Abstract
Purpose
Signet ring cell carcinoma (SRCC) is rare. The aim of this study is to understand the clinicopathological features and identify the possible prognostic factors in colorectal SRCC.
Methods
Patients with SRCC who underwent primary lesion resection at Fudan University Shanghai Cancer Center from September 2008 to July 2014 were retrospectively analyzed. Patient’s gender, age, tumor location, depth of invasion, lymph node metastasis, synchronous distant metastasis, perineural invasion, lymphovascular invasion, and E-cadherin expression were studied with prognosis, and the correlation between E-cadherin expression and clinicopathological features were analyzed. All clinicopathological and molecular factors were put into multivariate analysis using Cox proportional hazards model for detecting independent prognostic factors.
Results
59 patients accounting for 0.89% of total colorectal cancer patients met the criteria and were enrolled in the study. The median survival time is 28.9 months, and the 3-year survival rate is 62.7%. SRCC were seen more common in young male patients. Advanced stage was more common in SRCC, 58 (98.3%) patients had T3/T4 lesions, 52 (88.1%) patients had lymph node metastasis, and 14 (23.7%) patients had distant metastasis. Distant metastases were seen more common in peritoneal cavity. Distant metastasis (HR = 4.194, 95% CI: 1.297–13.567), lymphovascular invasion (HR = 2.888, 95% CI: 1.115–7.483), and E-cadherin expression (HR = 0.272, 95% CI: 0.096–0.768) were independent predictors for survival.
Conclusions
SRCC is a rare subtype of colorectal cancer with poor prognosis. Distant metastasis, lymphovascular invasion, and E-cadherin expression can predict prognosis of colorectal SRCCs independently. More precise therapy and more close surveillance are needed for these patients.
Introduction
Signet ring cell carcinoma (SRCC) is a rare type of adenocarcinoma, which is characterized by specific morphologic appearance of abundant intracytoplasmic mucin pushing nucleus to periphery and giving it a signet ring-like appearance. The World Health Organization classification of tumors has a clear definition for diagnosis of this subtype: SRCC is defined as presence of more than 50% of signet ring cells[1]. SRCCs are most commonly seen in stomach (95%) and occasionally found in colon, rectum, ovary, peritoneum, and gallbladder. SRCC in colorectum is rare, and the first case was reported by Laufman and Saphir in 1951[2]. Because of its rarity, the characteristics of this subtype are seldom described in detail. Till now, most of the reported articles are case reports or small series, and few investigations have explained the clinical behavior of this subtype. At present, it is generally acknowledged that colorectal SRCC has poorer prognosis and treatment response than conventional adenocarcinoma. The reported 5-year survival rate varied from 0% to 31%, and the median survival time was 15–45 months[3–11]. In fact, most of patients with colorectal SRCC were already in stage III or IV at diagnosis, and the reported percentage of patients diagnosed at early stage was only around 5%[11]. So clarifying SRCC’s clinicopathological features and identifying possible prognostic factors are essential in order to improve early detection, treatment and surveillance for this distinctive phenotype. E-cadherin is a cadherin family member and a calcium-dependent cell-to-cell adhesion molecule found mainly in epithelial tissue. It is thought to implicate embryogenesis, cellular migration, and cellular differentiation or dedifferentiation[12]. Many investigators have suggested the suppressor role of E-cadherin in tumor invasion[13]. Loss or diminished E-cadherin expression has been demonstrated in many epithelial cancers[14–16]. However, its function in colorectal SRCC has not been studied yet. Hence, we conducted a retrospective study on colorectal SRCC patients in our hospital to elucidate the clinicopathological features of colorectal SRCC and to identify the clinical correlation and possible prognostic factors including biological characteristics of E-cadherin in colorectal SRCC.
Materials and Methods
Patients
Patients with SRCC who underwent primary lesion resection at Fudan University Shanghai Cancer Center from September 2008 to July 2014 were reviewed in this study. Written informed consent was obtained from all study participants adhering to the local ethical guidelines prior to specimen collection. The study protocol and consent procedure were approved by the Ethics Committee of Fudan University Shanghai Cancer Center. All the patients met the criteria as follows: (1) the presence of signet ring cells in > 50% of total tumor cells; (2) having complete medical records including demographic information, clinical and pathological data, operation notes, and follow-up results; (3) having at least 6 months follow-up time after the operation. Patients who (1) had preoperative chemoradiotherapy before operation; (2) had other histological types including adenosquamous carcinoma, squamous carcinoma, neuroendocrine tumor, clear-cell carcinoma, spindle cell carcinoma, and anaplastic carcinoma; (3) had incomplete paraffin block were all excluded from the study. The cancer staging was based on the American Joint Committee on Cancer 7th edition. Patient’s gender, age, tumor location, depth of invasion, lymph node metastasis, distant metastasis, perineural invasion, lymphovascular invasion, and E-cadherin expression were analyzed in this study.
Immunohistochemial staining and interpretation
Tissues were embedded in paraffin. Sections were cut in 4 μm, dewaxed in xylene, and rehydrated in decreasing concentrations of ethanol. Prior to staining, sections were subjected to endogenous peroxidase blocking in 1% of H2O2 solution for 20 min and then to antigen retrieval treatment in 10 mM citrate buffer (pH 6.0) and in 95°C water bath for 40 min. Serum blocking was performed using 5% BSA for 20 min. Primary antibodies against E-cadherin (Clone NCH-38, Dako, Glostrup, Denmark) were incubated overnight at 4°C at 1:250 dilution. After washing, sections were incubated with mouse and horseradish-peroxidase-labeled streptavidin, respectively (1:200 dilution each). Diaminobenzidine hydrochloride (DAB) was used as the chromogen. Finally, they were counterstained with hematoxylin.
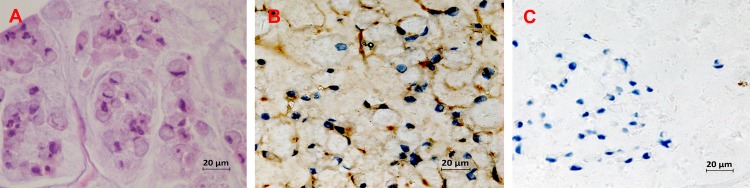
We randomly selected 10 fields at a high power magnification for evaluation. Generally, we applied a two grade scoring (negative or positive) for the expression of E-cadherin. We focused on the membranous staining pattern, and cytoplasmic or nuclear staining was not noted. Staining was scored as positive when immunoreactivity in the tumor region showed a similar membranous staining to its normal counterpart in more than 25% of the cells. Absence of membranous staining or positive immunoreactivity in less than 25% of the cells was graded as negative (Fig 1).
Fig 1.
Expression of E-cadherin in colorectal SRCC tissues (A) Hematoxylin and eosin staining; Examples of Immunohistochemical staining of E-cadherin at positive expression (B) and negative expression (C).
Statistical analysis
The clinicopathological features and E-cadherin expression status were analyzed using survival curves. Survival curves were generated by the Kaplan-Meier method, and univariate survival distributions were compared using log-rank test. The χ2 test was used to evaluate the statistical significance of the association of E-cadherin expression with patients’ clinicopathological parameters. All clinicopathological and molecular factors were included in multivariate analysis by using Cox proportional hazards model. The 2-tailed p value for significance was established at 0.05. All statistical analysis was performed by SPSS, version 19.0.0 (SPSS, Inc., Chicago, IL, USA).
Results
Clinicopathological characteristics
A total of 59 patients (38 men and 21 women) with colorectal SRCC met the criteria and were enrolled in the study, which accounts for 0.89% (59/6625) of the total resected colorectal cancer in our center. The median age of the diagnosis of SRCC was 46 y (range, 24–82 y). 58 (98.3%) patients had T3/T4 lesions, 52 (88.1%) patients had lymph node metastasis, and 14 (23.7%) patients had distant metastases. Among 14 patients with synchronous metastases, 5 (35.7%) patients had liver metastases only, 8 (57.1%) patients had diffusive intro-abdominal peritoneal metastases, and 1 (7.2%) patient had ovarian metastasis. With a median follow-up time of 19 months (range, 3.1–49.2 months), the median overall survival time is 28.9 months for all patients; the 3-year overall survival rate is 62.7%. The detailed clinicopathological characteristics are shown in Table 1.
Table 1. Patients' clinicopathologic features and survival (Kaplan-Meier).
| Variables | N(%) | 3-year survival rate(%) | P |
|---|---|---|---|
| Age | 0.871 | ||
| <40 | 16(27.1) | 43.0 | |
| ≥40 | 43(72.9) | 50.0 | |
| Gender | 0.702 | ||
| Male | 38(64.4) | 49.4 | |
| Female | 21(35.6) | 53.6 | |
| Location* | 0.475 | ||
| Distal | 45(76.3) | 42.8 | |
| Proximal | 14(23.7) | 70.1 | |
| Invasive depth | 0.896 | ||
| T1-T2 | 1(1.7) | 100 | |
| T3-T4 | 58(98.3) | 49.7 | |
| Lymph nodes metastasis | 0.691 | ||
| Yes | 52(88.1) | 47.4 | |
| No | 7(11.9) | 66.7 | |
| Distant metastasis | 0.005 | ||
| Yes | 14(23.7) | 16.1 | |
| No | 45(76.3) | 60.8 | |
| Perineural invasion | 0.475 | ||
| Yes | 43(72.9) | 45.2 | |
| No | 16(27.1) | 73.1 | |
| Lymphovascular invasion | 0.049 | ||
| Yes | 26(44.1) | 33.3 | |
| No | 33(55.9) | 63.5 | |
| Ecadherin expression | 0.010 | ||
| (+) | 23(39.0) | 75.0 | |
| (-) | 36(61.0) | 32.9 |
*Distal location includes descending colon, sigmoid colon and rectum; Proximal location includes cecum,ascending colon and transverse colon; P is calculated using unadjusted log-rank test.
By immunohistochemical staining, 23 (39.0%) patients were scored as E-cadherin positive, while 36 (61.0%) patients were scored as E-cadherin negative. E-cadherin expression was not related to patients’ clinicopathological features (Table 2).
Table 2. Patients’ clinicopathologic features and E-cadherin expression.
| Variables | N | E-cadherin expression | P | |
|---|---|---|---|---|
| negative (n = 36) | positive (n = 23) | |||
| Age | 0.371 | |||
| <40 | 16 | 8 | 8 | |
| ≥40 | 43 | 28 | 15 | |
| Gender | 0.405 | |||
| Male | 38 | 25 | 13 | |
| Female | 21 | 11 | 10 | |
| Location* | 0.734 | |||
| Distal | 45 | 28 | 17 | |
| Proximal | 14 | 8 | 6 | |
| Invasive depth | 1.000 | |||
| T1-T2 | 1 | 1 | 0 | |
| T3-T4 | 58 | 35 | 23 | |
| Lymph nodes metastasis | 0.899 | |||
| Yes | 52 | 32 | 20 | |
| No | 7 | 4 | 3 | |
| Distant metastasis | 0.532 | |||
| Yes | 14 | 10 | 4 | |
| No | 45 | 26 | 19 | |
| Perineural invasion | 0.647 | |||
| Yes | 43 | 27 | 16 | |
| No | 16 | 9 | 7 | |
| Lymphovascular invasion | 0.942 | |||
| Yes | 26 | 16 | 10 | |
| No | 33 | 20 | 13 | |
*Distal location includes descending colon, sigmoid colon and rectum; Proximal location includes cecum,ascending colon and transverse colon; P is based on χ2 test/Fisher’s exact test.
Survival analyses
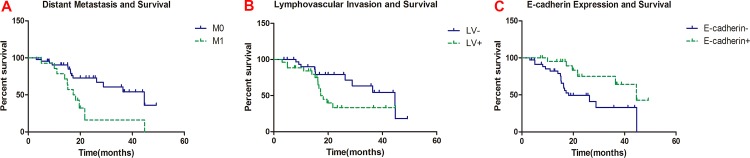
The clinicopathological characteristics and E-cadherin expression were included in univariate analysis. Distant metastasis (p = 0.005), lymphovascular invasion (p = 0.049), and E-cadherin expression (p = 0.010) were found related to patients’ overall survival (Table 1, Fig 2). All clinicopathological and molecular variables were included in multivariate analysis. The result showed that distant metastasis (HR = 4.194, 95% CI: 1.297–13.567), lymphovascular invasion (HR = 2.888, 95% CI: 1.115–7.483), and E-cadherin expression (HR = 0.272, 95% CI: 0.096–0.768) were independent prognostic factors for patients’ overall survival (Table 3).
Fig 2. Patient’s clinicopathologic features/E-cadherin expression and survival.
(A) Distant metastasis and survival;(B) Lymhovascular invasion and survival; (C) E-cadherin expression and survival.
Table 3. Multivariate analysis of survival in colorectal SRCC.
| Factor | HR | P | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| Gender | 1.547 | 0.439 | 0.512 | 4.675 |
| Age | 1.506 | 0.509 | 0.447 | 5.077 |
| Location | 0.657 | 0.508 | 0.189 | 2.281 |
| Lymph node metastasis | 1.289 | 0.789 | 0.201 | 8.246 |
| Distant metastasis | 4.194 | 0.017 | 1.297 | 13.567 |
| Perineural invasion | 1.695 | 0.411 | 0.482 | 5.962 |
| Lymphovascular invasion | 2.888 | 0.029 | 1.115 | 7.483 |
| E-cadherin expression | 0.272 | 0.014 | 0.096 | 0.768 |
Abbreviation: HR,harzard ratio; CI, confidence interval; P is based on Cox regression test.
Discussion
Colorectal SRCC is a rare disease, which comprises approximately 0.1–2.6% of all colorectal malignancies[7–10]. In our surgical series, SRCC comprised 0.89% (59/6625) of the resected colorectal cancer, being 0.90% (27/3009) of colon cancer and 0.88% (32/3616) of rectal cancer, which was comparable to the reported incidence in Western countries.
SRCC was reported more prevalent in patients at younger age (≤ 40 y) than conventional colorectal adenocarcinoma[4,6,17,18]. In our study, the median age of colorectal SRCC was 46 years old, and 66% of patients were younger than 40 years old, which is obviously higher than conventional colorectal adenocarcinoma. Some studies found that colorectal SRCC is slightly more prevalent in women than in men[7,19], while others demonstrated a clear male predominance[9,20–23]. In this study, we observed more male SRCC patients than female, with a ratio of 1.5: 1. More reported colorectal SRCC cases are localized exclusively in the rectum[8,10,17,19] whereas some studies reported a right-sided predominance[9,23]. In our study, more SRCCs were located in rectum, but all sites were involved and the distribution of cases between colon and rectum was similar.
Advanced stage is more common in SRCC, and most patients develop lymph node metastasis or distant metastasis when diagnosed[7–10]. In our study, almost all cases (98.2%) were T3 and T4, and only 1 case was T2. SRCCs are considered to have early invasion; possibly, this is because early SRCCs are usually asymptomatic and are normally a tumor arising in flat colonic mucosa but not following the adenocarcinoma sequence[24]. Thus, it is difficult to find SRCC by routine colonoscopy at early stage and hard to obtain a definitive diagnosis through biopsy. In our series, most cases (89%) had lymph node metastasis, and among them, 72.7% cases had 4 or more than 4 lymph node metastasis. This metastatic rate was consistent with previous studies[7–10]. Loss of early detection could partly explain the high rate of lymph node metastasis, and other reasons can be explained by SRCCs’ unique metastatic routes. In our study, 14 patients had distant metastasis. Among them, 5 (35.7%) patients had liver metastases, 8 (57.1%) patients had diffusive intro-abdominal peritoneal metastases, and 1 (7.1%) patient had ovarian metastasis. We also found distant metastasis was a significant and independent predictor for prognosis; median survival for patients with distant metastasis was only 17.3 months compared with patients without distant metastasis (44.6 months). As we have known, in conventional adenocarcinoma, liver metastasis and lung metastasis accounts for nearly 90% of total distant metastasis. However, in SRCC, distant metastasis in peritoneal cavity is more frequent. In gastric cancer, signet ring cell carcinoma also tends to develop peritoneal metastasis than other types more frequently[25]. Likewise, Pende et al. found that conventional colorectal adenocarcinoma containing signet ring cell component had a higher rate of peritoneal metastasis[26]. So, high incidence of peritoneal metastasis and a relatively low incidence of liver and lung metastasis are characteristic features of SRCC. Since liver/lung metastasis may still have chances for radical resection, whereas diffusive peritoneal metastasis means loss of surgical chances, this may lead to poor prognosis for SRCC.
In conventional adenocarcinoma, the percentage for lymphovascular invasion is around 20%, in our study, 26 (44.1%) patients had lymphovascular invasion, the median survival for LV+ patients were 17.3 months compared with LV- patients (44.6 months), and lymphovascular invasion was found to be an independent predictor for prognosis. So, the percentage of lymphovascular invasion in SRCC is higher than conventional adenocarcinoma. Some articles have already reported that lymphovascular invasion was a prognostic variable in conventional adenocarcinoma[27,28]. Since lymphovascular invasion means invasion of tumor cells into lymph or blood vessels, which is important in metastatic process, and SRCC has a high rate of metastasis, we have reasons to believe that lymphovascular invasion can be a good factor for predicting prognosis in colorectal SRCC.
E-cadherin plays a crucial role in cell-to-cell adhesion and maintaining epithelial morphology. Reduced expression of E-cadherin due to aberrant hypermethylation is important for metastases in multiple cancers. In conventional colorectal adenocarcinoma, the rate of loss of E-cadherin expression was reported between 13.0%-41.7% and down-regulated E-cadherin expression predicted worse prognosis[29–32]. In our study, the rate of loss of E-cadherin expression (61.0%) was higher than that reported in conventional adenocarcinoma. Survival analysis found the median survival time for E-cadherin- patients were 18.2 months compared with 44.6 months in E-cadherin+ patients. Multivariate analysis confirmed that loss of E-cadherin expression was a significant and independent predictor for poor prognosis. E-cadherin, a 120-kd transmembrane glycoprotein, is a calcium-dependent cell adhesion molecule involved in inducing and maintaining the epithelial cell polarity[33,34]. The cytoplasmic tail of E-cadherin binds to catenins and the formation of E-cadherin and β-catenin complexes is critical for epithelial cell functions and tissue integrity[35,36]. Loss of E-cadherin/catenin complex has been regarded as the cause of loss of epithelial differentiation or architecture and acquisition of a motile and invasive phenotype[37], which may allow certain cancer cells detached from the surrounding structure and become more infiltrative and metastasizing. Moreover, Wnt signaling pathway is the cause of approximately 93% of colorectal cancer[38] and β-catenin is a critical component of the Wnt signal pathway. The upregulation of Wnt signal pathway induces nuclear translocation of β-catenin and aberrant target gene expression[39,40]. Downregulation of E-cadherin may affect the decreased membrane expression of β-catenin and resulting in its nuclear shifting which may also enhance Wnt signal pathway and give rise to aberrant proliferation.
In our series, the overall 3-year survival rate was 62.7%, with a median survival of 28.9 months, which was significantly worse in comparison to the rates of conventional adenocarcinoma and was in accordance with previous studies[7–10]. Otherwise, we found that traditional prognostic variables such as depth of invasion and lymph node metastasis were not related to prognosis, this may partly because most cases were T3-T4 and N+, and there were too few early cases.
There are still limitations in our study. This is a retrospective study and some patients were excluded because of loss of follow-up or complete records. The adjuvant chemotherapy or radiation therapy was not completely disciplined because of the long study periods. And the follow-up periods were still not long enough for drawing a popular 5-year survival. Despite these limitations, our study represented one of the largest retrospective studies of colorectal SRCC.
In conclusion, colorectal SRCC is a rare type of colorectal cancer and is more commonly seen in young male patients. SRCC has poorer prognosis compared with conventional adenocarcinoma. Loss of E-cadherin expression was more common in colorectal SRCC and was one of important prognostic factors for predicting patients’ overall survival. Further studies were required to clarify the value and mechanism of E-cadherin expression in colorectal SRCC.
Supporting Information
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by grants from the National Natural Science Foundation of China(No.81101806) and Science and Technology Commission of Shanghai Municipality(No.134119a8602).
References
- 1.Bosman FT, World Health Organization and International Agency for Research on Cancer. WHO classification of tumours of the digestive system. International Agency for Research on Cancer; Lyon2010.
- 2.Laufman H, Saphir O. Primary linitis plastica type of carcinoma of the colon. AMA archives of surgery. 1951;62(1):79–91. [DOI] [PubMed] [Google Scholar]
- 3.Lee HS, Soh JS, Lee S, Bae JH, Kim KJ, Ye BD, et al. Clinical Features and Prognosis of Resectable Primary Colorectal Signet-Ring Cell Carcinoma. Intestinal research. 2015;13(4):332–8. 10.5217/ir.2015.13.4.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belli S, Aytac HO, Karagulle E, Yabanoglu H, Kayaselcuk F, Yildirim S. Outcomes of surgical treatment of primary signet ring cell carcinoma of the colon and rectum: 22 cases reviewed with literature. International surgery. 2014;99(6):691–8. 10.9738/INTSURG-D-14-00067.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inamura K, Yamauchi M, Nishihara R, Kim SA, Mima K, Sukawa Y, et al. Prognostic significance and molecular features of signet-ring cell and mucinous components in colorectal carcinoma. Annals of surgical oncology. 2015;22(4):1226–35. 10.1245/s10434-014-4159-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hugen N, Verhoeven RH, Lemmens VE, van Aart CJ, Elferink MA, Radema SA, et al. Colorectal signet-ring cell carcinoma: benefit from adjuvant chemotherapy but a poor prognostic factor. International journal of cancer Journal international du cancer. 2015;136(2):333–9. [DOI] [PubMed] [Google Scholar]
- 7.Secco GB, Fardelli R, Campora E, Lapertosa G, Gentile R, Zoli S, et al. Primary mucinous adenocarcinomas and signet-ring cell carcinomas of colon and rectum. Oncology. 1994;51(1):30–4. [DOI] [PubMed] [Google Scholar]
- 8.Nissan A, Guillem JG, Paty PB, Wong WD, Cohen AM. Signet-ring cell carcinoma of the colon and rectum: a matched control study. Diseases of the colon and rectum. 1999;42(9):1176–80. [DOI] [PubMed] [Google Scholar]
- 9.Ooi BS, Ho YH, Eu KW, Seow Choen F. Primary colorectal signet-ring cell carcinoma in Singapore. ANZ journal of surgery. 2001;71(12):703–6. [DOI] [PubMed] [Google Scholar]
- 10.Makino T, Tsujinaka T, Mishima H, Ikenaga M, Sawamura T, Nakamori S, et al. Primary signet-ring cell carcinoma of the colon and rectum: report of eight cases and review of 154 Japanese cases. Hepato-gastroenterology. 2006;53(72):845–9. [PubMed] [Google Scholar]
- 11.Thota R, Fang X, Subbiah S. Clinicopathological features and survival outcomes of primary signet ring cell and mucinous adenocarcinoma of colon: retrospective analysis of VACCR database. Journal of gastrointestinal oncology. 2014;5(1):18–24. 10.3978/j.issn.2078-6891.2013.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251(5000):1451–5. [DOI] [PubMed] [Google Scholar]
- 13.Nigam AK, Savage FJ, Boulos PB, Stamp GW, Liu D, Pignatelli M. Loss of cell-cell and cell-matrix adhesion molecules in colorectal cancer. British journal of cancer. 1993;68(3):507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin Y, Tang B, Hu CJ, Xiao YF, Xie R, Yong X, et al. An hTERT/ZEB1 complex directly regulates E-cadherin to promote epithelial-to-mesenchymal transition (EMT) in colorectal cancer. Oncotarget. 2016;7(1):351–61. 10.18632/oncotarget.5968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farmakovskaya M, Khromova N, Rybko V, Dugina V, Kopnin B, Kopnin P. E-Cadherin repression increases amount of cancer stem cells in human A549 lung adenocarcinoma and stimulates tumor growth. Cell cycle. 2016:0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Qin X, Chai S, Qu C, Wang X, Zhang H. Modulation of E-cadherin expression promotes migration ability of esophageal cancer cells. Scientific reports. 2016;6:21713 10.1038/srep21713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bittorf B, Merkel S, Matzel KE, Wein A, Dimmler A, Hohenberger W. Primary signet-ring cell carcinoma of the colorectum. Langenbeck's archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2004;389(3):178–83. [DOI] [PubMed] [Google Scholar]
- 18.Gopalan V, Smith RA, Ho YH, Lam AK. Signet-ring cell carcinoma of colorectum—current perspectives and molecular biology. International journal of colorectal disease. 2011;26(2):127–33. 10.1007/s00384-010-1037-z [DOI] [PubMed] [Google Scholar]
- 19.Giacchero A, Aste H, Baracchini P, Conio M, Fulcheri E, Lapertosa G, et al. Primary signet-ring carcinoma of the large bowel. Report of nine cases. Cancer. 1985;56(11):2723–6. [DOI] [PubMed] [Google Scholar]
- 20.Tung SY, Wu CS, Chen PC. Primary signet ring cell carcinoma of colorectum: an age- and sex-matched controlled study. The American journal of gastroenterology. 1996;91(10):2195–9. [PubMed] [Google Scholar]
- 21.Almagro UA. Primary signet-ring carcinoma of the colon. Cancer. 1983;52(8):1453–7. [DOI] [PubMed] [Google Scholar]
- 22.Anthony T, George R, Rodriguez-Bigas M, Petrelli NJ. Primary signet-ring cell carcinoma of the colon and rectum. Annals of surgical oncology. 1996;3(4):344–8. [DOI] [PubMed] [Google Scholar]
- 23.Messerini L, Palomba A, Zampi G. Primary signet-ring cell carcinoma of the colon and rectum. Diseases of the colon and rectum. 1995;38(11):1189–92. [DOI] [PubMed] [Google Scholar]
- 24.Ponz de Leon M, Di Gregorio C. Pathology of colorectal cancer. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2001;33(4):372–88. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Kim S, Lai JF, Hyung WJ, Choi WH, Choi SH, et al. Advanced gastric carcinoma with signet ring cell histology. Oncology. 2007;72(1–2):64–8. [DOI] [PubMed] [Google Scholar]
- 26.Pande R, Sunga A, Levea C, Wilding GE, Bshara W, Reid M, et al. Significance of signet-ring cells in patients with colorectal cancer. Diseases of the colon and rectum. 2008;51(1):50–5. [DOI] [PubMed] [Google Scholar]
- 27.Zhang CD, Wang JN, Sui BQ, Zeng YJ, Chen JQ, Dai DQ. Prognostic and Predictive Model for Stage II Colon Cancer Patients With Nonemergent Surgery: Who Should Receive Adjuvant Chemotherapy? Medicine. 2016;95(1):e2190 10.1097/MD.0000000000002190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parnaby CN, Scott NW, Ramsay G, MacKay C, Samuel L, Murray GI, et al. Prognostic value of lymph node ratio and extramural vascular invasion on survival for patients undergoing curative colon cancer resection. British journal of cancer. 2015;113(2):212–9. 10.1038/bjc.2015.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ngan CY, Yamamoto H, Seshimo I, Ezumi K, Terayama M, Hemmi H, et al. A multivariate analysis of adhesion molecules expression in assessment of colorectal cancer. Journal of surgical oncology. 2007;95(8):652–62. [DOI] [PubMed] [Google Scholar]
- 30.Seo KJ, Kim M, Kim J. Prognostic implications of adhesion molecule expression in colorectal cancer. International journal of clinical and experimental pathology. 2015;8(4):4148–57. [PMC free article] [PubMed] [Google Scholar]
- 31.Kang H, Min BS, Lee KY, Kim NK, Kim SN, Choi J, et al. Loss of E-cadherin and MUC2 expressions correlated with poor survival in patients with stages II and III colorectal carcinoma. Annals of surgical oncology. 2011;18(3):711–9. 10.1245/s10434-010-1338-z [DOI] [PubMed] [Google Scholar]
- 32.Roca F, Mauro LV, Morandi A, Bonadeo F, Vaccaro C, Quintana GO, et al. Prognostic value of E-cadherin, beta-catenin, MMPs (7 and 9), and TIMPs (1 and 2) in patients with colorectal carcinoma. Journal of surgical oncology. 2006;93(2):151–60. [DOI] [PubMed] [Google Scholar]
- 33.McNeill H, Ozawa M, Kemler R, Nelson WJ. Novel function of the cell adhesion molecule uvomorulin as an inducer of cell surface polarity. Cell. 1990;62(2):309–16. [DOI] [PubMed] [Google Scholar]
- 34.Behrens J, Mareel MM, Van Roy FM, Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. The Journal of cell biology. 1989;108(6):2435–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nollet F, Berx G, van Roy F. The role of the E-cadherin/catenin adhesion complex in the development and progression of cancer. Molecular cell biology research communications: MCBRC. 1999;2(2):77–85. [DOI] [PubMed] [Google Scholar]
- 36.Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. The EMBO journal. 1989;8(6):1711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanby AM, Chinery R, Poulsom R, Playford RJ, Pignatelli M. Downregulation of E-cadherin in the reparative epithelium of the human gastrointestinal tract. The American journal of pathology. 1996;148(3):723–9. [PMC free article] [PubMed] [Google Scholar]
- 38.Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–7. 10.1038/nature11252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morin PJ. beta-catenin signaling and cancer. BioEssays: news and reviews in molecular, cellular and developmental biology. 1999;21(12):1021–30. [DOI] [PubMed] [Google Scholar]
- 40.Barker N, Clevers H. Catenins, Wnt signaling and cancer. BioEssays: news and reviews in molecular, cellular and developmental biology. 2000;22(11):961–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.