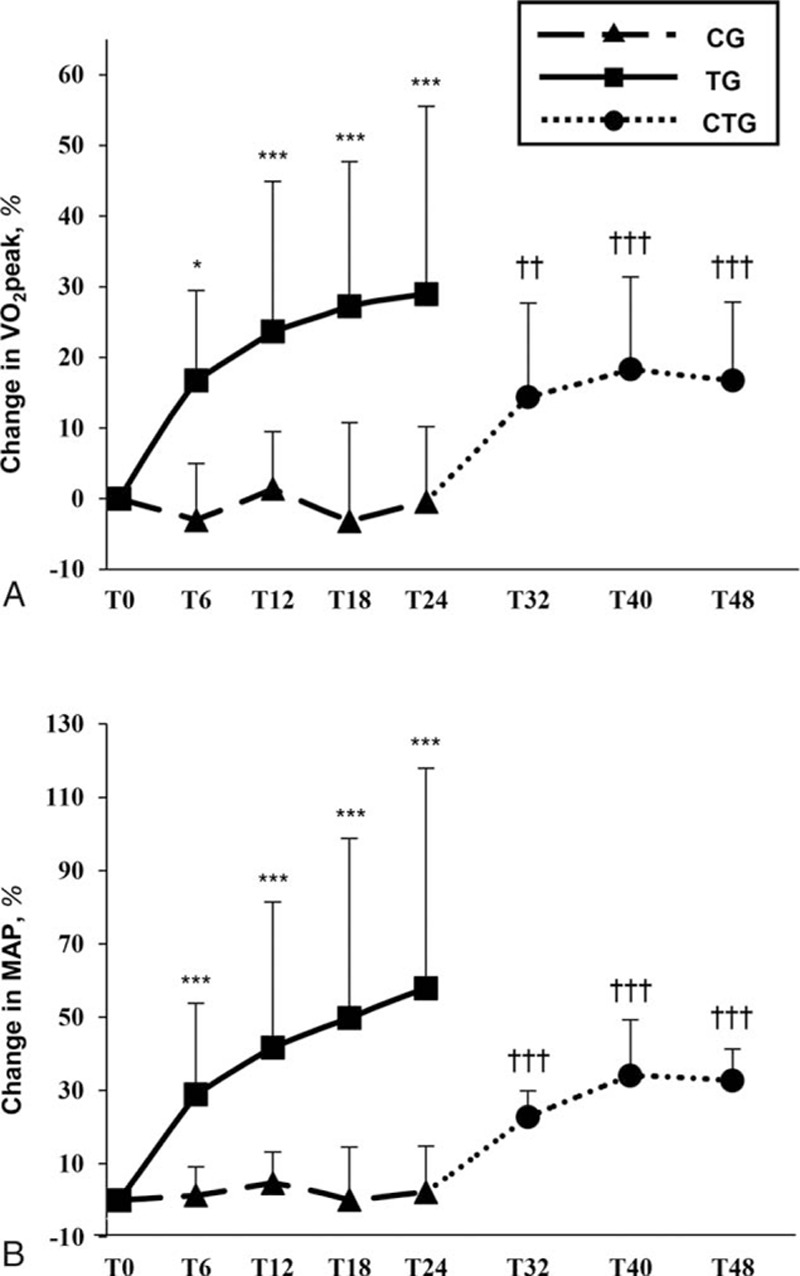
At T24, MVC at rest and muscle endurance were significantly greater versus T0 in TG (mean difference in change, 15%, P = 0.014 and 23%, P = 0.018, respectively, Table 2, Fig. 5A and B). In CTG, MVC was unchanged after 24 weeks of training although muscle endurance increased by 11% (P = 0.007, Fig. 5A and B). No changes in either MVC or muscle endurance were observed for CG during the 24-week invention. Changes in both MVC and MAP in TG were correlated with changes in muscle endurance (r = 0.74, P < 0.001 and r = 0.60, P = 0.001, respectively) and also between changes in MVC and changes in VO2 peak (r = 0.38, P = 0.048). No training effect was found for voluntary activation (Table 2) or evoked torque responses during the QIF-test in any group (data not shown).
Figure 5.
Training effects on neuromuscular, motor, and subjective parameters. Mean changes in (A) isometric maximal voluntary strength, (B) number of submaximal repetitions (muscle endurance), (C) 6-min walking test distance, and (D) experienced fatigue (Fatigue Severity Scale score) in the training group versus control group in the randomized controlled trial and in the control-training group in the nonrandomized trial. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 for within-group differences in the randomized controlled trial compared to T0 (2-way repeated measures ANOVA); †P < 0.05, ††P < 0.01, for differences compared to T24 in the nonrandomized trial (1-way repeated measures ANOVA). CG = control group; CTG = control-training group; MVC = maximal voluntary contraction; T = time (in weeks from baseline measures, T0); TG = training group.
3.3. Six-minute walking test
Over 24 weeks, 6-minute walking test distance increased in TG (mean difference in change at T24, 14%, P = 0.013, Table 2, Fig. 5C). Similar increases were seen in CTG at T48 (13%, P = 0.007) compared to T24 (Fig. 5C). No changes in 6-minute walking test distance were observed for CG over the 24 weeks. In TG, significant correlations were found between changes in 6-minute walking test distance and VO2 peak (r = 0.62, P = 0.001), MAP (r = 0.72, P < 0.001), and MVC (r = 0.68, P < 0.001).
3.4. Quality of life
Quality of life scores from the Short Form Health Survey (SF-36) did not change significantly in either group during the randomized controlled trial (Table 2) or in CTG (data not shown). Fatigue determined by Fatigue Severity Scale questionnaire (Table 2) decreased significantly in TG (mean difference in change T24, –38%, P = 0.001, Fig. 5D) and CTG by 22% (P = 0.001, Fig. 5D) at T48. An inverse relationship was found between changes in Fatigue Severity Scale and changes in Short Form Health Survey mental health component (r = –0.44, P = 0.023). Changes in Fatigue Severity Scale were also correlated with changes in the VO2 peak (r = –0.42, P = 0.001), MAP (r = –0.47, P = 0.012), muscle endurance (r = –0.58, P = 0.002), MVC (r = –0.56, P = 0.002), and 6-minute walking test distance (r = –0.55, P = 0.003). Subjective daily ratings of soreness and tiredness were unchanged across groups (data not shown).
3.5. Creatine kinase
Serum creatine kinase concentrations 24 h after maximal cycling and QIF tests were <1000 IU·L−1 at all time points for all groups. In 1 patient in TG with baseline values >1000 IU·L−1, post-exercise values were between 1000 and 2000 IU·L−1 at all time points.
3.6. Muscle biopsy parameters
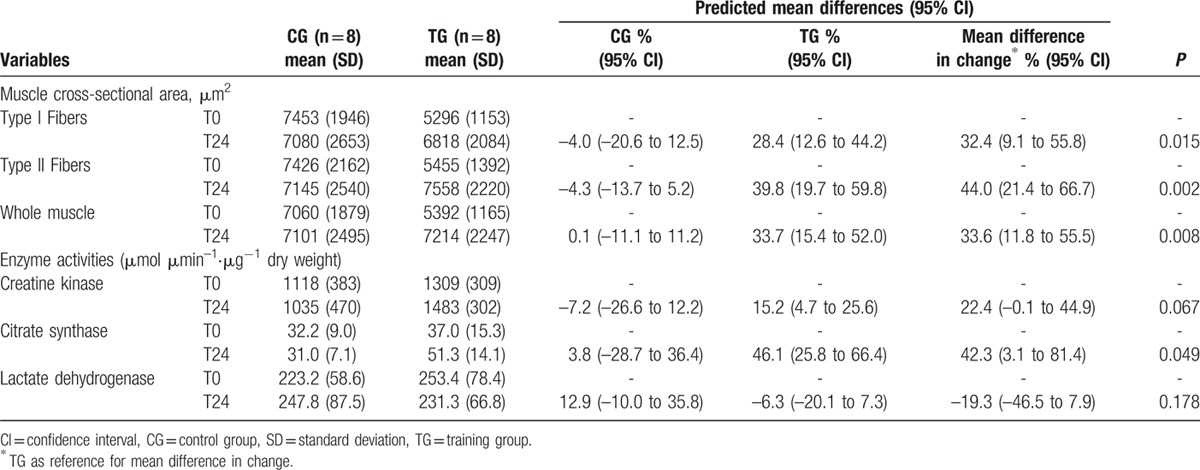
Muscle biopsy parameter data are presented in Table 3. Muscle fiber cross-sectional area increased in TG relative to unchanged cross-sectional area in CG (all P < 0.05). Citrate synthase enzymatic muscular activities also increased from T0 to T24 in TG (P = 0.005) whereas the increase in creatine kinase from T0 to T24 in TG approached significance (P = 0.055). No change was observed on muscle fiber type composition (data not shown) or lactate dehydrogenase enzymatic activity in either CG or TG.
Table 3.
Changes in muscle cross-sectional area and enzyme activities in the randomized controlled trial.
4. Discussion
This study is the first clinical randomized controlled trial evaluating the safety and efficacy of combined aerobic, high-intensity interval and strength training on motor function, muscle histological and biochemical characteristics and quality of life over 24 weeks in FSHD patients. The main findings are that combined home-based strength and endurance training (i) increases VO2 peak, MAP, and citrate synthase enzyme activity, (ii) increases muscle strength and endurance and muscle fiber cross-sectional area, (iii) improves motor function (walking speed), and (iv) reduces experienced fatigue. The training intervention did not alter general quality of life or muscle integrity (serum creatine kinase concentrations and muscle morphology). Together, these findings confirm our hypothesis that combined training is a well-tolerated, safe, and effective long-term method to induce functional gains without muscle damage in FSHD patients.
The present training program was more than twice the length of most previous FSHD training studies. Despite the longer duration, training adherence (>80%) was similar to shorter FSHD[8,9] and neuromuscular disease[24,25] studies. This contrasts a recent 16-week training intervention[6] where 39% of FSHD patients had unacceptable adherence, with travel time to the study site stated as 1 reason for lower adherence levels. In the present study, all sessions were closely supervised in person or by telephone. As training was performed at home, time constraints imposed by traditional rehabilitation programs at clinical centers were avoided. This result supports a therapeutic approach for FSHD patients based on closely supervised home-based training to promote patient participation.
A major concern was whether FSHD patients could perform long-term exercise training safely and without muscle damage. In TG, subjective ratings of perceived soreness and fatigue and serum creatine kinase concentrations remained unchanged during the intervention, consistent with observations that creatine kinase returned to baseline by 24 h following high-intensity cycling.[26] Serum creatine kinase results in the present study suggest an absence of muscle structure changes indicating that sarcolemmal integrity was not degraded with training. Thus, long-term exercise training appears to be a safe therapeutic method without muscle damage or adverse effects.
As expected, the VO2 peak and MAP increased in both training groups (Fig. 4A and B). Improvements in the VO2 peak were accompanied by greater muscle citrate synthase (+46%) and a near-significant increase in creatine kinase (+15%) activity in TG. Creatine kinase plays a key role in the regulation of aerobic metabolism[27] and citrate synthase and creatine kinase work together to meet the ATP requirements of contracting muscles. These results suggest improved oxidative phosphorylation with training. Interestingly, VO2 peak and MAP improvements at T12 were greater than in most dystrophy studies and 12-week FSHD aerobic training interventions.[8,9] Another FSHD randomized controlled trial reported no aerobic power improvement after 16 weeks of aerobic training.[6] One potential explanation for the comparatively large increase in aerobic capacity in the present study is initial patient fitness level; however, training type was likely crucial. High-intensity interval training induces greater improvements in aerobic power compared to continuous aerobic training.[28] In 3 previous FSHD studies,[6,8,9] continuous aerobic training was used whereas our study combined continuous aerobic exercise with interval training. It can be hypothesized that high-intensity aerobic intervals are more effective in FSHD patients. Moreover, we observed improvements in VO2 peak and MAP at T6 and further increases to 24 weeks. MAP increases after T12 underscore the potential for long-term continuous aerobic power improvements in FSHD. Thus, our study advances previous reports of short-term aerobic capacity improvements by demonstrating aerobic power can be improved beyond 3 months with combined aerobic and interval training. This has important practical consequences in designing training programs for FSHD patients.
Unlike previous FSHD studies,[10,11] combined training improved maximal quadriceps strength (+14% at T24). Although some neuromuscular disease studies have shown strength gains after resistance training, it was unclear whether neural and/or muscle adaptation (e.g., hypertrophy) mechanisms underpinned this improvement. High maximal voluntary activation was observed in both TG and CG at baseline (∼95%), confirming recent findings.[12] It was thus unsurprising that training effects were not observed, suggesting strength gains were due to structural changes. This was confirmed by muscle biopsy analysis showing a 34% increase in muscle fiber cross-sectional area at T24. Muscle hypertrophy has been reported to play a major role in strength enhancement.[29]
Recently, lower quadriceps endurance was reported in FSHD patients compared to healthy controls.[12] Significant improvements in both TG and CTG were found for muscle endurance and motor function. As absolute QIF-test target torques were maintained throughout the 24-week intervention, muscle endurance improvement might be explained by concomitant improvements in aerobic capacity and quadriceps muscle strength. This is supported by the significant positive correlations between muscle endurance and both MAP and MVC. A positive relationship between quadriceps endurance and strength in FSHD patients has previously been demonstrated with both parameters positively correlated with functional parameters.[30] Interestingly, another study reported mitochondrial disruption and oxidative stress are important causes of muscle weakness and functional muscle impairment in FSHD.[30] Thus, any intervention limiting mitochondrial impairment might be useful for maintaining muscle function. In the present study, increased VO2 peak suggests possible activation of mitochondrial biogenesis since citrate synthase increased with training.
Beneficial changes were also observed for motor function (6-minute walking test) in both TG and CTG and these changes were positively correlated with changes in VO2 peak, MVC, and muscle endurance, suggesting increases in both VO2peak and strength contribute to improved motor function. Subjective fatigue is a critical symptom for FSHD patients as it is directly associated with lower functional capacity and quality of life.[31] In agreement with another recent randomized controlled trial,[6] the present study observed decreased subjective fatigue with training that was correlated with increases in VO2 peak, MAP, MVC, muscle endurance, and 6-minute walking test distance and improvements in mental health (Short Form Health Survey). Meanwhile, quality of life scores (Short Form Health Survey) were unchanged, indicating that despite the progressive and incurable nature of FSHD and the load of the study, quality of life during the 24-week training intervention was maintained. This suggests the beneficial effects of a long-term training program on experienced fatigue impact motor function and quality of life in FSHD patients.
The main limit of this trial is patient number. However, analysis of the primary endpoint (VO2 peak) from 16 patients showed larger than expected improvements in TG compared to CG, suggesting that the results are reliable and provide valid conclusions while encouraging further study.
This study demonstrates clinically relevant benefits of a long-term training program combining (low-intensity with high-intensity intervals) aerobic and strength training in FSHD patients. These benefits occurred without inducing muscle damage or adverse effects and in spite of the progressive nature of FSHD. Clinicians are thus encouraged to promote an active lifestyle based on regular home-based exercise compatible with patients’ daily professional and social activities as a therapeutic approach. Based on the time-course of adaptations, long-term exercise programs should be prescribed in order to optimize the training effects.
Acknowledgments
The authors sincerely acknowledge all FSHD patients for their participation. They gratefully acknowledge technical support from Régis Bonnefoy, Marie Pierre Blanc, and Dominique Gouttefangeas and clinical research assistants Arnaud Garcin and Karine Ferraud for completing the documents necessary for clinical trial authorization. They also sincerely acknowledge Drs Emmeline Lagrange, Célia Boutte, Christophe Vial, Philippe Petiot, Alain Furby, and Pascal Edouard for performing medical inclusions.
Footnotes
Abbreviations: ANOVA = analysis of variance, CG = control group, CTG = control-training group, FSHD = facioscapulohumeral muscular dystrophy, MAP = maximal aerobic power, MVC = maximal voluntary contraction, T = time (in weeks from baseline measures, T0), TG = training group, VO2 peak = peak oxygen uptake.
Authorship: LCB, GYM, BW, SV, FK, and LF contributed to the conception and design of the study. LCB, GYM, JT, DB, MR, SV, FK, EP, and LF contributed to data collection, analysis, and interpretation. LCB, GYM, JT, FK, and LF drafted the manuscript. All authors critically revised the manuscript for important intellectual content.
Funding: This study was supported by the Association Française contre la Myopathie (AFM). The funding source had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Landry-Cyrille Bankolé was supported by a doctoral research grant from Örebro University and by the Association Française contre la Myopathie (AFM). John Temesi was supported by a doctoral research grant from the Rhône-Alpes Region.
The authors have no conflicts of interest to disclose.
References
- 1.Tawil R, van der Maarel S, Padberg GW, et al. 171st ENMC international workshop: standards of care and management of facioscapulohumeral muscular dystrophy. Neuromuscul Disord 2010; 20:471–475. [DOI] [PubMed] [Google Scholar]
- 2.Tawil R. Facioscapulohumeral muscular dystrophy. Neurotherapeutics 2008; 5:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCrory MA, Kim HR, Wright NC, et al. Energy expenditure, physical activity, and body composition of ambulatory adults with hereditary neuromuscular disease. Am J Clin Nutr 1998; 67:1162–1169. [DOI] [PubMed] [Google Scholar]
- 4.Wright NC, Kilmer DD, McCrory MA, et al. Aerobic walking in slowly progressive neuromuscular disease: effect of a 12-week program. Arch Phys Med Rehabil 1996; 77:64–69. [DOI] [PubMed] [Google Scholar]
- 5.Cup EH, Pieterse AJ, Ten Broek-Pastoor JM, et al. Exercise therapy and other types of physical therapy for patients with neuromuscular diseases: a systematic review. Arch Phys Med Rehabil 2007; 88:1452–1464. [DOI] [PubMed] [Google Scholar]
- 6.Voet N, Bleijenberg G, Hendriks J, et al. Both aerobic exercise and cognitive-behavioral therapy reduce chronic fatigue in FSHD: an RCT. Neurology 2014; 83:1914–1922. [DOI] [PubMed] [Google Scholar]
- 7.Voet NB, van der Kooi EL, Riphagen II, et al. Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev 2013; 7:CD003907. [DOI] [PubMed] [Google Scholar]
- 8.Olsen DB, Orngreen MC, Vissing J. Aerobic training improves exercise performance in facioscapulohumeral muscular dystrophy. Neurology 2005; 64:1064–1066. [DOI] [PubMed] [Google Scholar]
- 9.Andersen G, Prahm KP, Dahlqvist JR, et al. Aerobic training and postexercise protein in facioscapulohumeral muscular dystrophy: RCT study. Neurology 2015; 85:396–403. [DOI] [PubMed] [Google Scholar]
- 10.Colson SS, Benchortane M, Tanant V, et al. Neuromuscular electrical stimulation training: a safe and effective treatment for facioscapulohumeral muscular dystrophy patients. Arch Phys Med Rehabil 2010; 91:697–702. [DOI] [PubMed] [Google Scholar]
- 11.van der Kooi EL, Vogels OJ, van Asseldonk RJ, et al. Strength training and albuterol in facioscapulohumeral muscular dystrophy. Neurology 2004; 63:702–708. [DOI] [PubMed] [Google Scholar]
- 12.Bachasson D, Temesi J, Bankole C, et al. Assessement of quadriceps strength, endurance and fatigue in FSHD and CMT: benefits and limits of femoral nerve magnetic stimulation. Clin Neurophysiol 2014; 125:396–405. [DOI] [PubMed] [Google Scholar]
- 13.Bachasson D, Millet GY, Decorte N, et al. Quadriceps function assessment using an incremental test and magnetic neurostimulation: a reliability study. J Electromyogr Kinesiol 2013; 23:649–658. [DOI] [PubMed] [Google Scholar]
- 14.Henriksson KG. Semi-open muscle biopsy technique. A simple outpatient procedure. Acta Neurol Scand 1979; 59:317–323. [PubMed] [Google Scholar]
- 15.Peltier SL, Vincent L, Millet GY, et al. Effects of carbohydrates-BCAAs-caffeine ingestion on performance and neuromuscular function during a 2-h treadmill run: a randomized, double-blind, cross-over placebo-controlled study. J Int Soc Sports Nutr 2011; 8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King S, Wessel J, Bhambhani Y, et al. Validity and reliability of the 6 minute walk in persons with fibromyalgia. J Rheumatol 1999; 26:2233–2237. [PubMed] [Google Scholar]
- 17.Aaronson NK, Acquadro C, Alonso J, et al. International Quality of Life Assessment (IQOLA) Project. Qual Life Res 1992; 1:349–351. [DOI] [PubMed] [Google Scholar]
- 18.El Mhandi L, Millet GY, Calmels P, et al. Benefits of interval-training on fatigue and functional capacities in Charcot–Marie–Tooth disease. Muscle Nerve 2008; 37:601–610. [DOI] [PubMed] [Google Scholar]
- 19.Krupp LB, LaRocca NG, Muir-Nash J, et al. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46:1121–1123. [DOI] [PubMed] [Google Scholar]
- 20.Feasson L, Stockholm D, Freyssenet D, et al. Molecular adaptations of neuromuscular disease-associated proteins in response to eccentric exercise in human skeletal muscle. J Physiol 2002; 543 (pt 1):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charifi N, Kadi F, Feasson L, et al. Enhancement of microvessel tortuosity in the vastus lateralis muscle of old men in response to endurance training. J Physiol 2004; 554 (pt 2):559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charifi N, Kadi F, Feasson L, et al. Effects of endurance training on satellite cell frequency in skeletal muscle of old men. Muscle Nerve 2003; 28:87–92. [DOI] [PubMed] [Google Scholar]
- 23.Vincent L, Oyono-Enguelle S, Feasson L, et al. Effects of regular physical activity on skeletal muscle structural, energetic, and microvascular properties in carriers of sickle cell trait. J Appl Physiol 2012; 113:549–556. [DOI] [PubMed] [Google Scholar]
- 24.Sveen ML, Jeppesen TD, Hauerslev S, et al. Endurance training: an effective and safe treatment for patients with LGMD2I. Neurology 2007; 68:59–61. [DOI] [PubMed] [Google Scholar]
- 25.Taivassalo T, De Stefano N, Argov Z, et al. Effects of aerobic training in patients with mitochondrial myopathies. Neurology 1998; 50:1055–1060. [DOI] [PubMed] [Google Scholar]
- 26.Andersen SP, Sveen ML, Hansen RS, et al. Creatine kinase response to high-intensity aerobic exercise in adult-onset muscular dystrophy. Muscle Nerve 2013; 48:897–901. [DOI] [PubMed] [Google Scholar]
- 27.Saks VA, Ventura-Clapier R, Aliev MK. Metabolic control and metabolic capacity: two aspects of creatine kinase functioning in the cells. Biochim Biophys Acta 1996; 1274:81–88. [DOI] [PubMed] [Google Scholar]
- 28.Helgerud J, Hoydal K, Wang E, et al. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc 2007; 39:665–671. [DOI] [PubMed] [Google Scholar]
- 29.Mackey AL, Esmarck B, Kadi F, et al. Enhanced satellite cell proliferation with resistance training in elderly men and women. Scand J Med Sci Sports 2007; 17:34–42. [DOI] [PubMed] [Google Scholar]
- 30.Turki A, Hayot M, Carnac G, et al. Functional muscle impairment in facioscapulohumeral muscular dystrophy is correlated with oxidative stress and mitochondrial dysfunction. Free Radic Biol Med 2012; 53:1068–1079. [DOI] [PubMed] [Google Scholar]
- 31.Kalkman JS, Zwarts MJ, Schillings ML, et al. Different types of fatigue in patients with facioscapulohumeral dystrophy, myotonic dystrophy and HMSN-I. Experienced fatigue and physiological fatigue. Neurol Sci 2008; 29 suppl 2:S238–240. [DOI] [PubMed] [Google Scholar]


