Abstract
18F-fluoro-2-deoxy-D-glucose (FDG) positron emission tomography (PET) scan is used to evaluate various kinds of tumors. While most studies on PET findings of the colon focus on the colonic uptake pattern, studies regarding background colonic uptake on PET scan are rare. The purpose of this study was to identify the association between the background colonic uptake and the presence of colorectal adenoma (CRA), which is a frequent precancerous lesion. We retrospectively reviewed the medical records of 241 patients with gynecologic malignancy who had received PET or PET/computed tomography (CT) scan and colonoscopy at the same period as a baseline evaluation. Background colonic 18F-FDG uptake was visually graded and the maximal standardized uptake values (SUVmax) of 7 different bowel segments were averaged. In univariate analysis, older age at diagnosis (≥ 50 years, p = 0.034), overweight (BMI ≥ 23 kg/m², p = 0.010), hypercholesterolemia (≥ 200 mg/dL, p = 0.027), and high grade background colonic uptake (p = 0.009) were positively associated with the prevalence of CRA. By multiple logistic regression, high grade background colonic uptake was independently predictive of CRA (odds ratio = 2.25, p = 0.021). The proportion of CRA patients significantly increased as background colonic uptake grade increased from 1 to 4 (trend p = 0.015). Out of the 138 patients who underwent PET/CT, the proportion of CRA patients in the group with high SUVmax (> 2.25) was significantly higher than in the low SUVmax group (27.5% vs. 11.6%, p = 0.031). In conclusion, high grade of background colonic 18F-FDG uptake is significantly associated with the prevalence of CRA.
Introduction
18F-fluoro-2-deoxy-D-glucose (FDG) positron emission tomography (PET) scan is a functional imaging modality using the characteristics of FDG, which is accumulated more in tissues with increased glycolysis than in normal tissues. This is conceptually different from conventional structural imaging methods [1].
18F-FDG-PET is used in diagnosing various kinds of tumor, assessing tumor stage, and evaluating the treatment response [1]. In real clinical practice, baseline staging examinations for most kinds of cancer usually do not include colonoscopic evaluation, and some patients with gastrointestinal symptoms or possibility of colonic lesion in the radiographic imaging tend to undergo an additional colonoscopy. In colon, many studies focus on the FDG uptake pattern [1,2]. FDG uptake is classified into three patterns: focal, segmental, and diffuse. It is reported that focal uptake pattern is frequently associated with neoplasm such as colorectal adenoma (CRA) or colorectal cancer (CRC), and the segmental uptake pattern is more likely to be found in colonic inflammation such as colitis or inflammatory bowel disease [3–6]. Diffuse uptake pattern is usually considered as physiologic uptake [3,5,6].
To our knowledge, there have been few studies regarding background colonic uptake on PET. Underlying pathophysiology, related medical conditions, and clinical significance remain unknown. Recently, some studies reported that factors such as intestinal smooth muscle uptake, stool uptake, mucosal uptake, and lymphoid tissue uptake may affect physiologic intestinal 18F-FDG uptake [3,7–9]. In addition, the hypothesis that luminal bacteria and dyslipidemia affect background intestinal 18F-FDG uptake has been raised recently [10,11].
Therefore, we aimed to identify the clinical significance of background colonic 18F-FDG uptake on PET scan in real practice and establish the necessity of recommendation for colonoscopic evaluation in patients with increased background colonic uptake on PET. Accordingly, we analyzed the association between background FDG uptake grade on PET and the prevalence of CRA, which is a frequent precancerous lesion in the colon.
Materials and Methods
Study design and subjects
Patients' medical records from January 2006 to February 2015 in Ewha Womans University Mokdong Hospital, Seoul, Korea, were retrospectively reviewed. To evaluate the findings of PET scan and colonoscopy performed at the same period, this study included patients with gynecologic malignancy, whom our institute routinely performs both examinations for the initial baseline study. Patients with ovarian malignancies were excluded, because ovarian cancer itself or its peritoneal seeding can be overlapped or confused with colonic uptake. Patients with a history of infectious or inflammatory bowel disease, colonic malignancy, or metastatic colon lesion were excluded. We also excluded patients with age under 30 years old, incomplete medical records of colonoscopic or histopathologic findings, insufficient colonoscopy procedure, or poor bowel preparation.
Collection of clinical data
For the medical record review, underlying diseases, age at diagnosis, gender, alcohol and smoking history, family history of colon cancer, height, and body weight were retrieved, and the laboratory findings within average of 6 days before or after 18F-FDG PET scan, including plasma glucose, serum triglyceride (TG), and total cholesterol, were also collected.
We calculated body mass index (BMI) as ‘body weight (kg) / height (m)2’ and a BMI value of 23 kg/m2 or greater was considered overweight in the Korean population. Glucose intolerance was defined as a fasting plasma glucose level of 100 mg/dL or higher, hypertriglyceridemia as a serum TG level of 150 mg/dL or higher, and hypercholesterolemia as a serum total cholesterol level of 200 mg/dL or higher.
18F-FDG PET/CT and image analysis
All patients were evaluated with 18F-FDG PET (103 patients) or PET/CT (138 patients). Before the 18F-FDG injection, patients fasted at least 6 hours and blood glucose level was confirmed to be < 140 mg/dL. The injected dose of 18F-FDG was 5.18 MBq/kg. After the 18F-FDG injection, patients were strictly instructed to rest for one hour. For 18F-FDG PET, a transmission scan for attenuation correction was obtained using the point source of 137Cs, and then followed by an emission scan, using an Allegro PET scanner (Philips-ADAC Medical Systems, Cleveland, OH). The emission scan was acquired for 3 minutes per bed position with 3D mode and reconstructed using a 3D OSEM iterative algorithm (4 iterations and 8 subsets).
For 18F-FDG PET/CT, a low-dose CT for attenuation correction was obtained first, using loss reduction software (CARE Dose, Siemens Medical Solutions, Erlangen, Germany) without any contrast agent, after which an emission PET scan was performed from the skull base to the thigh, using a dedicated PET/CT (Biograph mCT, Siemens Medical Solutions, Erlangen, Germany). The emission scan was acquired for 2 minutes per bed position with 3D mode and reconstructed using a 3D OSEM iterative algorithm (2 iterations and 21 subsets) with time of flight (TOF) and point spread function (PSF).
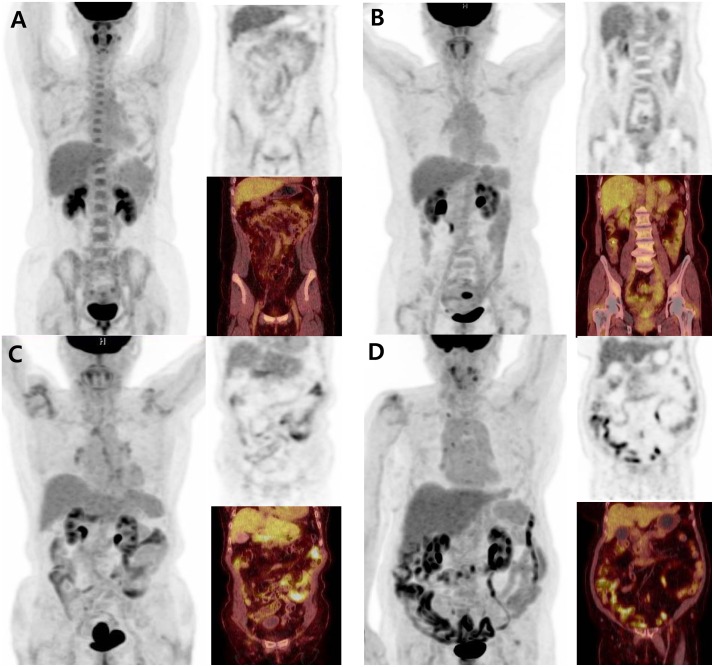
For qualitative and quantitative evaluation of background colonic uptake, the degree of non-focal diffuse uptake of colon was evaluated to minimize the effect by focal colonic adenoma. Background colonic 18F-FDG uptake was visually graded by two nuclear medicine specialists using a four-point scale; grade 1: background colonic 18F-FDG uptake of the whole colon is lower than that of the liver, grade 2: background colonic 18F-FDG uptake of at least one segment is equal to that of the liver, grade 3: background colonic 18F-FDG uptake of at least one segment is higher than that of the liver, grade 4: background colonic 18F-FDG uptake of almost all of the large intestine is higher than that of the liver (Fig 1). Grading was performed according to a consensus reached by the two readers, who were strictly blinded to other clinical information. For the quantitative analysis, the maximal standardized uptake value (SUVmax) in 7 different bowel segments (i.e., duodenum, jejunum, ileum, cecum, hepatic flexure, splenic flexure, and descending colon-sigmoid junction) were measured after placement of a three-dimensional volume of interest (VOI) [11]. Then, the SUVmax of the 7 bowel segments were averaged to obtain the total bowel (TB) SUVmax, which was used for further statistical analysis.
Fig 1.
(A) The representative case of grade 1 background colonic 18F-FDG uptake on PET/CT scan (TB SUVmax of 1.9) is shown. (B) The representative case of grade 2 background colonic uptake on PET/CT scan (TB SUVmax of 2.0) is shown. (C) The representative case of grade 3 background colonic uptake on PET/CT scan (TB SUVmax of 3.4) is shown. (D) The representative case of grade 4 background colonic uptake on PET/CT scan (TB SUVmax of 5.3) is shown.
Colonoscopy and diagnosis
All patients maintained a fasting state over 8 hours and bowel preparation was performed with polyethylene glycol before undergoing full colonoscopy. Electronic high resolution colonoscopy (CF-H260AL or CF-Q260AL, Olympus, Tokyo, Japan) was used for the examination. The colon was thoroughly observed from cecum to rectum, with withdrawal time more than 6 minutes. The grade of bowel preparation was classified into five levels according to the Aronchick scale (excellent, good, fair, poor, and inadequate) [12]. Adequate bowel preparation was defined as ‘fair’ or higher.
Six experienced endoscopists who had previously carried out colonoscopy more than 500 cases performed all colonoscopies, and recorded the size, location, and number of any colorectal neoplasm (CRN) that was identified. After removal of the CRN, histopathologic assessments were carried out by experienced pathologists. Inflammatory and hyperplastic polyps were classified as benign disease, as distinguished from CRA and CRC. Advanced CRA was defined as a CRA greater than 1 cm in diameter, CRA with high-grade dysplasia or containing a villous component.
Statistical analysis
For comparison of clinical factors between the group with CRA and the group without CRA (non-CRA), the Chi-square test or Fisher’s exact test was performed for categorical variables, and the Student t test was used for continuous variables. Multivariate logistic regression analysis was performed to determine independent factors for the presence of CRA. The inter-observer agreement for visual grading of background colonic 18F-FDG uptake on PET was analyzed using kappa statistics. The correlation between visually classified background colonic 18F-FDG uptake grade and TB SUVmax on PET was analyzed using Spearman’s correlation. All statistical analyses were performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA) and a value of p < 0.05 was considered statistically significant.
Ethics statement
This study was approved by the institutional review board of Ewha Womans University Mokdong Hospital (IRB file No. 2015-05-015). Written informed consent could not be given by participants because this study was conducted in a retrospective manner. However, patients’ records were anonymized and deidentified prior to analysis to protect their privacy.
Results
Baseline clinical factors of study subjects: colorectal adenoma vs. non-adenoma
There were 517 patients who had received PET scan in the gynecology department between January 2006 and February 2015. Of these patients, 250 patients with ovarian malignancy or without colonoscopy were excluded. After excluding patients with age under 30 years old, a history of other colonic disease, poor or inadequate bowel preparation, incomplete colonoscopy, and no medical records of colonoscopy or biopsy findings (N = 26), a total of 241 patients were finally included in the present study (Fig 2).
Fig 2. A flow diagram of patient selection in this study.
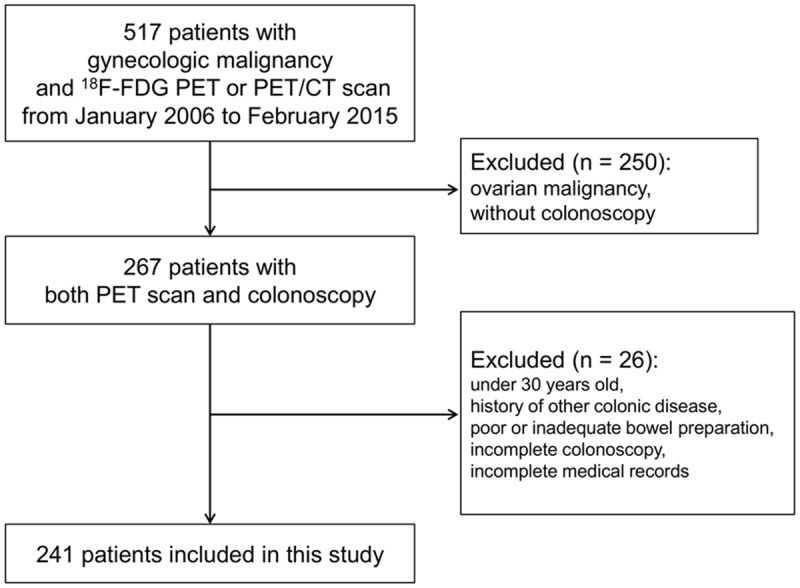
FDG, fluoro-2-deoxy-D-glucose; PET, positron emission tomography; CT, computed tomography.
In our study, the pathologic report of all patients with CRN showed adenoma or benign lesions, and there were no subjects with colorectal malignancy. Among a total of 241 study subjects, demographic data and clinical factors were compared between the CRA group and the non-CRA group (Table 1). The age at diagnosis was significantly higher in the CRA group than non-CRA group (61.1 ± 13.3 vs. 52.3 ± 12.2, p < 0.001). However, gender, alcohol use, smoking habits, and family history of CRC were not different between the two groups. Regarding the BMI, fasting plasma glucose, serum TG, and serum total cholesterol, only serum TG level was significantly higher in the CRA group than non-CRA group (116.8 ± 61.6 vs. 97.2 ± 49.9, p = 0.025), and other values did not show any considerable difference.
Table 1. Baseline Demographics and Clinical Factors of Study Subjects (N = 241).
| CRA (n = 45) | Non-CRA (n = 196) | p—value | |
|---|---|---|---|
| Age at diagnosis (years)a | 61.1 ± 13.1 | 52.3 ± 12.2 | < 0.001 |
| Alcohol use, n (%) | 0.387b | ||
| Non drinker | 45 (100) | 188 (95.9) | |
| Social drinker | 0 (0) | 6 (3.1) | |
| Heavy drinker | 0 (0) | 2 (1.0) | |
| Cigarette smoking, n (%) | 0.786b | ||
| Never smoker | 44 (97.8) | 189 (96.4) | |
| Ex-smoker | 0 (0) | 2 (1.0) | |
| Current smoker | 1 (2.2) | 5 (2.6) | |
| Family history of CRC, n (%) | 0.512 | ||
| Presence | 1 (2.2) | 2 (1.0) | |
| Absence | 44 (97.8) | 194 (99.0) | |
| Type of gynecologic disease, n (%) | 0.664 | ||
| Cervical cancer | 28 (62.2) | 134 (68.4) | |
| Endometrial cancer | 17 (37.8) | 59 (30.1) | |
| Others | 0 (0) | 3 (1.5) | |
| Body mass index (kg/m²)a | 24.8 ± 3.2 | 23.9 ± 3.6 | 0.125 |
| Fasting plasma glucose (mg/dL)a | 102.7 ± 31.7 | 97.5 ± 19.9 | 0.293 |
| Serum triglyceride (mg/dL)a | 116.8 ±61.6 | 97.2 ± 49.9 | 0.025 |
| Serum total cholesterol (mg/dL)a | 180.9 ± 39.8 | 177.8 ± 37.1 | 0.619 |
| Background colonic 18F-FDG uptake, n (%) | 0.013b | ||
| Grade 1 | 9 (20.0) | 63 (32.1) | |
| Grade 2 | 12 (26.7) | 69 (35.2) | |
| Grade 3 | 22 (48.9) | 60 (30.6) | |
| Grade 4 | 2 (4.4) | 4 (2.0) |
CRA, colorectal adenoma; non-CRA, non-colorectal adenoma; CRC, colorectal cancer; 18F-FDG, 18F-fluoro-2-deoxy-D-glucose.
aMean ± standard deviation
b trend p value by linear-to-linear association analysis
According to the visual grade of background colonic 18F-FDG uptake on PET, the inter-observer agreement was almost perfect (Cohen’s weighted kappa value of 0.817): 29.9% of patients were classified as grade 1, 33.6% were grade 2, 34.0% were grade 3, and 2.5% were grade 4. For further statistical analysis, grade 1 and grade 2 were considered the low grade group (63.5%), while grade 3 and grade 4 were considered the high grade group (36.5%).
Association of background colonic uptake grade on PET with the prevalence of colorectal adenoma
To identify the clinical factors associated with the prevalence of CRA, univariate analyses were performed for age at diagnosis, alcohol use, cigarette smoking, family history of CRC, BMI, fasting glucose, TG, total cholesterol, and background colonic uptake grade on PET scan (Table 2, univariate analysis). Consequently, the proportion of patients with older age at diagnosis (≥ 50 years, p = 0.034), overweight (BMI ≥ 23 kg/m², p = 0.010), and hypercholesterolemia (total cholesterol ≥ 200 mg/dL, p = 0.027) was significantly higher in the CRA group compared to the non-CRA group. In addition, high grade background colonic 18F-FDG uptake on PET was positively associated with the prevalence of CRA (p = 0.009). Multivariate analysis was performed adjusting all possible variables to identify independent predictors for the prevalence of CRA (Table 2, multivariate analysis). By multiple logistic regression model with adjusted other variables including age at diagnosis, alcohol use, cigarette smoking, family history of CRC, BMI, plasma glucose, serum TG, and total cholesterol, high grade background colonic 18F-FDG uptake was independently predictive of the possibility of having CRA. The odds of having CRA was 2.25 times higher in the high background colonic uptake grade group than low grade group (adjusted odds ratio (OR), 2.25, 95% confidence interval (CI), 1.13–4.49, p = 0.021).
Table 2. Association of background colonic uptake grade on PET with the prevalence of colorectal adenoma.
| Univariate analysis (Chi-square test) | Multivariate analysis (Logistic regression analysis) | |||||
|---|---|---|---|---|---|---|
| CRA | Non-CRA | p value | aOR* | 95% CI | p value | |
| Age at diagnosis, n (%) | 0.034 | |||||
| < 50 years | 12 (12.2) | 86 (87.8) | 1 (reference) | |||
| ≥ 50 years | 33 (23.1) | 110 (76.9) | 1.83 | 0.83–4.01 | 0.134 | |
| Alcohol use, n (%) | 0.168 | |||||
| Non drinker | 45 (19.3) | 188 (80.7) | 1 (reference) | |||
| Social and heavy drinker | 0 (0) | 8 (100) | 0 | 0 | 0.999 | |
| Cigarette smoking, n (%) | 0.649 | |||||
| Non smoker | 44 (18.9) | 189 (81.1) | 1 (reference) | |||
| Ex- or current smoker | 1 (12.5) | 7 (87.5) | 0.54 | 0.05–5.38 | 0.595 | |
| Family history of CRC, n (%) | 0.512 | |||||
| Absence | 44 (18.5) | 194 (81.5) | 1 (reference) | |||
| Presence | 1 (33.3) | 2 (66.7) | 4.28 | 0.34–53.40 | 0.259 | |
| Body mass index, n (%) | 0.010 | |||||
| < 23 kg/m² | 11 (11.0) | 89 (89.0) | 1 (reference) | |||
| ≥ 23 kg/m² | 34 (24.1) | 107 (75.9) | 1.91 | 0.88–4.15 | 0.101 | |
| Plasma glucose, n (%) | 0.226 | |||||
| < 100 mg/dL | 28 (16.7) | 140 (83.3) | 1 (reference) | |||
| ≥ 100 mg/dL | 17 (23.3) | 56 (76.7) | 1.17 | 0.56–2.42 | 0.676 | |
| Triglyceride, n (%) | 0.067 | |||||
| < 150 mg/dL | 35 (16.9) | 172 (83.1) | 1 (reference) | |||
| ≥ 150 mg/dL | 10 (30.3) | 23 (69.7) | 1.71 | 0.69–4.23 | 0.244 | |
| Total cholesterol, n (%) | 0.027 | |||||
| < 200 mg/dL | 26 (15.2) | 145 (84.8) | 1 (reference) | |||
| ≥ 200 mg/dL | 19 (27.5) | 50 (72.5) | 1.90 | 0.92–3.91 | 0.081 | |
| Background colonic 18F-FDG uptake, n (%) | 0.009 | |||||
| Low grade (1, 2) | 21 (13.7) | 132 (86.3) | 1 (reference) | |||
| High grade (3, 4) | 24 (27.3) | 64 (72.7) | 2.25 | 1.13–4.49 | 0.021 | |
CRA, colorectal adenoma; non-CRA, non-colorectal adenoma; aOR, adjusted odds ratio, CI, confidence interval; CRC, colorectal cancer; 18F-FDG, 18F-fluoro-2-deoxy-D-glucose.
*presence of colorectal adenoma as the dependent variable
We analyzed the prevalence of advanced and non-advanced CRAs according to the grade of background uptake; the prevalence of advanced CRA was not significantly different between low (Grade 1, 2) and high grade background uptake (Grade 3, 4) (p = 0.274). We also analyzed the number of CRAs according to the grade of background uptake; 3 or more CRAs was not significantly different than 1 or 2 CRAs (p = 0.578) (S1 Table).
Patients with colorectal adenoma according to the grade of background colonic uptake on PET scan
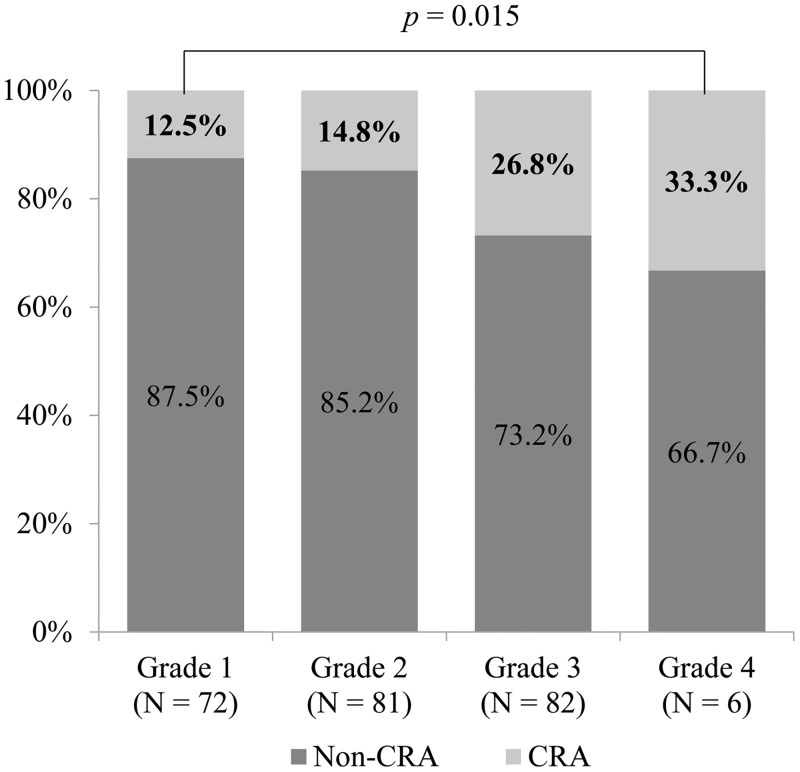
Percentages of patients with CRA according to the grade of background colonic 18F-FDG uptake on PET scan were analyzed (Fig 3). The proportion of patients with CRA was 12.5% in grade 1, 14.8% in grade 2, 26.8% in grade 3, and 33.3% in grade 4. Thus, the proportion of patients with CRA significantly increased as the grade of background colonic uptake became higher (trend p = 0.015 by linear-by-linear association analysis).
Fig 3. The proportion of patients with colorectal adenoma demonstrated rising trend with increasing grade of background colonic 18F-FDG uptake on PET scan (trend p = 0.015 by linear-by-linear association analysis).
CRA, colorectal adenoma.
Out of a total of 241 subjects in this study, TB SUVmax values were calculated for the 138 patients who underwent PET/CT scan. In quantitative analysis, TB SUVmax was 2.5 ± 0.68 in the CRA group, and 2.3 ± 0.55 in the non-CRA group. TB SUVmax was higher in the CRA group than non-CRA group, but it did not reach a statistical significance (p = 0.080). However, when the study subjects were classified according to the cut-off value of TB SUVmax, statistical significance was observed in further analyses. A ROC curve was obtained regarding TB SUVmax values and the cut-off value was 2.25. Subsequently, a Chi-square test was performed to determine the proportion of patients with CRA among those with SUVmax > 2.25 (high SUVmax group, N = 63) compared with those with SUVmax ≤ 2.25 (low SUVmax group, N = 75). The proportion of CRA patients was significantly higher in the high SUVmax group than in the low SUVmax group (27.5% vs. 11.6%, p = 0.031). Additionally, the correlation between the visual grade of background colonic 18F-FDG uptake and TB SUVmax was also statistically significant (Spearman’s rho = 0.719, p < 0.001).
By multiple logistic regression model with age at diagnosis, alcohol use, cigarette smoking, family history of CRC, BMI, plasma glucose, serum TG, and total cholesterol, and TB SUVmax, high TB SUVmax (> 2.25) was independently predictive of the possibility of having CRA (aOR 2.89, p = 0.038) (S2 Table).
Discussion
The purpose of this study was to identify the clinical significance of background colonic 18F-FDG uptake on PET scan. The results demonstrated that background colonic 18F-FDG uptake on PET was an independent factor associated with the prevalence of CRA, and that the proportion of patients with CRA became higher with an increase in the grade of background colonic uptake in a dose-dependent manner.
A recent study suggested that background intestinal 18F-FDG uptake on PET can be associated with gut flora [10]. In patients with nongastrointestinal lymphoma who had PET/CT scan after taking rifaximin, a nonabsorbed antibiotic, for 2 days, cecal SUVmax and the proportion of colonic 18F-FDG uptake greater than grade 1 during the post-rifaximin scan were significantly lower than those during the pre-rifaximin scan [10]. Considering several reports demonstrating that rifaximin changes the gut microbiota [13,14], the decrease in background intestinal 18F-FDG uptake could be explained by alteration of the gut flora. In addition, it has been reported that the microbiota of the colon in patients with and without CRC or CRA is different [15–17]. Taken together, we assumed that the contribution of gut flora may explain the significant association between background intestinal 18F-FDG uptake and the prevalence of CRA in the present study. However, this was an observational study to identify the association between background colonic FDG uptake and CRA. Thus, further translational and experimental studies, including metagenomic analyses, are necessary to clarify the underlying mechanism.
On the other hand, obesity and hypertriglyceridemia can be another underlying mechanism for our results. Yoon et al. [11] showed that obesity and hypertriglyceridemia may be related to high background intestinal 18F-FDG uptake on PET scan. In non-diabetic and non-hypertensive breast cancer patients, BMI and TG levels were higher as the visual grade of background intestinal 18F-FDG uptake and TB SUVmax increased [11]. It is already known that obesity is related to the prevalence of CRA [18–25]. Obesity is closely related to hyperinsulinemia and insulin resistance, which is associated with an increase in insulin like growth factor (IGF) levels [26]. It was identified that high levels of insulin and IGFs can promote cancer development through insulin/IGF axis, and especially that IGF-1 plays an important role in the inhibition of apoptosis and promotion of cell-cycle progression [27–29]. In addition, some studies demonstrated that hypertriglyceridemia is significantly associated with an increase in the prevalence of CRA [23,30]. This association of hypertriglycemia with CRA can be explained by the insulin/IGF-1 pathway [31], oxidative stress [32], and proinflammatory cytokines such as tumor necrosis factor α and interleukin (IL)-6 [33]. In the correlation analysis in our study, BMI (r = 0.155, p = 0.016) and TG levels (r = 0.151, p = 0.019) showed weak positive correlations with background colonic FDG uptake. Considering the significant association between background FDG uptake and obesity/hypertriglyceridemia, it could be assumed that background intestinal FDG uptake may be associated with the prevalence of CRA. However, further observational and experimental studies are warranted to explain its underlying mechanisms and obtain direct evidence.
There are several limitations in this study. First, it was a retrospective study reviewing medical records of subjects who had received both PET scan and colonoscopy. There were some missing data in the baseline characteristics including underlying disease and laboratory results. Among them, some medication may have influenced the physiologic intestinal 18F-FDG uptake. Particularly, the effect of metformin intake in diabetic patients has been reported by several previous studies [8,9]. According to our reviewing medical records, 4 diabetic patients were prescribed metformin among the 241 patients. A Fisher’s exact test did not show a significant difference in background colonic uptake based on metformin intake (p = 0.624). However, the small sample size of the metformin intake group could contribute to the statistical insignificance. If the retrospective chart review missed the exact number of diabetic patients with metformin intake, it could be one of limitations of this study. Second, how to exclude uptake by CRA from background colonic 18F-FDG uptake on PET scan could raise a possibility of bias. Though CRA can show variable degree of FDG uptake, the most common uptake pattern of CRA is a focal nodular uptake [5,34]. Thus, we evaluated non-focal diffuse uptake of colon to minimize the effect by CRA. For the low grade group, we believe that most of CRA uptake can be avoided by evaluating non-focal diffuse colonic uptake. However, in the high background colonic uptake group, it is difficult to discriminate focal colonic uptake from background colonic uptake. Even if the discrimination of focal colonic uptake is impossible due to high background colonic uptake, and if the measurement was performed at the site of colonic adenoma, diffuse background colonic uptake would be high enough to mask focal uptake of CRA. Third, the colonoscopies were performed by several endoscopists, not by a single person. This may have acted as a bias because the adenoma detection rate may differ according to the level of competence and experience of the endoscopists. However, to minimize this limitation, this study included only examinations performed by experienced endoscopists, each of whom had performed more than 150 colonoscopic examinations. Furthermore, only examinations with a withdrawal time of more than 6 minutes and a level of bowel preparation 'fair' or higher on the Aronchick scale were included to improve the quality of the study.
Conclusion
This study shows that a high grade of background colonic 18F-FDG uptake is significantly associated with a high possibility of having CRA. In addition, as the grade of background colonic 18F-FDG uptake increases, the proportion of CRA increases in a dose-dependent manner. Therefore, although patients undergo PET scans for many other indications, physicians should be alert to the possibility of finding high background colonic FDG uptake and considering its implications.
Supporting Information
The prevalence of advanced CRA and the number of CRA according to the grade of background uptake was analyzed.
(DOCX)
Multiple logistic regression analysis was performed with the presence of colorectal adenoma as the dependent variable.
(DOCX)
Data Availability
All relevant data are within the paper.
Funding Statement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number, NRF-2014R1A1A2058325) and (grant number, NRF-2012M3A9B6055379) and the Ewha Womans University Research Grant of 2014.
References
- 1.Valk PE, Abella-Columna E, Haseman MK, Pounds TR, Tesar RD, et al. (1999) Whole-body PET imaging with [18F]fluorodeoxyglucose in management of recurrent colorectal cancer. Arch Surg 134: 503–511; discussion 511–503. [DOI] [PubMed] [Google Scholar]
- 2.Falk PM, Gupta NC, Thorson AG, Frick MP, Boman BM, et al. (1994) Positron emission tomography for preoperative staging of colorectal carcinoma. Dis Colon Rectum 37: 153–156. [DOI] [PubMed] [Google Scholar]
- 3.Kim S, Chung JK, Kim BT, Kim SJ, Jeong JM, et al. (1999) Relationship between Gastrointestinal F-18-fluorodeoxyglucose Accumulation and Gastrointestinal Symptoms in Whole-Body PET. Clin Positron Imaging 2: 273–279. [DOI] [PubMed] [Google Scholar]
- 4.Tatlidil R, Jadvar H, Bading JR, Conti PS (2002) Incidental colonic fluorodeoxyglucose uptake: correlation with colonoscopic and histopathologic findings. Radiology 224: 783–787. [DOI] [PubMed] [Google Scholar]
- 5.Gutman F, Alberini JL, Wartski M, Vilain D, Le Stanc E, et al. (2005) Incidental colonic focal lesions detected by FDG PET/CT. AJR Am J Roentgenol 185: 495–500. [DOI] [PubMed] [Google Scholar]
- 6.Kei PL, Vikram R, Yeung HW, Stroehlein JR, Macapinlac HA (2010) Incidental finding of focal FDG uptake in the bowel during PET/CT: CT features and correlation with histopathologic results. AJR Am J Roentgenol 194: W401–406. 10.2214/AJR.09.3703 [DOI] [PubMed] [Google Scholar]
- 7.Cook GJ, Wegner EA, Fogelman I (2004) Pitfalls and artifacts in 18FDG PET and PET/CT oncologic imaging. Semin Nucl Med 34: 122–133. [DOI] [PubMed] [Google Scholar]
- 8.Oh JR, Song HC, Chong A, Ha JM, Jeong SY, et al. (2010) Impact of medication discontinuation on increased intestinal FDG accumulation in diabetic patients treated with metformin. AJR Am J Roentgenol 195: 1404–1410. 10.2214/AJR.10.4663 [DOI] [PubMed] [Google Scholar]
- 9.Gontier E, Fourme E, Wartski M, Blondet C, Bonardel G, et al. (2008) High and typical 18F-FDG bowel uptake in patients treated with metformin. Eur J Nucl Med Mol Imaging 35: 95–99. [DOI] [PubMed] [Google Scholar]
- 10.Franquet E, Palmer MR, Gifford AE, Selen DJ, Chen YC, et al. (2014) Rifaximin suppresses background intestinal 18F-FDG uptake on PET/CT scans. Nucl Med Commun 35: 1026–1031. 10.1097/MNM.0000000000000170 [DOI] [PubMed] [Google Scholar]
- 11.Yoon HJ, Kim HN, Yun Y, Kim Y, Ha AN, et al. (2015) Background Intestinal 18F-FDG Uptake Is Related to Serum Lipid Profile and Obesity in Breast Cancer Patients. PLoS One 10: e0141473 10.1371/journal.pone.0141473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aronchick CA, Lipshutz WH, Wright SH, Dufrayne F, Bergman G (2000) A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc 52: 346–352. [DOI] [PubMed] [Google Scholar]
- 13.Xu D, Gao J, Gillilland M 3rd, Wu X, Song I, et al. (2014) Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology 146: 484–496.e484. 10.1053/j.gastro.2013.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DuPont HL (2016) Review article: the antimicrobial effects of rifaximin on the gut microbiota. Aliment Pharmacol Ther 43 Suppl 1: 3–10. 10.1111/apt.13434 [DOI] [PubMed] [Google Scholar]
- 15.Kasai C, Sugimoto K, Moritani I, Tanaka J, Oya Y, et al. (2016) Comparison of human gut microbiota in control subjects and patients with colorectal carcinoma in adenoma: Terminal restriction fragment length polymorphism and next-generation sequencing analyses. Oncol Rep 35: 325–333. 10.3892/or.2015.4398 [DOI] [PubMed] [Google Scholar]
- 16.Dulal S, Keku TO (2014) Gut microbiome and colorectal adenomas. Cancer J 20: 225–231. 10.1097/PPO.0000000000000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakatsu G, Li X, Zhou H, Sheng J, Wong SH, et al. (2015) Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun 6: 8727 10.1038/ncomms9727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim BC, Shin A, Hong CW, Sohn DK, Han KS, et al. (2012) Association of colorectal adenoma with components of metabolic syndrome. Cancer Causes Control 23: 727–735. 10.1007/s10552-012-9942-9 [DOI] [PubMed] [Google Scholar]
- 19.Bird CL, Frankl HD, Lee ER, Haile RW (1998) Obesity, weight gain, large weight changes, and adenomatous polyps of the left colon and rectum. Am J Epidemiol 147: 670–680. [DOI] [PubMed] [Google Scholar]
- 20.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, et al. (1995) Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 122: 327–334. [DOI] [PubMed] [Google Scholar]
- 21.Kang HW, Kim D, Kim HJ, Kim CH, Kim YS, et al. (2010) Visceral obesity and insulin resistance as risk factors for colorectal adenoma: a cross-sectional, case-control study. Am J Gastroenterol 105: 178–187. 10.1038/ajg.2009.541 [DOI] [PubMed] [Google Scholar]
- 22.Kim Y, Kim Y, Lee S (2009) An association between colonic adenoma and abdominal obesity: a cross-sectional study. BMC Gastroenterol 9: 4 10.1186/1471-230X-9-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu CS, Hsu HS, Li CI, Jan CI, Li TC, et al. (2010) Central obesity and atherogenic dyslipidemia in metabolic syndrome are associated with increased risk for colorectal adenoma in a Chinese population. BMC Gastroenterol 10: 51 10.1186/1471-230X-10-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yun KE, Chang Y, Jung HS, Kim CW, Kwon MJ, et al. (2013) Impact of body mass index on the risk of colorectal adenoma in a metabolically healthy population. Cancer Res 73: 4020–4027. 10.1158/0008-5472.CAN-12-3477 [DOI] [PubMed] [Google Scholar]
- 25.Nagata N, Sakamoto K, Arai T, Niikura R, Shimbo T, et al. (2014) Visceral abdominal fat measured by computed tomography is associated with an increased risk of colorectal adenoma. Int J Cancer 135: 2273–2281. 10.1002/ijc.28872 [DOI] [PubMed] [Google Scholar]
- 26.Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444: 840–846. [DOI] [PubMed] [Google Scholar]
- 27.Bardou M, Barkun AN, Martel M (2013) Obesity and colorectal cancer. Gut 62: 933–947. 10.1136/gutjnl-2013-304701 [DOI] [PubMed] [Google Scholar]
- 28.Kaaks R, Lukanova A (2001) Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc 60: 91–106. [DOI] [PubMed] [Google Scholar]
- 29.Aaronson SA (1991) Growth factors and cancer. Science 254: 1146–1153. [DOI] [PubMed] [Google Scholar]
- 30.Yang MH, Rampal S, Sung J, Choi YH, Son HJ, et al. (2013) The association of serum lipids with colorectal adenomas. Am J Gastroenterol 108: 833–841. 10.1038/ajg.2013.64 [DOI] [PubMed] [Google Scholar]
- 31.Siddiqui AA (2011) Metabolic syndrome and its association with colorectal cancer: a review. Am J Med Sci 341: 227–231. 10.1097/MAJ.0b013e3181df9055 [DOI] [PubMed] [Google Scholar]
- 32.Cowey S, Hardy RW (2006) The metabolic syndrome: A high-risk state for cancer? Am J Pathol 169: 1505–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esteve E, Ricart W, Fernandez-Real JM (2005) Dyslipidemia and inflammation: an evolutionary conserved mechanism. Clin Nutr 24: 16–31. [DOI] [PubMed] [Google Scholar]
- 34.Yasuda S, Fujii H, Nakahara T, Nishiumi N, Takahashi W, et al. (2001) 18F-FDG PET detection of colonic adenomas. J Nucl Med 42: 989–992. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The prevalence of advanced CRA and the number of CRA according to the grade of background uptake was analyzed.
(DOCX)
Multiple logistic regression analysis was performed with the presence of colorectal adenoma as the dependent variable.
(DOCX)
Data Availability Statement
All relevant data are within the paper.