Abstract
Malaria parasites dramatically alter the rheological properties of infected red blood cells. In the case of Plasmodium vivax, the parasite rapidly decreases the shear elastic modulus of the invaded RBC, enabling it to avoid splenic clearance. This study highlights correlation between rosette formation and altered membrane deformability of P. vivax-infected erythrocytes, where the rosette-forming infected erythrocytes are significantly more rigid than their non-rosetting counterparts. The adhesion of normocytes to the PvIRBC is strong (mean binding force of 440pN) resulting in stable rosette formation even under high physiological shear flow stress. Rosetting may contribute to the sequestration of PvIRBC schizonts in the host microvasculature or spleen.
Author Summary
While Plasmodium vivax is generally not as virulent as P. falciparum; severe manifestations of vivax malaria do occur. While little is known about the mechanisms underlying the pathobiology of P. vivax, most agree its ability to increase the deformability of stiff host reticulocytes is key adaptation to avoid splenic clearance. We show that P. vivax-infected red blood cells (PvIRBCs) rosette irreversibly with normocytes and are significantly more stiff than non-rosetting PvIRBCs. We discuss how these stiff PvIRBC rosettes are removed from the peripheral circulation and its rheopathological consequences.
Introduction
Plasmodium spp. derived changes to the rheology of infected red blood cells (IRBCs) play a central role in the pathogenesis of human malaria. Malaria parasite remodelling of IRBCs dramatically alter their deformability and cytoadhesive properties [1]. Interestingly, for all four non-zoonotic causes of human malaria (P. falciparum, P. vivax, P. ovale and P. malariae) IRBCs cytoadhere to uninfected RBCs forming distinctive ‘rosettes’ [2–4]. While the precise role of rosetting in malaria pathogenesis remains contentious, many believe that this adaptation may play important roles in the survival of parasites within the circulation [5]. Rheological studies on P. falciparum rosettes show them to be stable and the binding force between the IRBC and the uninfected RBCs tends to be very strong (>300pn) [6]. Indeed, most studies on rosetting have focused on P. falciparum, leading to the discovery of rosetting ligands such as PfEMP1 [7], STEVOR [8], and RIFINs [9]. Although rosette formation has been reported to be a common phenomenon in P. vivax [2, 10, 11], the rosetting ligand of this species has yet to be discovered. Despite recent evidence showing cytoadhesive potential for P. vivax-infected RBCs [12], most consider this species to be much less adhesive than P. falciparum, as it lacks any orthologue to the PfEMP1 protein (the key cytoadhesive ligand in P. falciparum) and the knobby IRBC ultrastructure (which concentrate and display PfEMP-1) that facilitate binding of IRBCs to the vascular endothelium under physiological shear flow [13]. Therefore, although P. vivax rosettes are relatively commonly observed, it is not known whether they are stable structures or ephemeral ex-vivo formations that break apart in the haemodynamic environment of the circulation in vivo. The objective of this study was to examine the rheological consequences of rosetting on PvIRBCs and specifically quantify the binding strength of normocytes to PvIRBCs.
Methods
Ethics statement
Blood samples of vivax malaria patients from the Northwestern Thailand were collected under the following ethical guidelines and approved protocols: OXTREC 027–025 (University of Oxford, Centre for Clinical Vaccinology and Tropical Medicine, UK) and MUTM 2008–215 from the Ethics Committee of Faculty of Tropical Medicine, Mahidol University, Thailand. Experiments were conducted in Singapore Immunology Network (SIgN) and National University of Singapore (NUS), Singapore. All adult subjects provided informed written consent, and a parent or guardian of any child participant provided informed written consent on their behalf. Ten clinical samples were collected from malaria patients of SMRU clinics in Northwestern Thailand using BD Vacutainer with lithium heparin anticoagulant. Thick and thin blood smears were prepared for each sample to determine the species of malaria parasite, the parasitemia, and the predominating developmental stage of the parasite. White blood cells were depleted with cellulose (Sigma-Aldrich) packed columns. Blood samples containing predominantly ring-stage parasites (≥ 70%) were cryopreserved with Glycerolyte 57 (Fenwal). For experiments, cryopreserved isolates were thawed and the parasites matured in vitro [14]. When the parasite population reached late erythrocytic stages (late trophozoite and schizont), 50 μl of the culture suspension was taken for rosetting assay using a wet mount method as described elsewhere[11]. Rosetting rate (percentage of rosette-forming IRBCs) was determined by examining the number of of rosettes per 200 IRBCs observed. Subsequently, 1 μl packed RBCs were suspended in 1 ml of 1X PBS supplemented with 1% BSA for micropipette aspiration and microfluidic assays.
Micropipette aspiration was modified from Hochmuth et al [15]. Briefly, aspiration was performed at 32°C to 37°Cand observed using an oil immersion objective (1000 x magnification) with an Olympus research inverted microscope IX73. Borosilicate glass micropipettes (diameter 1.5±0.2 μm) were used to hold or aspirate RBCs. Rosetting and non-rosetting IRBCs were individually selected for measurements. Individual RBCs were aspirated at a pressure drop rate of 0.5 Pa/s for 100s. The corresponding cell membrane deformation was recorded using the Dual CCD Digital Camera DP80 (Olympus) at an image taking rate of one frame/s. Images were processed by cellSens Dimension (Olympus). Hemispherical cap model was used to calculate the membrane shear elastic modulus, as a quantitative surrogate measure of the rigidity of RBC membrane skeleton [15].
To quantify the binding force between RBCs and an IRBC in a P. vivax rosette, a double pipette aspiration method was used as described previously [6]. A rosette was held by a micropipette (diameter = 2.0±0.2μm). A second micropipette was used to aspirate the uninfected RBCs of the rosette at a gradually increased aspirating pressure. The force (F) to detach an RBC from an IRBC was calculated as F = πr2 × P; where r is the inner diameter of the second micropipette, and P is the pressure required to detach two cells. The aspiration pressure was measured by a pressure transducer (P61 model, Validyne Engineering) and recorded by USB-COM Data logger (Validyne Engineering). The process was recorded using a Dual CCD Digital Camera DP80 (Olympus) at one frame/s. Recorded images were analyzed with cellSens Dimension (Olympus).
To characterize the ability of PvIRBCs to move through narrow channels, polydimethylsiloxane (PDMS) microfluidic chips with 4μm slits were used. To avoid RBCs from interacting with (or adhering to) the walls of the microfluidic chip, channels were pre-filled and incubated with 1X PBS supplemented with 1% BSA for one hour prior to the experiment being performed. Subsequently, 1μl of RBC suspension was injected into the microfluidic channel. Cells were forced through the channel at a constant pressure gradient of 0.1 Pa/μm. Numbers of RBCs that blocked at the openings of the microfluidic channels in each experiment were recorded. Videos of the microfluidic assay were recorded using a Dual CCD Digital Camera DP80 (Olympus). Data were subsequently analyzed using the cellSens Dimensions software (Olympus). GraphPad Prism 5 was used for statistical analysis of all experimental data. The one-way ANOVA test was used to compare differences between different experimental groups.
Results
In keeping with previous report [11], cryopreserved P. vivax isolates showed rosetting, albeit with lower frequency than the fresh isolates. The rosettes found in these cryopreserved isolates were generally small. A mode of three uninfected normocytes were involved in rosettes (Fig 1). Similar to the previous study [11], rosetting in this study was only observed with RBCs infected with the late erythrocytic stages (predominantly schizonts).
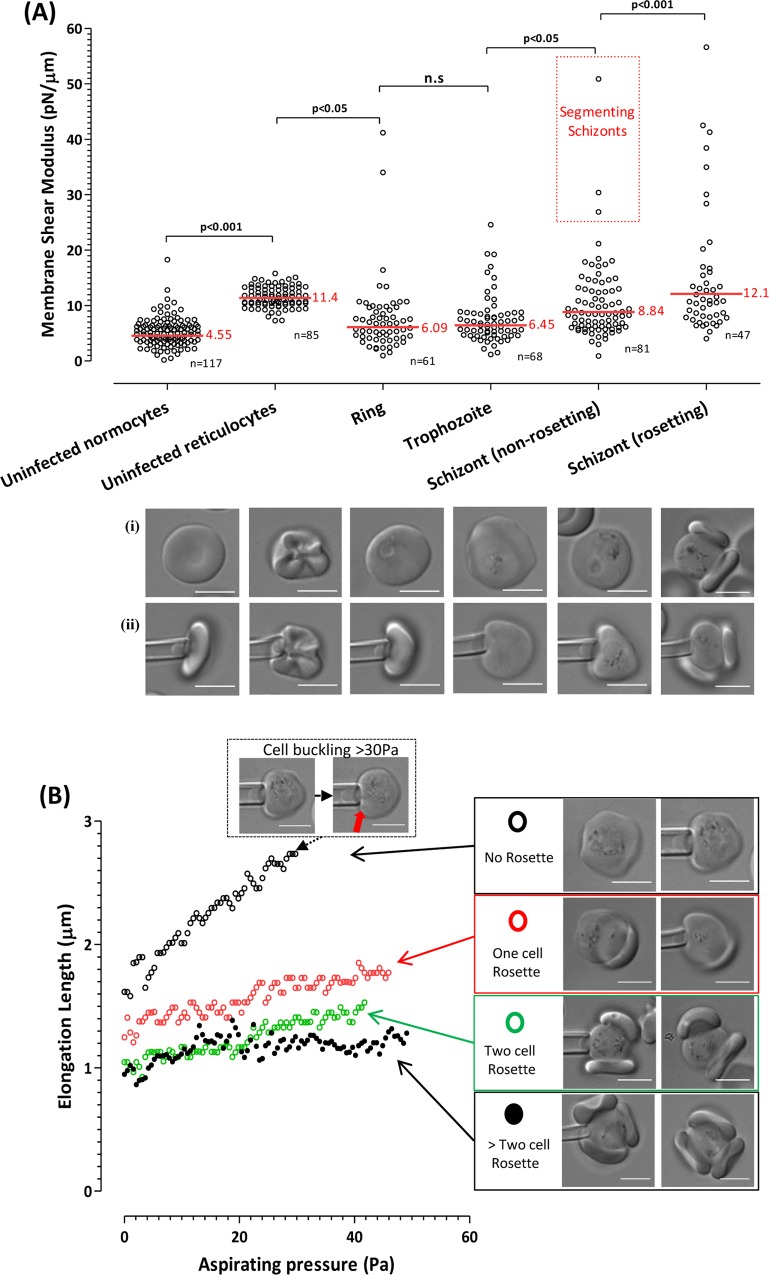
Fig 1.
(A) The effect of P. vivax invasion, development and rosetting on the deformability of the infected reticulocyte membrane (normocytes are shown as a comparator). Plot showing membrane shear moduli (SM) (a higher SM indicates a reduced membrane deformability) of different cell types and stages of P. vivax erythrocytic development, with geometric mean (overall of 10 isolates) SM of each group indicated by a red line (each dot represents an individual cell measurement the total ‘n = x’). Pictures of respective cell types before (i) and during (ii) membrane shear modulus measurement by micropipette aspiration are shown under the graph. Mean (Geometric) shear moduli was compared using ANOVA (Bonferroni correction) and multiple comparison test (tukey). Uninfected normocytes were significantly more deformable than uninfected reticulocytes (P< 0.001). However both ring and trophozoite P. vivax stages become progressively more deformable (P< 0.05) until schizont stage (the very mature schizonts ‘segmenters’ were especially rigid). When normocytes adhered (rosette) with schizonts the infected cell membrane became significantly more rigid than non-rosetting schizonts (P< 0.001). (B) The number of normocytes involved in the rosette had no significant effect on the mean deformability kinetics (aspirated length versus the suction pressure) of the IRBCs. Pictures of cells before (i) and during (ii) measurement are shown under the graph. Measurements were done with increasing aspirating pressure until cells became structurally unstable under that pressure point (buckling effect), as shown by pictures (inset).
Membrane shear elastic modulus measurements were used to quantify IRBC membrane deformability (Fig 1A). Uninfected reticulocytes showed significantly higher membrane shear moduli than uninfected normocytes (11.40±1.85 pN/μm vs. 4.55±2.58 pN/μm; P < 0.001). Interestingly, the membrane shear elastic moduli of P. vivax ring-infected reticulocytes were reduced to values similar to uninfected normocytes (6.09±6.45 pN/μm). The membrane shear elastic moduli of IRBCs remained virtually unchanged at the trophozoite stage (6.45±4.31 pN/μm). The membrane shear elastic modulus of non-rosetting schizonts were significantly higher than measurements recorded by trophozoites (8.84±6.88 pN/μm; P < 0.05). Measurements performed on rosetting schizonts (12.1±11.36 pN/μm) were significantly higher than those of non-rosetting schizonts (P < 0.01).
All RBCs showed an increased elongation length (i.e. increased deformability) with increasing aspiration pressure (Fig 1B). The attachment of a single uninfected RBC caused a significant reduction in deformability of the IRBC (P < 0.05). However, a Spearman’s rank correlation analysis showed that the attachment of additional RBCs did not result in further decreases to IRBC deformability, regardless of the size of the rosettes formed (Fig 1B).
From dual micropipette aspiration assays (Fig 2A) (S1 Video), the shear force to separate uninfected RBCs from a rosetting complex was 440±197.4pN, which was similar to that reported previously for P. falciparum [6] (Fig 2B). In microfluidic experiments (Fig 2C), RBCs infected with either P. vivax ring, trophozoite or schizonts (early schizont and mature segmenting schizont) stages (Three clinical isolates in total were used) were injected into microfluidic channels as previously shown (S2–S5 Videos) [16]. The only cells observed blocking the microfluidic restrictions were rosetting and very mature segmenting schizonts. Rosettes blocking the microfluidic restrictions did not lose cells under pulsed shear flow pressure up to of 1.0 Pa/μm.
Fig 2.
(A) Binding affinity of the rosetting complex using dual micropipette aspiration technique (B) Comparison of binding forces recorded from P. vivax rosettes (from this study) and P. falciparum rosettes (Nash et al 1992). (C) Examples of IRBCs capable of moving through 4 um microfluidic channel openings (Trophozoites (first image)) at 0.1 Pa and those that are trapped mature schizonts.
To better determine if the act of rosetting directly causes changes to the IRBC shear modulus (as opposed to IRBCs with a higher shear modulus are more likely to form rosettes) we measured the shear modulus of rosetting IRBCs, then using the dual micropipette we carefully peeled off the uninfected normocytes and repeated the measurement on the denuded IRBC. As the rosetting cells strongly bind to the IRBC, the separation process usually resulted the destruction of the IRBC. We were able to conduct 5 successful paired rosette separations, showing a significant reduction in the mean geometric shear modulus of the IRBC from 13.3pN (Rosetting) to 9.5pN (Non-Rosetting) (P<0.05, t = 2.8, df = 4(Paired t-test)).
Discussion
Plasmodium vivax, the most globally-widespread cause of human malaria, has a specific tropism for the rigid CD71+ve reticulocytes generally found in the bone marrow [14, 17]. Within six hours post invasion, P. vivax remodels the IRBC membrane and cytoskeleton, causing it to become as deformable as an uninfected normocyte [14, 18]. In contrast to P. falciparum, RBCs infected with trophozoite and early schizont stages of P. vivax retain a relatively low shear modulus (compared to reticulocytes and P. falciparum IRBCs), and are able to deform and pass through micro-capillaries and 2μm sinusoidal slits [16]. It is thought that P. vivax increases the deformability of the host cell to avoid splenic clearance [18].
Our results show that rosetting with at least one uninfected RBC is closely associated with a a significant increase in the rigidity of the P. vivax IRBCs. While it is difficult to demonstrate direct causality, we were able to demonstrate that the removal of rosetting RBCs, restores the deformability of the IRBC to the levels usually seen in non rosetting IRBCs.
It is Important to understand that these rosettes are stable even under shear stress, and on encountering microfluidic constrictions they not only block the restriction, but also retain their full complement of attached uninfected red cells. The only other P. vivax IRBCs that tend to block the microfluidic restrictions are very mature schizonts. Traditionally these very late stage schizonts are referred to as ‘segmenters’, because the merozoites are fully mature and clearly defined within the schizont complex. In P. falciparum, late stage asexual parasites become rigid due to a range of proteins such as RESA, KHARP, MESA, PfEMP3 and STEVOR interacting with the IRBC cytoskeleton and membrane[1, 19–23]. In P. vivax we do not understand the molecular basis driving the switch from a relatively deformable early schizonts, to a rigid segmenter. However, as this change occurs an hour or so before schizonts rupture; we speculate the rigidity in P. vivax segmenters is due to osmotic deregulation (as opposed to the incorporation of crosslinking proteins into the cytoskeleton) as the IRBC membrane degenerates prior to merozoite release. In any case, our study clearly demonstrates that segmenting schizonts and rosetting are the only events responsible for significant rigidity of the P. vivax IRBCs.
Recent studies in Brazilian individuals infected with P. vivax reveal a disparate and unexpected disappearance of schizonts from the circulation [24]. Although this may be partially due to cytoadherence to endothelial receptors expressed on the surface of the vascular endothelium [12], we suggest that the increased rigidity of segmenters and rosetting IRBCs is a major factor behind the paucity of P. vivax schizonts in the circulation. The ligands responsible for P. vivax rosetting remain unknown. The vir proteins of P. vivax have been associated with endothelial cytoadhesion [12].
While we still expect to see spontaneous rosette formation occurring in the circulation, our study suggests that a large proportion P. vivax rosettes will be sequestered. Although the incidence and rate of P. vivax rosetting is high, we are still unsure how this phenomenon contributes to the pathology of vivax malaria[25]. It is important to understand that while rosetting has been observed in most forms of human malaria[2–4, 26], we only have a a clear understanding of this process in P. falciparum. Future studies should strive to understand the pathobiological process behind non-falciparum and possible develop therapeutics that disrupt their formation[27, 28].
Supporting Information
Force required to dissociate the rosette was recorded.
(AVI)
The video showed the unblocked flow condition, where cells moved through the channel openings rapidly and identity of the cells (infected and uninfected) cannot be differentiated clearly from the video.
(AVI)
(AVI)
Other cells were seen passing through the channel opening.
(AVI)
Participating uninfected erythrocytes of the rosette did not detach from the blockade to move freely, showing the stability of the rosetting complex.
(AVI)
Acknowledgments
The authors would like to express gratitude to the staff of SMRU who assisted in the management of this study. Presented in part at the Australian Society for Parasitology (ASP) Annual Conference, Canberra, Australia, 30 June– 3 July 2014; Challenges in Malaria Research: Core Science & innovation, Oxford, UK, 22–24 September 2014; and the 12th NUS-Nagasaki Joint Symposium, Singapore, 11–12 June 2015. 4th Singapore Malaria Network Meeting, 18–19 February 2016.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study received financial supports from the following funds: WCL, RS and LR were supported by funding from SIgN, A*STAR. RZ and BR were funded by the National University of Singapore (AcRF Tier 1 FRC T1-2013 Apr 13) and a Singapore Ministry of Education Tier 2 Grant (MOE2013-T2-1-145). YLL was supported by University of Malaya High Impact Research (HIR) Grant (UM.C/HIR/MOHE/MED/16) from Ministry of Higher Education, Malaysia. SMRU is part of the Mahidol-Oxford University Research Unit, supported by the Wellcome Trust of the Great Britain. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cooke BM, Stuart J, Nash GB. The cellular and molecular rheology of malaria. Biorheology. 2014;51(2–3):99–119. 10.3233/BIR-140654 . [DOI] [PubMed] [Google Scholar]
- 2.Udomsangpetch R, Thanikkul K, Pukrittayakamee S, White NJ. Rosette formation by Plasmodium vivax. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1995;89(6):635–7. . [DOI] [PubMed] [Google Scholar]
- 3.Angus BJ, Thanikkul K, Silamut K, White NJ, Udomsangpetch R. Rosette formation in Plasmodium ovale infection. American Journal of Tropical Medicine and Hygiene. 1996;55(5):560–1. . [DOI] [PubMed] [Google Scholar]
- 4.Lowe BS, Mosobo MK, Bull PC. All four species of human malaria parasites form rosettes. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1998;92(5):526 . [DOI] [PubMed] [Google Scholar]
- 5.Rowe JA, Claessens A, Corrigan RA, Arman M. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Expert reviews in molecular medicine. 2009;11:e16 Epub 2009/05/27. 10.1017/s1462399409001082 ; PubMed Central PMCID: PMCPmc2878476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nash GB, Cooke BM, Carlson J, Wahlgren M. Rheological properties of rosettes formed by red blood cells parasitized by Plasmodium falciparum. British journal of haematology. 1992;82(4):757–63. . [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Barragan A, Fernandez V, Sundstrom A, Schlichtherle M, Sahlen A, et al. Identification of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) as the rosetting ligand of the malaria parasite P. falciparum. The Journal of experimental medicine. 1998;187(1):15–23. Epub 1998/01/31. ; PubMed Central PMCID: PMCPmc2199182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niang M, Bei AK, Madnani KG, Pelly S, Dankwa S, Kanjee U, et al. STEVOR is a Plasmodium falciparum erythrocyte binding protein that mediates merozoite invasion and rosetting. Cell host & microbe. 2014;16(1):81–93. Epub 2014/07/11. 10.1016/j.chom.2014.06.004 ; PubMed Central PMCID: PMCPmc4382205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goel S, Palmkvist M, Moll K, Joannin N, Lara P, Akhouri RR, et al. RIFINs are adhesins implicated in severe Plasmodium falciparum malaria. Nature medicine. 2015;21(4):314–7. Epub 2015/03/10. 10.1038/nm.3812 . [DOI] [PubMed] [Google Scholar]
- 10.Chotivanich KT, Pukrittayakamee S, Simpson JA, White NJ, Udomsangpetch R. Characteristics of Plasmodium vivax-infected erythrocyte rosettes. American Journal of Tropical Medicine and Hygiene. 1998;59(1):73–6. . [DOI] [PubMed] [Google Scholar]
- 11.Lee WC, Malleret B, Lau YL, Mauduit M, Fong MY, Cho JS, et al. Glycophorin C (CD236R) mediates vivax malaria parasite rosetting to normocytes. Blood. 2014;123(18):e100–9. 10.1182/blood-2013-12-541698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvalho BO, Lopes SC, Nogueira PA, Orlandi PP, Bargieri DY, Blanco YC, et al. On the cytoadhesion of Plasmodium vivax-infected erythrocytes. The Journal of infectious diseases. 2010;202(4):638–47. 10.1086/654815 . [DOI] [PubMed] [Google Scholar]
- 13.Cooke BM, Rogerson SJ, Brown GV, Coppel RL. Adhesion of malaria-infected red blood cells to chondroitin sulfate A under flow conditions. Blood. 1996;88(10):4040–4. . [PubMed] [Google Scholar]
- 14.Malleret B, Li A, Zhang R, Tan KS, Suwanarusk R, Claser C, et al. Plasmodium vivax: restricted tropism and rapid remodeling of CD71-positive reticulocytes. Blood. 2015;125(8):1314–24. 10.1182/blood-2014-08-596015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochmuth RM. Micropipette aspiration of living cells. Journal of biomechanics. 2000;33(1):15–22. . [DOI] [PubMed] [Google Scholar]
- 16.Handayani S, Chiu DT, Tjitra E, Kuo JS, Lampah D, Kenangalem E, et al. High deformability of Plasmodium vivax-infected red blood cells under microfluidic conditions. The Journal of infectious diseases. 2009;199(3):445–50. 10.1086/596048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malleret B, Xu F, Mohandas N, Suwanarusk R, Chu C, Leite JA, et al. Significant biochemical, biophysical and metabolic diversity in circulating human cord blood reticulocytes. PloS one. 2013;8(10):e76062 10.1371/journal.pone.0076062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suwanarusk R, Cooke BM, Dondorp AM, Silamut K, Sattabongkot J, White NJ, et al. The deformability of red blood cells parasitized by Plasmodium falciparum and P. vivax. The Journal of infectious diseases. 2004;189(2):190–4. 10.1086/380468 . [DOI] [PubMed] [Google Scholar]
- 19.Crabb BS, Cooke BM, Reeder JC, Waller RF, Caruana SR, Davern KM, et al. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell. 1997;89(2):287–96. . [DOI] [PubMed] [Google Scholar]
- 20.Maier AG, Rug M, O'Neill MT, Brown M, Chakravorty S, Szestak T, et al. Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell. 2008;134(1):48–61. 10.1016/j.cell.2008.04.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moxon CA, Grau GE, Craig AG. Malaria: modification of the red blood cell and consequences in the human host. British journal of haematology. 2011;154(6):670–9. Epub 2011/06/01. 10.1111/j.1365-2141.2011.08755.x ; PubMed Central PMCID: PMCPmc3557659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rug M, Prescott SW, Fernandez KM, Cooke BM, Cowman AF. The role of KAHRP domains in knob formation and cytoadherence of P falciparum-infected human erythrocytes. Blood. 2006;108(1):370–8. 10.1182/blood-2005-11-4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanyal S, Egee S, Bouyer G, Perrot S, Safeukui I, Bischoff E, et al. Plasmodium falciparum STEVOR proteins impact erythrocyte mechanical properties. Blood. 2012;119(2):e1–8. Epub 2011/11/23. 10.1182/blood-2011-08-370734 ; PubMed Central PMCID: PMCPmc3257022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopes SC, Albrecht L, Carvalho BO, Siqueira AM, Thomson-Luque R, Nogueira PA, et al. Paucity of Plasmodium vivax mature schizonts in peripheral blood is associated with their increased cytoadhesive potential. The Journal of infectious diseases. 2014;209(9):1403–7. 10.1093/infdis/jiu018 . [DOI] [PubMed] [Google Scholar]
- 25.Marín-Menéndez A, Bardají A, Martínez-Espinosa FE, Bôtto-Menezes C, Lacerda MVG, Ortiz J, et al. Rosetting in Plasmodium vivax: a cytoadhesion phenotype associated with anaemia. PLoS Neglected Tropical Diseases. 2013;7(4):e2155 10.1371/journal.pntd.0002155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.David PH, Handunnetti SM, Leech JH, Gamage CP, Mendis KN. Rosetting: a new cytoadherence property of malaria-infected erythrocytes. American Journal of Tropical Medicine and Hygiene. 1988;38(2):289–97. . [DOI] [PubMed] [Google Scholar]
- 27.Ch'ng JH, Moll K, Quintana Mdel P, Chan SC, Masters E, Moles E, et al. Rosette-Disrupting Effect of an Anti-Plasmodial Compound for the Potential Treatment of Plasmodium falciparum Malaria Complications. Sci Rep. 2016;6:29317 10.1038/srep29317 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang R, Suwanarusk R, Malleret B, Cooke BM, Nosten F, Lau YL, et al. A Basis for Rapid Clearance of Circulating Ring-Stage Malaria Parasites by the Spiroindolone KAE609. The Journal of infectious diseases. 2015. 10.1093/infdis/jiv358 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Force required to dissociate the rosette was recorded.
(AVI)
The video showed the unblocked flow condition, where cells moved through the channel openings rapidly and identity of the cells (infected and uninfected) cannot be differentiated clearly from the video.
(AVI)
(AVI)
Other cells were seen passing through the channel opening.
(AVI)
Participating uninfected erythrocytes of the rosette did not detach from the blockade to move freely, showing the stability of the rosetting complex.
(AVI)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.