Abstract
As a novel experimental animal model, tree shrews have received increasing attention in recent years. Despite this, little is known in regards to the time phases of their embryonic development. In this study, surveillance systems were used to record the behavior and timing of copulations; embryos at different post-copulation stages were collected and cultured in vitro; and the developmental characteristics of both early-stage and in vitro cultured embryos were determined. A total of 163 females were collected following effective copulation, and 150 were used in either unilateral or bilateral oviduct embryo collections, with 307 embryos from 111 females obtained (conception rate=74%). Among them, 237 embryos were collected from 78 females, bilaterally, i.e., the average embryo number per female was 3.04; 172 fertilized eggs collected from 55 females, bilaterally, were cultured for 24-108 h in vitro for developmental observations; finally, 65 embryos from 23 bilateral cases and 70 embryos from 33 unilateral cases were used in embryo transplantation.
Keywords: Tree shrew, Embryonic developmental stage, Embryo culture
INTRODUCTION
Tree shrews (Tupaia belangeri) are currently placed in Order Scandentia. However, their taxonomic status has received considerable attention in recent years due to the unresolved debate regarding the phylogenetic relationship of tree shrews to primates. In 2013, in collaboration with BGI-Shenzhen, China, and other research institutions, the Kunming Institute of Zoology (KIZ) from the Chinese Academy of Sciences (CAS) released a high-quality genome sequence and annotation of the Chinese tree shrew (Tupaia belangeri chinensis). This provided genetic evidence regarding the taxonomic status and biological characteristics of the tree shrew and clarified the close relationship between tree shrews and primates (Zheng et al., 2014). The tree shrew has been used as an animal model over several decades. Compared with non-human primates, the tree shrew features a small adult body and short reproductive cycle, and can, therefore, partially replace non-human primates in biomedical research (Cao et al., 2003), including studies on HBV infection (Pang et al., 2014), H1N1 infection (Yang et al., 2013), depression (Wang et al., 2012), drug addiction (Sun et al., 2012), and breast cancer (Xia et al., 2012).
Understanding embryonic developmental phases has critical meaning in promoting the embryonic development and transgenic study of tree shrews. In the present research, to determine effective copulation, surveillance systems were applied to record animal behaviors. In addition, to observe the developmental stages in vitro, embryos at different developmental stages were collected and cultured from adult females after copulation.
MATERIALS AND METHODS
Experimental animals
Healthy, adult male (n=16) and female tree shrews (n=163) (average body weight=131 g) were provided by the Kunming Primate Research Center, KIZ, CAS. The animal rearing room was maintained at an illumination of 150-300 LX, light: dark cycle of 12 h:12 h (light: 0730-1930h), and noise level below 50 dB. The surveillance system was an infrared all-in-one video camera (2×107 pixel, Sunell Technology, Co., Ltd., Shenzhen, China). All experimental protocols were approved by the Animal Ethics Committee of KIZ, CAS.
Animal copulation
Each male tree shrew was caged with one female. All 16 cages were under surveillance with four sets of video cameras. Copulation behaviors were determined the following morning based on recorded images. The females that had copulated were replaced by new females.
Embryo collection and embryonic developmental stage observation
Fertilized eggs were collected from females that had effectively copulated. The tree shrews were anaesthetized with ketamine (0.2 mg/g, intramuscular injection). After shaving and skin sterilization, the abdominal cavity and reproductive system were exposed along the medioventral line. The oviduct, uterus and adnexa were collected, rinsed with 37 ℃ pre-warmed saline water and then twice rinsed with M2 culture fluid. The oviduct and uterus were separated under stereomicroscope using pointed tweezers to tear the oviduct and squeeze out the fertilized eggs. The uterus was rinsed with M2 culture fluid, and the collected embryos were placed in TCM199 culture fluid for future observation.
In vitro embryo culture
The culture medium used was TCM199 culture fluid with 20% fetal bovine serum. Fluid drops (20 uL) were prepared the night before culture. The fluid drops were covered with paraffin oil and balanced in an incubator. The collected embryos were rinsed once with TCM199 culture fluid and then placed in the pre-balanced fluid drops. Each fluid drop contained 2-5 embryos and was cultured in the incubators (37 ℃, 5% CO2) for future observation.
Embryo transplantation
Either bilateral allotransplantation (each receptor received 4-6 embryos) or unilateral autotransplantation (each receptor received 2-3 embryos) was conducted. The operation procedure was referenced on the transplantation procedure used in mice. After skin preservation and sterilization, the abdominal cavity and oviduct were exposed along the medioventral line. The peritoneal sheath was torn and transplantation was conducted at the morsus diaboli.
RESULTS
Because no obvious features (e.g., copulatory plug) can be found after copulation in tree strews, the timing of copulation can be difficult to determine. Therefore, a surveillance system was used to record both the behavior and timing of copulation in the present study. Effective copulation was considered only when the male presented at least three times with crawling or straddling, accompanied with copulatory behaviors and elongation of the genitals for over five minutes. Short copulatory behaviors were considered ineffective. The copulations of tree shrews were monitored from December 2014 to November 2015. Of the 150 cases of effective copulation, only four occurred in December, after which successful copulations increased and peaked from April, May and June (32, 34 and 39, respectively). Copulations decreased from July and no copulations were observed during October or November (Figure 1A). Of the 150 females that effectively copulated, embryos were obtained from 111, resulting in a conception ratio of 74% (111/150). No conceptions were found in females after ineffective copulation, with dissections in 15 animals finding no embryos. These results indicate that the criteria established to differentiate successful/failed copulations in tree shrews were objective and reliable. From January to March, embryos were obtained in approximately 65% of tree shrews; from April to June, the number of embryos obtained peaked at 85%; in July, the number of embryos obtained decreased to 50%, and in August and September dropped to 0%, even with effective copulation. No copulations were observed from October to November. In December, the rate of embryos obtained reached 100%; however, due to the small number (n=4) of copulations, statistical randomness could not be ruled out, and therefore this high rate was not considered typical tree shrew copulatory behavior for December (Figure 1B). The average monthly amount of obtained embryos from each female was compared (Figure 1C). The copulations were concentrated during the daytime and ceased at night. Copulation occurred from 0700-0900h in 24 cases, 0900-1100h in 12 cases, 1100-1300h in 35 cases, 1300-1500h in 29 cases, 1500-1700h in 21 cases, 1500-1900h in 26 cases, and 1900-2000h in 3 cases, with no cases observed from 2000-0700h (Figure 1D).
Figure 1.
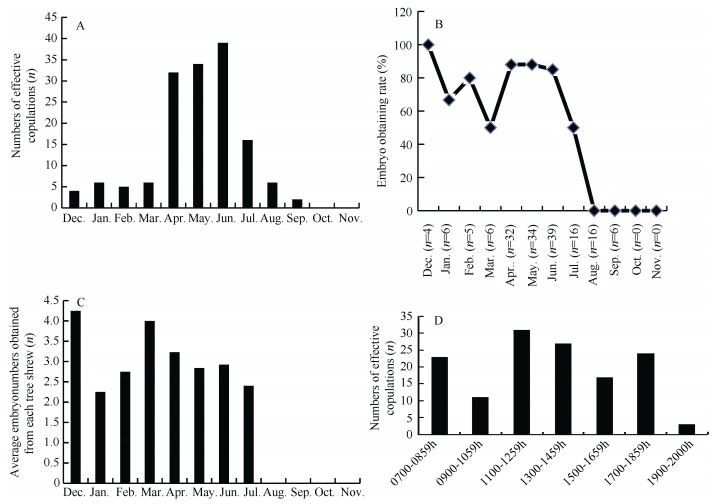
Copulation characteristics of tree shrews
Embryo collection was performed 22-80 h post-effective copulation in 150 tree shrews. For the early-stage embryos cultured in vitro, embryo collections were performed on females 22-34 h post-copulation. Among the 307 embryos obtained from the 111 females after effective copulation, 237 from 78 females were collected from bilateral oviducts (3.04 embryos per female) and 70 from 33 females were collected from unilateral oviducts. At 22-28 h post-copulation, 96% of collected eggs were at the 1-cell stage; at 29-34 h post-copulation, most eggs were at the 1-cell and 2-cell stages; at 41-50 h post-copulation, 71% of the collected eggs were at the 4-cell stage; at 60-75 h post-copulation, 87% of the collected eggs were at the 8-cell or morula stages; and at 72 h post-copulation, most eggs were at the blastula stage (Figure 2).
Figure 2.
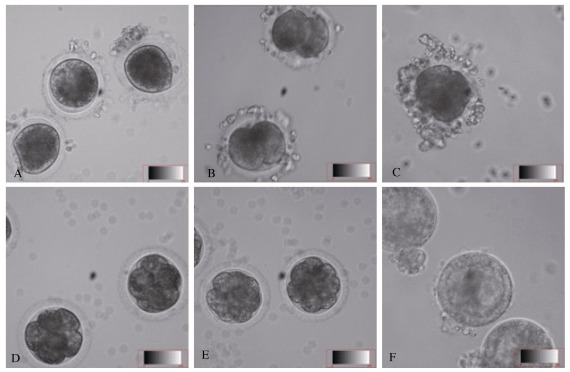
Embryo developmental stages of tree shrews
Table 1.
Embryos collected at different stages from 111 females after effective copulation
| Time post copulation | Numbers of embryos at different stages (n) | Numbers of females (n) | ||||
| 1-cell stage | 2-cell stage | 4-cell stage | 6-cell stage | Blastula | ||
| 22-28 h | 211 | 7 | 2 | 0 | 0 | 79 |
| 29-34 h | 25 | 21 | 9 | 0 | 0 | 21 |
| 41-46 h | 0 | 0 | 0 | 3 | 0 | 1 |
| 47-50 h | 2 | 0 | 12 | 0 | 0 | 5 |
| 60-64 h | 2 | 0 | 0 | 0 | 3 | 2 |
| 69-75 h | 0 | 0 | 0 | 6 | 4 | 3 |
In vitro culture was performed on the 172 collected embryos from 55 females (Table 2). Among the 127 embryos at the 1-cell stage, 57 developed into the 2-cell stage (cleavage rate=45%), 47 developed into the 4-cell stage, 29 developed into the 8-cell or morula stages, and 5 developed into blastulae. Of the 29 embryos at the 2- to 4-cell stages, 19 cleaved and developed into morula. About 50% of the embryos at the 8-cell and morula stages developed into blastulae. The blastulae collected 72 h post-copulation hatched after 17 h of culture in vitro (Figure 3). In general, during culture, 2-cell, 4-cell, morula and blastula were observed after 32-36, 44-50, 72 and 85 h, respectively.
Table 2.
In vitro culture and development of 172 embryos collected from 55 females after effective copulation
| Embryonic developmental stages | 1-cell stage | 2-cell stage | 4-cell stage | 8-cell stage | Morula | Morula | Hatching |
| Numbers of embryos (n) | 127 | 57 | 47 | 37 | 19 | 5 | 0 |
| 16 | 14 | 12 | 10 | 0 | 0 | ||
| 13 | 12 | 9 | 2 | 0 | |||
| 3 | 0 | 0 | 0 | ||||
| 9 | 6 | 6 | |||||
| 4 | 0 |
Figure 3.
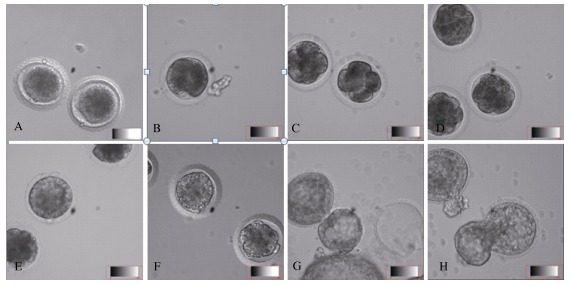
In vitro culture and development of tree shrew embryos
In tree shrews, embryo compaction, which is critical to embryo pre-implantation, begins from the late 8-cell stage. After the first cleavage, the effects of the maternal genes decrease, while the embryo genome activates and the morula compacts and develops into blastulae. In this study, after cleavage but before the 8-cell stage, the blastomeres were loosely compacted with clear boundaries (Figure 3B-C). Compaction occurred at the late 8-cell stage. With significant adhering and pressing, the blastomeres were stretched and elongated, and the entire embryo was tightly compacted (Figure 3D). With further development, polarization began (Figure 3E). The blastomeres in contact with teleblem were arranged in tight strips and distributed peripherally around the embryo. These cells were polarized and with blastocyst formation, developed into extra-embryonic tissues. The blastomeres in contact with the basilar membrane were arranged in gapped junctions. These non-polarized cells were found within the embryo and developed into an inner cell mass (Johnson & Ziomek, 1981).
The 65 fertilized eggs obtained from the bilateral oviducts of 23 females after effective copulation were used in transplantation. Another 13 females that had effectively copulated were used as receivers to accept 2-3 eggs on each side, bilaterally. However, no conception was found in either situation. The 70 eggs collected from the unilateral oviduct of 33 females were autotransplanted. Each female accepted 2-3 eggs obtained from the other side of its oviduct; however, no conception was observed either.
DISCUSSION
In the wild, copulation in tree shrews exhibits obvious seasonal patterns and is usually concentrated from March to June. In captive conditions, 60% of copulations occur from February to June, with the rate decreasing from September to December. Pups are born from March to August (Peng et al., 1991). There are a number of indicators of seasonal reproduction patterns in tree shrews. Cao (1990) reported that from October to the following January, the number of primordial follicles in the ovaries was far greater than that from April to July, whereas, from January to April, the number of vesicular follicles that turned into mature follicles was greater than that from July to October. By comparing testicle slices from males in different seasons, Collins et al. (1982) found that reproductive capacity changed with environmental conditions and seasons, indicating that the physiology and puberty secretions in male tree shrews were similar to those in non-human primates and humans (Collins & Tsang, 1987).
In this study, copulations in tree shrews from December to the following November were observed by surveillance. Even though copulations occurred in December, most effective copulations (70%) were concentrated from April to June (Figure 1A). The rate of fertilized egg procurement from April to June was about 85% and was relatively stable (Figure 1B). Copulations were rarely seen (only 1-2 times) from July to August and none was observed from August to October. However, no significant differences were found in the average number of obtained embryos from each tree shrew over different seasons (Figure 3C). The number of young born under both wild and captive conditions is usually 3-4 (Jiang et al., 2011; Peng et al., 1991). In this study, a total of 237 embryos were obtained from the bilateral oviducts of 78 females after effective copulation, i.e., average embryo number for each female was 3.04. These results are in accordance with previous findings on tree shrews in regards to seasonal reproduction, time and peak of reproduction, and number of young (Peng et al, 1991). According to Peng et al. (1991), copulations generally occur from 0700-0800h, 0830-1030h, and 1500-1600h. We found that the 150 cases of copulation were scattered from 0700-1800h, with no clear time concentration occurring over the day (Figure 1D). In total of 147 cases of copulation occurred before 1900h. Furthermore, there was a dramatic decrease in copulation behavior after 1900h.
The external genital tract of the female tree shrews in the estrus cycle is different with that of mice, i.e., a copulatory plug cannot be used to determine if copulation was successful or to estimate the time of fertilization. Although we were tried to diagnose the estrum of the female tree shrew by comparing the changes in color of its external genital tract in different stages, no regular pattern was observed, such as the light to dark to light pattern found in mice (Fu et al., 2005; Hubrecht & Kirkwood, 2010). To accurately diagnose the timing of copulation in tree shrews, we established effective copulation criteria based on surveillance recordings. Using these criteria, fertilized eggs were obtained from 74% of females after effective copulation, indicating the viability of this method in determining successful copulations in tree shrews. In this study, egg collection was concentrated 25-28 h post-copulation. Seventy-one percent of the eggs collected 41-50 h post-copulation were at the 4-cell stage; 87% collected 60-75 h post-copulation were at the 8-cell or morula stage; and blastulae with cavities were found 75 h post-copulation. In mice, eggs at the 2-cell and blastocyst stages were found 36 h and 84 h post copulatory plug occurrence. The gestational period in mice is 19 days, but is 42-45 days in tree shrews (Tsang & Collins, 1985). These findings indicate that even though early stage embryonic development is comparable between mice and tree shrews, the development after implantation was quite different (being much slower in tree shrews than in mice).
In this study, among the 127 in vitro cultured eggs, only 57 developed from the cleavage to the 2-cell stage, i.e., the cleavage rate was 45%. Among these 57 2-cell stage embryos, 37 developed into the 8-cell stage, i.e., the development rate was 64%. The possible reason for the significant difference between the cleavage and development rate is the accuracy in diagnosing effective fertilization. The basic criteria of determining fertilization are the occurrences of polocytes and nuclei. In tree shrews, however, cytoplasm oocytes are not only darkened and compact due to the enrichment of lipid droplets (Chen et al., 2002), but are also surrounded by a large amount of condensed granular cells. Even though we used the wash tube to rinse the embryos repeatedly, granular cells were not completely removed, which likely interfered in determining the development of cultured oocytes (Yue et al., 2004), as well as in observing the polocytes and nuclei. The application of other methods, e.g., low speed centrifugation, in fertilization diagnosis needs further exploration (Ma et al., 2007).
Among the 89 embryos at the 2-to 8-cell stages, 38 developed into morula, i.e., the development rate was 43%; among the 98 embryos after cleavage, only 17 (17%) developed into blastulae; 6 of the 9 morula that developed into blastulae were successfully hatched. The high cleavage to morula development rate indicates that the culture system was suitable in this study; however, the blastula rate was still low. Yue et al. (2004) obtained 14% blastulae following in vitro culture of mature oocytes. The low rate of blastula development may be due to degeneration and developmental arrest during in vitro culture. These phenomena generally exist in the in vitro culture of pre-implanted embryos in mammals, e.g., developmental arrest was found in 2-cell stage embryos in mice, in the 4-cell stage in pigs and in the 8-16 cell stages in goats and sheep (Li et al., 2005).
Morula compaction plays an important role in embryo development. Without it, embryos in vitro may be unable to reach the blastula stage or may develop into poor quality blastulae, with later implantation impacted. van Soom et al. (1997) found that development in in vivo embryos was more delayed than that in in vitro embryos. Thus, the occurrence of compaction should be considered when establishing in vitro culture systems. Before compaction, mice embryos cannot fully digest glucose and pyruvic or lactic acid is taken as the metabolism substrate. Whereas, after the 8- to 16-cell stages and compaction, glucose becomes vital to embryo development, e.g., Ding et al. (2007) reported that after adding glucose to the in vitro embryo culture system, the rate of blastula development increased significantly. Oviducts also are important in in vivo and in vitro embryo development, e.g., Mercader et al. (2001) successfully improved embryonic development by co-culturing endometrial epithelial cells with embryos. Another issue worth attention is the individual differences among experimental animals. In many previous studies, experimental tree shrews were captured in the wild with indeterminate ages and genetic backgrounds, and therefore, significant differences likely existed among them. It is, therefore, important to establish tree shrew strains with clear genetic backgrounds (Xu et al., 2013). In this study, we determined the differences in both the morphology and time phase between in vivo and in vitro cultured tree shrew embryos. Although no morphological changes were found, delayed development was observed in the in vitro embryos. Under in vivo conditions, the 2-cell, 4-cell and blastula embryos were obtained at 27, 41 and 72 h post-copulation, respectively, whereas, under in vitro conditions, the embryos were obtained at 32, 44 and 75 h post-copulation, respectively.
The success of egg transplantation is closely correlated with proper receptors. In this study, oviduct transplantation was applied as per the method used in mice (Nagy et al., 2003) . The structure and direction of oviducts in the tree shrews are similar to those in mice, but the texture is tougher, and after copulation, the obvious expansion of ampulla in mice is not found in tree shrews. Thus, the presence of a copulatory plug cannot be used as proof of effective copulation. In this study, based on recorded images, compared with the donors, the receptors had staggered copulation within 1-2 hours. The 13 receivers for bilateral transplantation received 2-3 embryos on each side and were individually housed after operation; however, no conception was found a month later. Because tree shrews ovulate after copulation, it is possible that differences in developmental phases exist in the females with accordant copulation times. Moreover, the differences in genetic background may also cause immunological rejection. Therefore, we autotransplanted embryos from one side to the other within an individual; again, however, no successful conception was achieved. Tree shrews are easily stressed, and can become agitated with environmental stimuli. Stress can significantly affect copulation and reproduction in tree shrews, e.g., external stimuli can cause scent gland lesions in females and can induce miscarriage or infanticide (Peng et al., 1991). The transplantation procedure, which includes capture, anesthesia, and operation, may provoke stress in tree shrews and thus cause failure in transplantation. As such, embryo transplantation in tree shrews needs further exploration, with both individual differences and environmental stimuli taken into consideration.
Funding Statement
This study was supported by the Breakthrough Project of Strategic Priority Program of the Chinese Academy of Sciences (XDB13000000)
REFERENCES
- 1. Cao J, Yang EB, Su JJ, Li Y, Chow P. 2003. The tree shrews:adjuncts and alternatives to primates as models for biomedical research. Journal of Medical Primatology, 32 (2): 123- 130. [DOI] [PubMed] [Google Scholar]
- 2. Cao XM. 1990. Microscopic structure of ovary and ovarian activity of different seasons in tree shrew (Tupaia belangeri chinensis). Zoological Research, 11 (1): 17- 25. [Google Scholar]
- 3. Chen JQ, Yu YM, Chen J, Wu YQ, Cheng GX. 2002. Observation of reproductive organs and oocyte's character of female tree shrew anesthetized. Acta Laboratorium Animalis Scientia Sinica, 10 (4): 230- 232. [Google Scholar]
- 4. Collins PM, Tsang WN. 1987. Growth and reproductive development in the male tree shrew (Tupaia belangeri) from birth to sexual maturity. Biology of Reproduction, 37 (2): 261- 267. [DOI] [PubMed] [Google Scholar]
- 5. Collins PM, Tsang WN, Lofts B. 1982. Anatomy and function of the repro-ductive tract in the captive male tree shrew (Tupaia belangeri). Biology of Reproduction, 26 (1): 169- 182. [DOI] [PubMed] [Google Scholar]
- 6. Ding F, Zhou HL, Liu Y, Ma L, Su Y, Du L. 2007. Effects of glucose on development of ICR mouse embryos in vitro. Zoological Research, 28 (5): 501- 506. [Google Scholar]
- 7. Fu WD, Sun YC, Suo L, Gao JM. 2005. Observation of estrous cycle and choice of optimal superovulation time in mice. Journal of Beijing Agricultural College, 20 (2): 19- 21. [Google Scholar]
- 8. Hubrecht RC, Kirkwood J. 2010. The UFAW Handbook on the Care and Management of Laboratory and other Research Animals. 8th ed. Singapore:Robert Hubrecht and James Kirkwood Press, 262- 275. [Google Scholar]
- 9. Jiang QF, Kuang DX, Tong PF, Sun XM, Dai JJ. 2011. Scale breeding of tree shrews and the establishment of breeding population. Laboratory Animal Science, 28 (6): 35- 38. [Google Scholar]
- 10. Johnson MH, Ziomek CA. 1981. The foundation of two distinct cell lineages within the mouse morula. Cell, 24 (1): 71- 80. [DOI] [PubMed] [Google Scholar]
- 11. Li YJ, Ao H, Sun GJ, Zhao YZ. 2005. Study on the gene expression of different goat preimplantation embryos on given specific stage. Acta Veterinaria et Zootechnica Sinica, 36 (12): 1281- 1285. [Google Scholar]
- 12. Ma YZ, Hu TM, Xia GL, Bou SG. 2007. Transgic ovine embryos production by means of pronuclear microinjection. Journal of Inner Mongolia University, 38 (6): 660- 664. [Google Scholar]
- 13. Mercader A, Simón C, Galán A, Herrer R, Albert C, Remohi J, Pellicer A. 2001. An analysis of spontaneous hatching in a human endometrial epi-thelial coculture system:is assisted hatching justified?. Journal of Assisted Reproduction and Genetics, 18 (6): 315- 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nagy A, Gertsenstein M, Vintersten K, Behringer R. 2003. Manipulating the Mouse Embryo:A Laboratory Manual, New York: Cold Spring Harbor Laboratory Press; [Google Scholar]
- 15. Pang YQ, Feng Y, Sun XM, Liu L, Dai JJ, Xia XS. 2014. Research progress of tree shrew models of viral hepatitis and modeling strategy. Acta Laboratorium Animalis Scientia Sinica, 22 (2): 95- 102. [Google Scholar]
- 16. Peng YZ, Ye ZZ, Zou RJ, Wang YX, Tian BP, Ma YY, Shi LM. 1991. Biology of Chinese Tree Shrews, Kunming: Yunnan Science and Technology Press; [Google Scholar]
- 17. Sun YM, Yang JZ, Sun HY, Ma YY, Wang JH. 2012. Establishment of tree shrew chronic morphine dependent model. Zoological Research, 33 (1): 14- 18. [DOI] [PubMed] [Google Scholar]
- 18. Tsang WN, Collins PM. 1985. Techniques for hand-rearing Tree-shrews (Tupaia belangeri) from birth. Zoo Biology, 4 (1): 23- 31. [Google Scholar]
- 19. van Soom A, Boerjan ML, Bols PE, Vanroose G, Lein A, Coryn M, de Kruif A. 1997. Timing of compaction and inner cell allocation in bovine embryos produced in vivo after superovulation. Biology of Reproduction, 57 (5): 1041- 1049. [DOI] [PubMed] [Google Scholar]
- 20. Wang J, Zhou QX, Lü LB, Xu L, Yang YX. 2012. A depression model of social defeat etiology using tree shrews. Zoological Research, 33 (1): 92- 98. [DOI] [PubMed] [Google Scholar]
- 21. Xia HJ, Wang CY, Zhang HL, He BL, Jiao JL, Chen CS. 2012. Characteri-zation of spontaneous breast tumor in tree shrews (Tupaia belangeri chinenesis). Zoological Research, 33 (1): 55- 59. [DOI] [PubMed] [Google Scholar]
- 22. Xu L, Zhang Y, Liang B, Lü LB, Chen CS, Chen YB, Zhou JM, Yao YG. 2013. Tree shrews under the spot light:emerging model of human diseases. Zoological Research, 34 (2): 59- 69. [DOI] [PubMed] [Google Scholar]
- 23. Yang ZF, Zhao J, Zhu YT, Wang YT, Liu R, Zhao SS, Li RF, Yang CG, Li JQ, Zhong NS. 2013. The tree shrew provides a useful alternative model for the study of influenza H1N1 virus. Virology Journal, 10 (1): 111- 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yue HF, Su JJ, Li Y, Huang YJ, Yang C, Ban KC, Ou C, Cao J, Duan XX, Zhai DM. 2004. Study on in vitro maturation and fertilization of Tupaia glis oocytes. Heilongjiang Journal of Animal Reproduction, 12 (1): 5- 8. [Google Scholar]
- 25. Zheng YT, Yao YG, Xu L. 2014. The Basic Biology and Disease Models of Tree Shrews, Kunming: Yunnan Science and Technology Press; [Google Scholar]


