Abstract
High frequency heart rate variability (HRV) is a measure of neurocardiac communication thought to reflect predominantly parasympathetic cardiac regulation. Low HRV has been associated empirically with clinical and subclinical levels of anxiety and depression and, more recently, high levels of HRV have been associated with better performance on some measures of executive functioning (EF). These findings have offered support for theories proposing HRV as an index measure of a broad, self-regulatory capacity underlying aspects of emotion regulation and executive control. This study sought to test that proposition by using a structural equation modeling approach to examine the relationships of HRV to negative affect (NA) and EF in a large sample of U.S. adults ages 30s–80s. HRV was modeled as a predictor of an NA factor (self-reported trait anxiety and depression symptoms) and an EF factor (performance on three neuropsychological tests tapping facets of executive abilities). Alternative models also were tested to determine the utility of HRV for predicting NA and EF, with and without statistical control of demographic and health-related covariates. In the initial structural model, HRV showed a significant positive relationship to EF and a nonsignificant relationship to NA. In a covariate-adjusted model, HRV’s associations with both constructs were nonsignificant. Age emerged as the only significant predictor of NA and EF in the final model, showing inverse relationships to both. Findings may reflect population and methodological differences from prior research; they also suggest refinements to the interpretations of earlier findings and theoretical claims regarding HRV.
Keywords: heart rate variability, executive functioning, negative affect, anxiety, depression, structural equation modeling
1. Introduction
Autonomic and neuroendocrine mechanisms associated with negative emotion have been implicated in the development of mental and physical health problems (Brooks et al., 2011; Glick et al., 1965). Although the focus has been on sympathetic adrenomedullary activity associated with emotional volatility (Kreibig, 2010), a growing body of work examines parasympathetic nervous system influences on heart rate variability (HRV) thought to reflect emotion regulation (Berntson et al., 1997; Levy, 1990). High HRV, taken in this work to indicate greater parasympathetic (vagal) tone, is a possible marker for cardioprotective processes, whereas low HRV/less parasympathetic tone is a potential risk factor for cardiovascular disease (CVD), adverse cardiac events, and all-cause mortality (Dekker et al., 1997, 2000; Tsuji et al., 1994, 1996).
Inverse relationships have been reported between HRV and symptoms of anxiety and depression in physically healthy individuals (Friedman, 2007; Rottenberg, 2007). Both anxiety and depression are considered possible risk factors for CVD and other physical health problems (Gianaros & Sheu, 2009; Rozanzki et al., 1999; Suls & Bunde, 2005). There are also reports of positive relationships between HRV and executive functioning (EF; reviewed in Thayer et al., 2009), cognitive control processes that support planning and execution of goal-directed activity. These associations with negative affect (NA) and its sequelae, and with EF, are consistent with theories conceptualizing HRV as a measure of bidirectional neurocardiac communication that reflects adaptive mechanisms of affective and cognitive self-regulation (Bates & Buckman, 2013; Benarroch, 1997; Berntson et al., 2007).
The present study addressed several questions that emerge from the work outlined above by using structural equation modeling (SEM) to examine simultaneously the relationships of HRV to both NA and EF. A large, nonclinical sample of U.S. adults permitted a test of the HRV–NA relationship in individuals predominantly without anxiety or mood disorders or clinical CVD. It also afforded the opportunity to examine associations among HRV, NA, and EF across a broad age range. In addition, employing SEM allowed a more comprehensive representation of EF compared with most prior studies of its relationship to HRV.
1.1 Associations of HRV with anxiety and depression
Among the anxiety disorders, panic and post-traumatic stress disorder have shown the strongest relationships with low HRV, while less consistent results have been found for generalized anxiety disorder and phobias (Friedman, 2007). In nonclinical samples, low HRV has been associated with biased attention toward threat (Miskovic & Schmidt, 2010) and delay in disengaging attention from threat (Cocia et al., 2012). These cognitive responses have been linked to anxiety disorders, but are not measures of anxiety (Clark, 1999). In other research, low resting HRV has been found to predict exaggerated startle response under threat of shock (Melzig et al., 2009) and increased startle magnitude following neutral stimuli (Ruiz-Padial, 2003). These findings have been discussed in terms of anticipatory anxiety. However, while startle modulation has validity for measuring neurobiological aspects of emotion, it is not itself a measure of subjective NA. With regard to trait anxiety, which is a marker for chronic NA, an inverse association with HRV has been shown in medical patients (e.g., Kogan et al., 2012), but findings in physically healthy individuals have been inconsistent (Bleil et al., 2008; Dishman et al., 2000; Fuller, 1992; Virtanen et al., 2003; Watkins et al., 1998).
Turning to HRV’s relationship to depressive symptoms, much of the research has been conducted in CVD patients (e.g., Carney et al., 2001; Stein et al., 2000), raising the possibility that CVD confounds or moderates this association (Kemp et al., 2010). Studies of HRV and depression in physically healthy participants have shown more mixed and modest findings than the HRV–anxiety literature (Kemp et al., 2010; Rottenberg, 2007). Major considerations regarding the discrepancies include: (1) some antidepressant medications affect HRV, (2) unmeasured cardiovascular factors may be linked to both HRV and depression, and (3) unmeasured comorbid anxiety may account for effects on HRV. To our knowledge, only one study has examined HRV’s relationship to symptoms of both anxiety and depression in a large community sample. Bleil et al. (2008) reported that in young and middle-aged adults (N=653) depression and anxiety each independently predicted HRV and contributed to the higher-order latent variable of negative affect, which also predicted HRV.
1.2 Association of HRV with executive functioning
Executive functions are defined as cognitive control mechanisms for maintaining task goals and flexibly implementing task rules (e.g., Miller & Cohen, 2001). One prominent theoretical framework for characterizing individual differences in EF describes a three-factor model comprising (1) monitoring and updating information in working memory, (2) task shifting, and (3) inhibition of prepotent responses (Miyake et al., 2000). Factor analyses have shown these three EF components to be intercorrelated yet separable facets of the same underling construct (Miyake et al., 2000).
Several studies reported associations between resting HRV and performance on tasks involving EF (Thayer et al., 2009). Among young, male Norwegian Navy personnel, high-HRV participants showed superior accuracy on a computerized, two-back working memory measure, and faster responding, with a trend toward better accuracy, on particular components of a continuous performance task (Hansen, et al., 2003). Other studies using the same tasks and Navy population reported improved EF under stress only among low-HRV participants; also reported were coincident increases in HRV and improvements in EF task components after fitness training (Hansen et al., 2004, 2009). Similar to the latter finding, a study of older, sedentary adults found increased HRV and improvement on the Wisconsin Card Sorting Test only in the group assigned to an exercise regimen (Albinet et al., 2010).
This work provides initial support for a correlation between higher resting HRV and better performance on several tasks tapping facets of EF. However, the samples were relatively small (N = 24–65), predominantly male, and drawn from the extremes of the adult population in terms of age and physical fitness. Moreover, some continuous measures of HRV were dichotomized, and single-task or task-component measures reflect relatively narrow conceptualizations of EF. The present study aimed to clarify the HRV–EF relationship in a nonclinical adult population by (1) using a larger, more diverse sample than much prior research in this area; (2) treating HRV as a continuous variable; and (3) conceptualizing EF as a latent variable measured by a set of tasks tapping its major theorized components.
1.3 Relationship of age to HRV, NA, and EF
Age is a pervasive influence on HRV, NA, and EF, and is therefore an important factor in modeling their inter-relationships. There is a well-documented, inverse association between age and HRV (e.g., Kuo, 1999; O’Brien et al., 1986; Sinnreich et al., 1998; Voss et al., 2012). Age also is associated with a number of more firmly established CVD risk factors, including hypertension, heart disease, hypercholesterolemia, diabetes or hyperglycemia, body mass index (BMI), smoking, and low levels of physical activity (Davis et al., 2011; Lakatta & Levy, 2003); these factors have been linked in turn with low HRV (Thayer & Lane, 2007). Though research on age and adult anxiety and depression has yielded mixed results, studies controlling for risk factors (e.g., sex, education, marital status, socioeconomic status) generally have shown age-related declines in anxiety and depression risk (Jorm, 2000). EF has also shown an inverse association with age in research on age-related cognitive decline (e.g., Bryan & Luszcz, 2000; reviewed in Luszcz, 2011), though the picture is somewhat clouded by variations in definition and measurement of EF (Luszcz, 2011). One source of ambiguity is the possibility that global, age-related declines in processing speed, rather than EF specifically, might better explain patterns of cognitive aging (Salthouse, 1996). On the other hand, processing speed and efficiency may be inherent to the EF construct (e.g., Albinet et al., 2012; Borella et al., 2011).
1.4 Aims and hypotheses
In a large, nonclinical adult sample spanning six decades of age (30s–80s), SEM was used to examine the relationships of resting HRV to NA and EF. We sought to extend prior work by representing HRV as a continuous variable, examining anxiety and depression as indicators of a latent NA construct, and modeling EF as a latent construct reflecting three component cognitive abilities. This represents the first effort to operationalize and test theories proposing HRV as an index of individual differences in a broad set of affective and cognitive self-regulatory processes (Appelhans & Luecken, 2006; Porges, 2011; Thayer & Lane, 2009). It was hypothesized that: (1) HRV would show an inverse relationship to NA and a positive relationship to EF; (2) NA and EF would have an inverse association; and (3) controlling for other prominent CVD risk factors, age would be inversely associated with HRV, NA, and EF, and contribute, in part, to their interrelationships.
2. Materials and methods
2.1 Participants
Data were drawn from the second wave of the Midlife in the United States (MIDUS) study (MIDUS II; 2002–2006), which collected biomedical, psychosocial, cognitive, and psychophysiological data from a large, diverse sample of U.S. adults (N = 4,975) aged 33 to 84 years. MIDUS II included 9-year follow-ups of all four subsamples in MIDUS I: (1) a national random digit dialing (RDD) sample, (2) oversamples from 5 U.S. cities, (3) siblings of participants from the RDD sample, and (4) a national RDD sample of twin pairs. In addition, MIDUS II added an African-American subsample from Milwaukee, WI. To be eligible to participate, individuals had to be non-institutionalized English-speakers living in the continental U.S. and aged 25 to 74 when they took part in MIDUS I.
The current analyses used data that were drawn from two study components initiated in MIDUS II: the Biomarker Project (n = 1,255), which collected data on psychophysiological, biomedical, and psychosocial parameters; and the Cognitive Project (n = 4,512), which collected data on cognitive functioning. The eligible sample for the current study before exclusion criteria were applied included the subset of MIDUS II participants who took part in both of these new studies and had valid resting HRV data (n = 1,056).
Several exclusion criteria were applied to limit major confounding influences. First, eligible participants who did not deny a history of stroke were excluded (i.e., those who affirmed a history of stroke [n=20] or for whom data on history of stroke were missing [n = 59]). Stroke has been associated with low HRV, decrements in neurocognitive functioning, and increased risk of depression (Dütsch et al., 2007; Robinson, 2006). Individuals taking antidepressant medications (ADMs; n = 157) and those taking antihypertensive medications (n = 367) also were excluded, as these medications have been shown to affect autonomic cardiac influences (Lampert et al., 2003; Licht et al., 2008, 2009; Rottenberg, 2007; Toivonen, 1993). Table 1 presents demographic and clinical characteristics of the sample (N = 533).
Table 1.
Demographic and Clinical Characteristics of the Sample
| Demographics | N (%) | Mean (SD) |
|---|---|---|
| Age | — | 54.9 (10.7) |
| Sex | ||
| Male | 247 (46.3) | — |
| Female | 286 (53.7) | — |
| Racial Origin | ||
| White | 493 (92.5) | — |
| Black / African-American | 11 (2.1) | — |
| Native American or Alaska Native / Aleutian Islander / Eskimo | 8 (1.5) | — |
| Asian | 3 (0.6) | — |
| Other | 17 (3.2) | — |
| Refused | 1 (0.2) | — |
| Highest Level of Education | ||
| No high school diploma or equivalency certificate | 13 (2.5) | — |
| Graduated from high school or received equivalency certificate | 111 (20.8) | — |
| 1–2 Years of college, no degree | 78 (14.6) | — |
| 3 or more years of college, no degree | 25 (4.7) | — |
| Graduated from 2-year college, vocational school, or associate degree | 37 (6.9) | — |
| Graduated from a 4- or 5-year college, or bachelor degree | 138 (25.9) | — |
| Some graduate school or completed graduate degree | 130 (24.4) | — |
| Not reported | 1 (0.2) | — |
|
| ||
| Clinical Characteristics | ||
|
| ||
| Health-Related Covariates | ||
| Body Mass Index (BMI) | — | 28.18 (5.7) |
| Ever had heart disease | 17 (3.2) | — |
| Ever had high blood pressure/hypertension (12 mo) | 29 (5.4) | — |
| Ever had diabetes/high blood sugar (12 mo) | 22 (4.1) | — |
| Blood LDL cholesterol (mg/dL) | — | 110.2 (34.0) |
| Ever smoked cigarettes regularly | 203 (38.1) | — |
| Exercise at least 20 min. 3 times/wk | 493 (82.4) | — |
| Additional Health-Related Characteristics | ||
| Currently smoke cigarettes regularly | 59 (11.1) | — |
| Ever had depression | 74 (13.9) | — |
| Ever had cholesterol problems | 162 (30.4) | — |
| Taking corticosteroid medications | 52 (9.8) | — |
| Taking cholesterol medications | 85 (15.9) | — |
2.2 Data collection procedures
Data for the MIDUS II Biomarker Project were collected from July 2004 to June 2006. MIDUS II participants who completed the MIDUS I follow-up phone interview and self-administered questionnaire (Project 1) were eligible, except those drawn from the city oversamples. Biomarker Project participants traveled to one of 3 regional research centers for an overnight stay. All sites followed an identical protocol. The self-report questionnaires, including measures of anxious and depressive symptoms (STAI-T and CES-D), were completed in writing on the evening of the first day. The psychophysiological recording session was conducted in the morning on the second day, following a light breakfast with no caffeinated beverages (Ryff et al., 2010a).
HRV data were collected with electrocardiograph (ECG) electrodes placed on each shoulder and in the left lower quadrant. Simultaneously, respiration rate was measured using bands placed on the chest and abdomen. The participant remained seated for equipment positioning and calibration and during recording. Following the instruction to breathe normally, 10-min simultaneous baseline ECG and respiration recordings were obtained; ECG recordings were divided into two 5-min epochs for processing. As described elsewhere (Ryff et al., 2010a; Shcheslavskaya et al., 2010), a National Instruments A/D board was used to digitize the analog ECG signals at 500 Hz and to pass them to a microcomputer. Proprietary event detection software was used to submit the ECG waveform to an R-wave detection routine, which yielded an RR interval series. Errors in R-wave marking were corrected following established procedures (see Shcheslavskaya et al., 2010). The spectra of the RR interval series were calculated using an interval method for computing Fourier transforms, in which the mean of the RR interval series was first subtracted from each series value (see DeBoer, Karemaker, & Strackee, 1984). Next, the series was filtered using a Hanning window; estimates of spectral power were adjusted to offset any attenuation caused by this filter (Harris, 1978).
All MIDUS II participants were eligible for the Cognitive Project. Participants were administered the Brief Test of Adult Cognition by Telephone (BTACT) to collect data on six cognitive domains: episodic verbal memory, inductive reasoning, processing speed, working memory span, verbal fluency, and task-switching (Lachman & Tun, 2008; Tun & Lachman, 2006). A detailed description of BTACT administration is available (Tun & Lachman, 2006). Of note, the MIDUS 2 Biomarker Project and Cognitive Project are separately administered study components. As a result, the interval between the collection of biomarker data, including NA measures, and cognitive data varied among participants (M = 24.12 months, SD = 14.11 months).
Of several prominent cardiovascular risk factors that served as covariates in this study, most were obtained during participants’ lab stay for Biomarker Project data collection. On their first day at the lab, participants underwent a standardized medical history interview including questions about prior and present hypertension and heart disease, and current regular levels of physical activity, as well as other symptoms and conditions (Ryff et al., 2010b). On the next morning of the lab stay, prior to the collection of psychophysiological data (including HRV measures), participants received a physical exam including assessments of BMI and blood LDL cholesterol (mg/dL); the latter was measured from a fasting blood sample taken that morning (Ryff et al., 2010b). Data for two covariables, history of diabetes or hyperglycemia and lifetime history of regular cigarette smoking, were obtained via self-report during the MIDUS 2 Psychosocial Project, a follow-up to MIDUS 1 (MIDUS, 2007). The former represented the most complete source of relevant data for this subset of participants, while for the later, the Psychosocial Project was the only source.
2.3 Measures
2.3.1 Resting HRV
High-frequency R-R interval variability (HF-HRV; bandwidth 0.15–0.50 Hz, msec2) was used to measure resting HRV. The frequency domain measure was selected for these analyses because time-domain measures include some low-frequency contributions to R-R interval that may conflate sympathetic and vagal influences (Berntson et al., 2005). To maximize both sample size and between-subject consistency of HRV epoch duration, the first of two 5-min epochs in the baseline recording period was used in these analyses, which accords with standard guidelines’ recommended duration for collecting reliable, valid HRV data (Berntson et al., 1997; Task Force, 1996). Resting HRV has shown good test-reliability over intervals of three weeks (.81–.99; Bertsch et al., 2012) to several months (.76–.80; Sinnreich et al., 1998) in healthy adults.
2.3.2 Negative affect
The Spielberger State Trait Anxiety Inventory—Trait version (STAI-T) is a 20-item inventory that uses a 4-point Likert scale to measure anxiety symptoms elicited by potentially stressful situations (Spielberger, 1983, 1989). The trait scale has high test-retest reliability and good convergent validity with other self-report anxiety measures (Spielberger, 1983). Reliability in the current sample was adequate (Cronbach’s alpha = .75).
The Center for Epidemiologic Studies Depression Scale (CESD) is a 20-item measure of depressive symptoms designed for large-scale surveys (Radloff, 1977; Roberts & Vernon, 1983). It has a stable factor structure across large clinical and nonclinical samples, high internal and adequate test-retest reliability, and good convergent and discriminant validity (Contrada et al., 2006; Radloff, 1977). It has strong psychometric properties in older populations (Herzog et al., 1990). Cronbach’s alpha =.88 in the present sample.
2.3.3 Executive functioning
The Digits Backward task, which measures the longest series of digits an individual can mentally resequence in reverse order, is a common measure of attention and working memory (Tun & Lachman, 2006). This task demands sustained attention and active manipulation of information (Kaneko et al., 2011), making it a viable indicator of the executive capacity for continuous monitoring and updating information in working memory (Engle, 2002). Reliability data are unavailable for the present sample, who were tested only once. It has shown good test-retest reliability (r = .83; Weschler, 1981) in a large, demographically representative sample of U.S. adults.
The Red/Green task is a variant of the classic Go/No Go measure; it uses auditory cues and responses, so that it can be administered as part of the Brief Test of Adult Cognition by Telephone (BTACT). Like the classic task, it is thought to draw on the executive processes of task switching and inhibition of prepotent responses (Kramer et al., 1999; Tun & Lachman, 2006). It includes a “normal” block of trials (participants said “stop” when the examiner said red and “go” when the examiner said green) a “reverse” block (“stop”—green; “go”—red), and a mixed block. In the mixed block, participants were cued with the words normal and reverse to switch between response types at unpredictable intervals. Participants showed high accuracy (> 94%) across age groups and task conditions, and speed was not compromised for accuracy, even when controlling for age (Tun & Lachman, 2008). The present analyses used average response latencies of switch and non-switch trials during the mixed block. Latency values were subtracted from zero so that higher values would correspond to better performance as with other measures. Test-retest reliability over a 6-month interval in a representative subset of MIDUS II participants was adequate (r = .77) for the mixed-task condition (Tun & Lachman, 2008).
The Category Fluency task measures the number of unique items from a semantic category (e.g., animals) an individual can generate in 1 minute. This task is interpreted as a measure of the executive processes of active self-monitoring and inhibition (Lezak et al., 2004; Tun & Lachman, 2006). It has shown adequate test-retest reliability (r = .70) in middle-aged and elderly samples (Harrison et al., 2000; Snow et al., 1988).
2.4 Covariates
2.4.1 Demographic factors
Age and sex, which have been associated with resting HRV in previous research (e.g., Kou et al., 1999; Stein et al., 1997), were examined to account for their possible relationships with HRV, NA, and EF. Differences in resting HRV across racial groups also have been reported (e.g., Choi, 2006), but the racial makeup of the sample precluded analysis of this factor (see Table 1).
2.4.2 Cardiovascular health
A great number of factors related to cardiovascular health potentially influence HRV and its relationship to other factors, including NA and EF, so hypothesized covariates in this domain were first selected on a theoretical basis. Given that low HRV has been associated with CVD and is considered a possible CVD risk factor (Dekker et al., 1997, 2000; Tsuji et al., 1994, 1996), several other major CVD risk factors were considered eligible covariates based on their respective prior associations with low HRV (reviewed in Thayer & Lane, 2007). These included hypertension, heart disease, hypercholesterolemia, diabetes or hyperglycemia, BMI, smoking, and low levels of physical activity (NHLBI, 2012).
2.4.3 Covariate selection
Demographic and health-related covariates were controlled in order to guard against these factors causing spurious relationships between HRV and other constructs. In order to maintain relative parsimony in the adjusted models, analyses focused on the subset of hypothesized covariates significantly linked to HRV in this sample. First, the demographic and CVD-related covariates’ respective bivariate relationships to HRV were examined. After the initial structural model was analyzed, the subset of potential covariates showing significant bivariate associations with HRV (age, BMI, presence of diabetes or hyperglycemia) was included in the adjusted model to account for potential extraneous demographic- or CVD-related explanations for relationships between HRV and NA or EF. Including covariates significantly related to HRV helped to yield more interpretable adjusted models, but it is nonetheless important to note that even covariates showing non-significant relationships to HRV from a statistical standpoint may exert a substantial influence in clinical or practical terms, either individually or collectively.
2.5 Statistical analyses
The general mode of analysis involved structural equation modeling (SEM), which incorporates both measured and latent variables and can represent simultaneously the hypothesized interrelationships between multiple variables, in order to test the overall model’s fit to the data. The maximum likelihood (ML) method was used to analyze covariance matrices. This method uses all available data to estimate parameters, standard errors, and test statistics that are unbiased when data are missing at random or completely at random and are multivariate normal (Brown, 2006); it also has performed well relative to other methods when data are nonnormal (Savelei & Bentler, 2005).
The various indices used to evaluate models in SEM account for different facets of model fit. Consequently, the convention followed in these analyses is to evaluate and report a standard set of fit indices for each model to compensate for the limitations of particular indices and minimize Type I and Type II error (Hu & Bentler, 1999; Kline, 2005). Model fit was evaluated using the normed chi square (χ2/df), comparative fit index (CFI), root mean square error of approximation (RMSEA), and normed fit index (NFI; Kline, 2005). According to standard criteria, values of (χ2/df) < 5 are considered a good fit and interpreted in light of factors such as sample size, the number of parameters estimated, magnitude of associations between variables, and results for other fit indices (Bollen, 1989). For RMSEA, model fit is considered good at values ≤ 0.05, reasonable at values 0.05 to 0.08, or poor at values > 0.10 (Browne & Cudeck, 1993). CFI and NFI values ≥ 0.95 each are considered good fit (Hu & Bentler, 1999). For additional details about these fit indices see Browne and Cudeck (1993), Hu and Bentler (1999), and Kline (2005). Nested models were compared using the chi-square difference test, in which a significant difference indicates that the additional parameters estimated in the more complex model have improved the model’s fit to the data enough to justify the decline in parsimony compared to the simpler model (Hoyle, 2012). In addition, the models’ Akaike information criterion (AIC) and Browne-Cudeck criterion (BCC) values were compared, with a decrease on these measures of at least 10 units indicating a significantly better fitting model (Burnham & Anderson, 2004).
3. Results
3.1 Preliminary analyses
Preliminary analyses were performed using SPSS Statistics 20 (IBM Corporation, 2012) to check for skew, kurtosis, and univariate outliers with z-scores greater than ±4.0, as recommended for large samples (Kline, 2005). HRV, CESD, and STAI data were not normally distributed; a logarithmic transformation improved normality. Two variables (BMI and Red/Green task response latency) included univariate outliers with z-scores greater than ±4.0; these were set to their respective, highest non-outlier values. Descriptive statistics and simple bivariate correlations are shown in Table 2. Multicollinearity was evaluated using tolerance values (O’Brien, 2007) and found to be inconsequential.
Table 2.
Main Variables’ Descriptive Statistics and Simple Bivariate Correlations
| Mean (SD) | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1. HF-HRV† | 309 (815) | −.031 | .021 | .064 | .053 | .108* |
| 2. STAI† | 32.7 (8.55) | — | .727** | −.105* | −.041 | −.033 |
| 3. CESD† | 7.23 (7.12) | — | −.067 | −.042 | −.046 | |
| 4. Category Fluency | 20.44 (5.82) | — | .143** | .281** | ||
| 5. Digits Backward | 5.18 (1.42) | — | .159** | |||
| 6. Red/Green | 1.03 (.201) | — |
p < 0.05;
p <0.01.
Note: All descriptive statistics reflect raw data.
For the three non-normally distributed variables, bivariate correlations reflect the log-transformed data used in the analyses. CESD = Center for Epidemiologic Studies Depression Scale; HF-HRV = high-frequency heart rate variability (0.15–0.50 Hz, msec2); STAI = Spielberger Trait Anxiety Inventory–Trait scale.
3.2 Measurement model
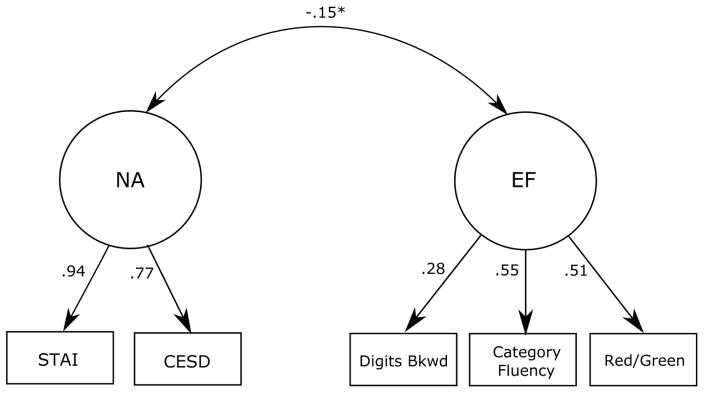
Modeling analyses were conducted with AMOS 21 software (Arbuckle, 2012). As has been recommended, the measurement model was examined first, followed by the structural model (Kline, 2005). The measurement model included two correlated latent variables, NA and EF. NA was modeled with two indicators: the CESD and STAI-T, and EF was modeled with three indicators: category fluency, backward digit span, and the Red/Green task. Results are shown in Figure 1. Based on accepted standards (Kline, 2005), the model showed good fit to the data (χ2 = 2.723, df = 4, p = .605, NFI = .994, CFI = 1.00, RMSEA < .001). All indicators had significant factor loadings (p = .011–.001). NA and EF showed a significant inverse relationship as expected (r = −.15, p =.04).
Figure 1.
3.3 Structural model
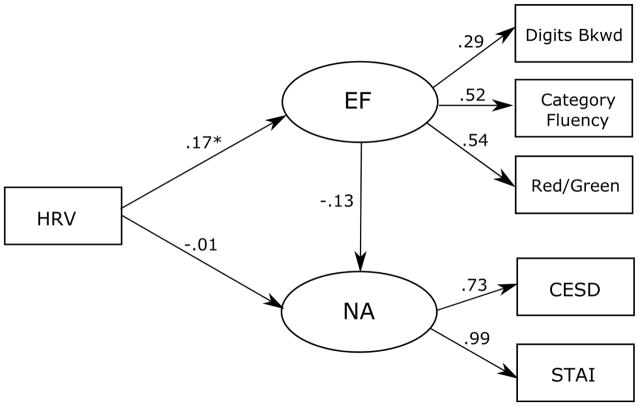
The full model showed good fit to the data (χ2 = 5.981, df = 7, p = .542, NFI = .998, CFI = 1.00, RMSEA < .001; Figure 2). As expected, the path from HRV to executive functioning was positive and significant (β = .17, B = .015, SE = .006, p = .02). However, the path from HRV to NA was not significant (β = −.01, B = −.015, SE = .023, p = .51), nor was the path from EF to NA (β = −.13, B = −.771, SE = .527, p = .05).
Figure 2.
3.4 Covariate-adjusted model
As a means of taking into account their potential confounding effects, an alternative model was then evaluated that incorporated the subset of hypothesized demographic- and CVD-related covariates that showed significant bivariate correlations with HRV. Sex, hypertension, heart disease, hypercholesterolemia, smoking, and low physical activity all showed very small and nonsignificant associations with HRV (r = −.07–.05) and were not included in the covariate-adjusted model. Age (r = − .31, p < .001), BMI (r = −.12, p = .004), and the presence of hyperglycemia or diabetes in the past 12 months (r = −.10, p = .02) showed small but significant bivariate correlations with HRV and were included. These three variables were allowed to intercorrelate because they tend to co-occur as risk factors for both CVD and type 2 diabetes (Lindström & Tuomilehto, 2003; NHLBI, 2012; Yusuf et al., 1998), were hypothesized to correlate with HRV (Thayer & Lane, 2007), and were examined as predictors of NA and EF.
The covariate-adjusted model yielded a significant chi-square (χ2 = 32.52, df = 16, p = .01), nominally indicating inadequate fit (Figure 3). However, the chi-square test is known to be inflated by large sample size and the presence of high correlations within a model (Kline, 2005). The remaining fit indices subsequently were examined to determine whether the significant chi-square value accurately reflected inadequate fit or was more likely a Type II error due to the large sample size and the introduction of significantly correlated covariates. These other indices uniformly indicated good model fit (NFI = .951, CFI = .973, RMSEA = .044), supporting the interpretation of an inflated chi-square statistic and good model fit to the data (Kline, 2005). In this model, the path from HRV to EF was no longer significant (β =.01, B = .001, SE =.005, p =.92), and the path from HRV to NA remained nonsignificant. Age significantly predicted EF (β = −.44, B = −.005, SE =.001, p < .001) and NA (β =−.31, B =−.022, SE =.005, p < .001). The path from EF to NA was significant (β = −.27, B = −1.899, SE =.659, p = .004). Each covariate retained its significant inverse relationship to HRV (age: r = −.31, p < .001; diabetes/hyperglycemia: r = −.10, p = .02; BMI: r= −.12, p = .001.). In addition, diabetes/hyperglycemia correlated significantly with age (r= .11, p = 01.) and BMI (r = .14, p = .001.). The correlation between age and BMI was not significant (r = −.03, p = .49).
Figure 3.
3.5 Model comparison
Finally, the covariate-adjusted model was compared to an alternative model in which the covariates were allowed to intercorrelate but their relationships with the variables of interest (HRV, NA, and EF) were constrained to zero. Retaining the full set of paths in the model while constraining the covariates’ relationships to key variables allows for a direct comparison of two structurally identical, nested models: one in which the covariates’ relationships to HRV are accounted for, and a second, nested within the first, in which HRV is the sole predictor of NA and EF. This comparison was designed to indicate whether including covariates in the model improved its fit sufficiently to justify the increase in complexity.
The constrained model showed poor fit to the data across all indices (χ2 = 174.855, df = 25, p < .001, NFI = .738, CFI = .759, RMSEA = .106). A chi-square difference test yielded a significant result (χ2diff = 142.334, dfdiff = 9, p < .001), indicating that the model including estimates of the covariates’ relationships to key variables provided significantly better fit compared to the model in which these relationships were constrained to zero. The AIC and BCC values likewise showed that the model in which covariates’ relationships to the variables of interest were estimated (AIC = 108.521, BCC = 109.977) was superior to the structurally equivalent model in which these paths were constrained to zero (AIC = 232.855, BCC = 233.966).
4. Discussion
A measurement model representing NA and EF as latent constructs manifested in multiple observed indicators showed adequate fit to the data. These findings indicated that conditions were met to assess relationships linking HRV to separate, multifaceted NA and EF constructs. In an initial structural model that showed good fit with the data, resting HRV showed the hypothesized positive association with EF. But neither HRV nor EF showed expected, inverse relationships with NA. A second structural model that added demographic and health-related covariates also showed good fit. In this model, HRV was no longer related to EF, and the expected inverse association emerged between EF and NA. Age showed inverse associations with HRV, NA, and EF, and HRV. A third model constraining the covariates’ associations with HRV, EF, and NA to zero showed poor fit to the data. Model comparison statistics showed the second model, in which associations were permitted between the covariates and the variables of interest, to be superior to the constrained model.
Thus, it was the covariate-adjusted model that was best supported by the data. The EF–NA relationship it contains is consistent with the literature showing that lower levels of executive control are associated with subclinical levels of anxiety and depression (Kaiser et al., 2014; Koster et al., 2006). It also accords with theories conceptualizing executive control as a contributor to self-regulatory processes including emotion regulation (Hofmann et al., 2012; Zelazo & Cunningham, 2007). The relationships involving age are also consistent with previous theory and research. Theoretical and methodological implications of the findings are discussed below.
4.1 Relationship between HRV and NA
In this study, HRV showed a negligible relationship to NA. This was true with and without statistical control for demographic and health-related variables. This contrasts with prior findings linking low HRV to symptoms of anxiety and depression. Among likely explanations for this disparity are population differences. Most reports of associations between low HRV and NA were based on clinical or mixed samples (e.g., Friedman, 2007; Rottenberg, 2007; but see Bleil, 2008), in contrast to the current epidemiological, largely nonclinical sample. One possibility is that HRV is not related to subclinical negative affect at effect sizes that could be detected in the current study. Alternatively, the relationship of low HRV to clinical depression in some previous work may be overstated due to insufficient controls for use of antidepressant medication (ADM). Findings from the Netherlands Study of Depression and Anxiety, which examined very large samples (N > 2000) of psychologically disordered, remitted, and healthy individuals, showed that ADM use, rather than clinical symptoms, accounted for associations of HRV with major depression (Licht et al., 2008) and anxiety disorders (Licht et al., 2009). Another possibility is that the exclusion of MIDUS II participants taking ADMs in the present study prevented the detection of a real but modest HRV–NA relationship driven by persistent, clinical levels of NA among excluded participants (Kemp, 2012). A further population difference concerns CVD. The relative absence of CVD or CVD risk factors appears to characterize study samples in which associations between HRV and depression have been more mixed and modest (Kemp et al., 2010; Rottenberg, 2007). Similarly, relationships between HRV and anxiety symptoms in physically healthy samples have been inconsistent (Bleil et al., 2008; Dishman et al., 2000; Fuller, 1992; Virtanen et al., 2003; Watkins et al., 1998).
Although inconsistent, low HRV has shown stronger associations with anxiety than with depression, including some work in nonclinical samples (Kemp et al., 2010; Rottenberg, 2007), suggesting that factors other than population differences may be at work. One issue concerns alternative operationalizations of anxiety. Associations of low HRV with greater emotion-modulated startle magnitude and potentiation (e.g., Melzig et al., 2009; Ruiz-Padial, 2003) may reflect anxiety-related neurobiological processes that are not tightly coupled with self-reported anxiety symptoms such as were examined in the present study. This suggestion is consistent with a lack of relationship between trait anxiety and fear-potentiated startle in nonclinical samples (Cook et al., 1992; Grillon et al., 1993), which supports a conservative interpretation of startle-related measures as a function of fear elicited by the immediate stimulus, rather than an indicator of generally higher anxiety levels (Grillon & Baas, 2003). Similarly, the kind of attentional measures implicated in cognitive models of anxiety, which have been related to HRV in previous studies (Cocia et al., 2012; Miskovic & Schmidt, 2010), are associated with, but separable from, stable, subjective reports of anxiety symptoms.
The absence of association between HRV and NA in the present study failed to support the notion that HRV indexes a broad capacity for adaptive responding that underlies cognitive and affective self-regulation (Porges, 2011, Thayer & Lane, 2009). One implication of this, as suggested by the foregoing discussion, is that such relationships between HRV and self-regulatory capacities may characterize populations with anxiety disorders, mood disorders, and/or CVD and its risk factors, but not the relatively healthy population represented by the current sample. If this speculation were accurate, it would suggest that a higher order flexible responding capacity (Porges, 2011, Thayer & Lane, 2009) describes an individual difference dimension that emerges only in the context of certain mental and physical health problems. Another possible implication is that the absence of a HRV–NA relationship in the present study identifies a boundary condition, such that the adaptive capacity indexed by HRV is narrower than has been theorized, lacking a simple, direct connection to affective self-regulation and specific to cognitive aspects of self-regulation. We now turn to that possibility.
4.2 Relationship between HRV and EF
The relationship between HRV and EF in this study depended on whether the covariables (age, BMI, and diabetes/hyperglycemia) were included in the model. Without covariates (see Figure 2), an initial structural model showed a significant positive relationship between HRV and EF. No other paths were significant, yet this the model fit the data well.
In the covariate-adjusted model (Figure 3), addition of the covariates eliminated the HRV–EF relationship. This appeared to be due largely to the addition of age, which showed significant, substantial inverse associations with HRV, EF, and NA, in accord with existing theory and empirical precedents. An inverse association between age and HRV has been documented (e.g. Kuo, 1999; O’Brien et al., 1986; Sinnreich et al., 1998). An inverse association between age and EF is consistent with evidence of differential age-related decline in various cognitive abilities (e.g., Bryan & Luszcz, 2000; reviewed in Luszcz, 2011) and with evidence that normal aging is associated with structural and functional changes in frontal lobe structures that subserve executive abilities (Raz et al., 1997, 2005; Spreng et al, 2010; West, 1996, 2000).
The null association between HRV and EF after controlling for covariates contrasts with HRV-EF relationships found in smaller, more homogenous samples in which such controls were not required (reviewed in Thayer et al., 2009). However, it is consistent with a recent investigation of HRV and executive functioning using MIDUS II data (Kimhy et al., 2013), which likewise reported that significant relationships between a multifaceted EF factor and various HRV measures were reduced to nonsignificance by the addition of demographic covariates (age, sex, education). It is also consistent with a recent investigation of relationships between regional cerebral blood flow (rCBF), resting HF-HRV, and performance on a battery of seven EF measures (Jennings et al., 2014). This study reported significant associations between HRV and rCBF in several brain areas related to vagal control yet found no relationship of HRV to EF performance or to EF–rCBF associations.
One interpretation of the current result is that the significant HRV–EF association in the initial structural model (Figure 2) may have been attributable to both variables’ significant age-related decline. A caveat regarding this interpretation is that age, a strong predictor of HRV, EF, and NA in that model, summarizes a multitude of biological and psychological changes, as well as possible cohort and period effects, without reference to mechanisms. Therefore, controlling for age, particularly across a broad range, risks “washing out” subtle associations between age-affected processes (Type II error; Consonni et al., 1997). The finding that HRV and EF each showed stronger relationships to age than to one another does not preclude the possibility that their initial significant association reflected a meaningful link in which both variables also show significant age-related change.
In addition to the possible role of age in driving the HRV–EF relationship, key differences between MIDUS and prior studies (Thayer et al., 2009) may help to explain discrepancies. First, prior studies generally collected HRV and EF data at the same time points (Hansen et al., 2003, 2004, 2009), whereas in MIDUS II sometimes several years intervened. Whereas resting HRV is reasonably stable over time (Bertsch et al., 2012; Kleiger et al., 1991; Sinnreich et al., 1998), extraneous influences may have obscured a relationship that would have been evident had the measures been collected at closer time points.
A second methodological difference is that some prior studies (e.g., Hansen et al., 2003, 2009) used median splits based on their respective HRV measures to define “high” versus “low” HRV groups for comparison on cognitive tasks. Although the use of median splits in this manner has been criticized (e.g. Cohen, 1983; MacCullum, 2002), dichotomizing HRV may better capture the functional form of the HRV–EF relationship than the use of linear models representing HRV as a continuous measure (e.g., Kimhy et al., 2013).
Thirdly, the discrepant findings are based on different approaches to operationalizing EF. The present study and Kimhy et al.’s (2013) recent MIDUS study each conceived of EF as a multifaceted capacity measured with multiple cognitive tests selected to tap different facets of executive functioning (Miyake, 2000; Tun & Lachman, 2006). In contrast, the few prior studies in which HRV predicted aspects of EF have examined narrower EF measures. These analyses parsed subtasks of continuous performance and two-back working memory tasks, conducting separate analyses of response times on specific component measures and on rates of true and false positive responses for task components (Hansen et al., 2003, 2004, 2009). These findings split moment-to-moment performance into executive and nonexecutive components, highlighting HRV-related differences in the former.
The present study sought to extend Hansen and colleagues’ work by testing resting HRV, as a continuous variable, as a predictor of performance on a multifaceted EF construct in a large, diverse sample. The lack of a significant HRV–EF relationship under these conditions contrasts with Hansen et al.’s work, yet it parallels findings by Kimhy et al. (2013), whose multifaceted EF construct included the current study’s three EF indicators plus two additional tests tapping processing speed and reasoning ability. Their covariate-adjusted models also showed no relationship of either resting or post-challenge HRV to EF, while exploratory post hoc analyses of individual cognitive measures revealed one nominally significant relationship: Faster vagal recovery from stress (but not resting HRV) was significantly related to faster response on the mixed-trial Red/Green task (Kimhy et al., 2013). This task’s demands for rapid task-switching and inhibition of rote, prepotent responses bear notable resemblance to the demands of Hansen and colleagues’ continuous performance task measure, suggesting that HRV may correlate with rapid switching between rote tasks under time pressure, though the extent to which this relationship is due to executive demands or other task features is unclear.
In sum, the significant relationship between HRV and EF in the initial structural model was eliminated by the addition of covariates (age, BMI, diabetes/hyperglycemia). The covariate-adjusted model showed age as the strongest predictor of EF, which is broadly consistent with research on age-related decline in both executive abilities (e.g. Lusczc, 2011) and HRV (e.g., Voss et al., 2012), and therefore with the possibility that controlling their relationship for age across a broad range may represent a form of “overcontrol” (Type II error). The lack of relationship between HRV and EF in the covariate-adjusted model contrasts with prior findings in studies using smaller, more homogenous samples; simultaneous collection of cognitive and HRV data; dichotomized HRV measures; and narrower EF conceptualizations (Hansen et al., 2003, 2004, 2009). It is consistent with the results of Kimhy et al. (2013), whose multifaceted EF measures likewise showed no HRV– EF relationship after covariate adjustments.
4.3 Limitations
The literature on HRV and its many potential correlates includes a variety of findings that have yet to be fully integrated and resolved. This work includes recent studies supporting associations between HRV and either EF or NA in particular clinical populations (e.g., Hovland et al., 2012; Pittig et al., 2013), and HRV reactivity research showing task-related differences (e.g., Shcheslavskaya et al., 2010). Debate is ongoing with regards to the respective significance of task-based measures of HRV reactivity versus HRV measured at a resting baseline as meaningful indicators of neurocardiac interaction and parasympathetic tone (Berntson et al., 2007). Also under discussion is whether and when it may be useful, inconsequential, or problematic to control HRV for respiration (Denver et al., 2007). On this point, recent theoretical and empirical work suggests that such controls may be unnecessary (Denver et al., 2007; Thayer et al., 2011) particularly for baseline measures taken at rest (Houtveen et al., 2002), like those in the present study. Nonetheless, the mixed results and related debates underscore the need for caution in interpreting the findings of any single study of HRV.
In this vein, several limitations of the present study should be kept in mind. First, the characteristics of SEM are important to consider: It can probe causal relationships but it cannot demonstrate causality; it poses challenges for examining nonlinear relationships, which may characterize autonomic functioning; and it shares with other methods the potential to yield misleading conclusions if influential variables are omitted (Berntson et al., 1994; Tomarkin & Waller, 2005). Nonetheless, its ability to provide a global test of model fit for a set of linear relationships and to compare nested models makes it appropriate for testing theory-driven predictions about such relationships between a physiological variable, HRV, and the multifaceted psychological constructs of NA and EF.
Second, the use of cross-sectional data precludes causal or developmental inferences about the observed associations (Kraemer et al., 2000), a common limitation in research on HRV’s associations with EF or NA. As future waves of MIDUS gather physiological and cognitive data using the protocols initiated in MIDUS II, analyses of longitudinal change in the variables of interest will become possible. In those analyses, it will be necessary to address cohort effects, as higher levels of anxiety have been found in younger compared to older cohorts at comparable ages (Twenge, 2000). This effect complicates the inference of developmental change from the inverse age–NA relationship, pending longitudinal data on MIDUS II Biomarker Project measures. Similar cohort effects on EF are possible, in relation to educational levels. Older participants with lower HRV (Stein et al., 1997) also may tend to be less educated than younger participants, based on trends in U.S. educational attainment (U.S. Bureau of the Census, 1977, 1987; U.S. Department of Education, 1996). Given that education level has shown associations with executive skills (e.g., Tun & Lachman, 2008; van Hooren et al., 2007), it may interact with age as a confounding influence on EF. However, controlling EF for education level also could artificially reduce real EF variance, because associations between education and many aspects of cognitive functioning is theoretically bidirectional (Cesi, 1991).
Third, the present sample poses limits on generalizability. There was low representation of racial and ethnic minorities among MIDUS II participants for whom both cognitive and biomarker data were collected, which is important given the differences in resting HRV and in its patterns of age-related change between U.S. racial groups (Choi, 2006; Liao et al., 1995). Also under-represented were individuals in poor physical health (Radler & Ryff, 2010). As a result, older participants may have had lower NA than older adults in the U.S. population at large, given that among older individuals, ill health is among the strongest predictors of anxiety and depression (Jorm, 2000; Wade & Cairney, 2000). Similarly, although medication-based exclusion criteria were imposed to avoid confounding effects on HRV, they may have enhanced the age–NA relationship and/or attenuated the age–EF relationship. Both possibilities reflect the increased risk of relatively poorer cardiovascular health among excluded individuals, which is associated with increased risk of depression and anxiety as well as with a vascular risk profile thought to confer executive impairments (Raz et al., 2003; Vicario et al., 2005). The age–NA relationship also may be specific to the portion of the lifespan represented in MIDUS II data (midlife to old age), given that, across the adult lifespan, a quadratic relationship has been described in which NA is relatively low during early adulthood, peaks at midlife, and declines in old age (Blanchflower & Oswald, 2008). Notwithstanding these considerations, the representativeness of the present sample compares favorably with many previous studies of HRV’s relationship to EF or NA.
5. Conclusions
Under the conditions of the current study, in the structural model that showed the best fit with the data, resting high frequency HRV had no predictive value in relation to either NA or EF after accounting for other influences, particularly age. Population and methodological differences may explain the discrepancy between these findings and those previously reported. It is also possible that statistical control for age obscures meaningful, psychologically relevant individual differences in HRV, including links facets of EF. Nonetheless, the study provided an opportunity to confirm suggestions that individual differences in HRV are broadly predictive of cognitive and affective outcomes (e.g., Porges, 2011; Thayer et al., 2009) and it failed to do so.
Instead, the findings suggest that refinements in the understanding of linkages of HRV to NA and EF could be informed by (1) more fine-grained analysis of specific component elements of NA and EF and their possible differential associations with HRV, particularly the potential value of parsing the broad EF construct into specific abilities including processing speed (Luszcz, 2011) and subtask measures that differentially capture particular executive functions (Kimhy et al., 2013); (2) explicit examination of processes associated with chronological age in relation to HRV and its hypothesized correlates; for example, age-related changes in vasculature that have been linked to affective disorders, cognitive ability, and cardiovascular functioning (e.g., Debette et al., 2011; Kivimäki et al., 2012; Kovacic et al., 2011; Matsuo et al., 2005); (3) more large-scale tests of those interpretations in demographically diverse samples that allow controls for medication effects; and (4) targeted studies of specific populations in which theory provides a rationale for particular hypotheses concerning the significance of individual differences in HRV.
Highlights.
HRV was tested as a predictor of negative affect (NA) and executive functioning (EF).
The initial SEM showed a positive HRV-EF relationship; HRV-NA was nonsignificant.
After covariate adjustment, HRV’s associations with NA and EF were nonsignificant.
Population and methodological differences from prior research are discussed.
Results suggest refinements to the interpretations of prior findings and HRV theory.
Acknowledgments
This article is based on a thesis submitted by Sarah Mann, under the supervision of Dr. Contrada, in partial fulfillment of the requirements for the degree of Master of Science in the Clinical Psychology Ph.D. Program at Rutgers University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Edward A. Selby, Email: edward.selby@rutgers.edu.
Marsha E. Bates, Email: mebates@rutgers.edu.
Richard J. Contrada, Email: contrada@rci.rutgers.edu.
References
- Albinet CA, Boucard G, Bouquet CA, Audiffren M. Increased heart rate variability and executive performance after aerobic training in the elderly. Eur ] Appl Physiol. 2010;109:617–24. doi: 10.1007/s00421-010-1393-y. [DOI] [PubMed] [Google Scholar]
- Albinet CT, Boucard G, Bouquet CA, Audiffren M. Processing speed and executive functions in cognitive aging: how to disentangle their mutual relationship? Brain Cognition. 2012;79(1):1–11. doi: 10.1016/j.bandc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Rev Gen Psychol. 2006;10(3):229. [Google Scholar]
- Arbuckle JL. Amos (Version 21.0) [Computer Program] Chicago: SPSS; 2012. [Google Scholar]
- Bates ME, Buckman JF. Integrating body and brain systems in addiction neuroscience. In: Miller P, editor. Encyclopedia of Addictive Behaviors. New York: Elsevier; 2013. [Google Scholar]
- Benarroch EE. The central autonomic network. In: Low PA, editor. Clinical Autonomic Disorders. 2. Philadelphia: Lippincott-Raven; 1997. pp. 17–23. [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Grossman P. Whither vagal tone. Biol Psychol. 2007;74(2):295–300. doi: 10.1016/j.biopsycho.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS, Fabro VT. Autonomic space and psychophysiological response. Psychophysiology. 1994;31(1):44–61. doi: 10.1111/j.1469-8986.1994.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Berntson G, Lozano D, Chen Y. Filter properties of root mean square successive difference (RMSSD) for heart rate. Psychophysiology. 2005;42:246–252. doi: 10.1111/j.1469-8986.2005.00277.x. [DOI] [PubMed] [Google Scholar]
- Bertsch K, Hagemann D, Naumann E, Schächinger H, Schulz A. Stability of heart rate variability indices reflecting parasympathetic activity. Psychophysiology. 2012;49:672–682. doi: 10.1111/j.1469-8986.2011.01341.x. [DOI] [PubMed] [Google Scholar]
- Blanchflower DG, Oswald AJ. Is well-being U-shaped over the life cycle? Soc Sci Med. 2008;66:1733–1749. doi: 10.1016/j.socscimed.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Bleil ME, Gianaros PJ, Jennings JR, Flory JD, Manuck SB. Trait negative affect: toward an integrated model of understanding psychological risk for impairment in cardiac autonomic function. Psychosom Med. 2008;70(3):328–337. doi: 10.1097/PSY.0b013e31816baefa. [DOI] [PubMed] [Google Scholar]
- Bollen KA. Structural Equations with Latent Variables. New York: Wiley; 1989. [Google Scholar]
- Borella E, Ghisletta P, de Ribaupierre A. Age differences in text processing: The role of working memory, inhibition, and processing speed. J Gerontol B Psychol Sci Soc Sci. 2011;66B(3):311–320. doi: 10.1093/geronb/gbr002. [DOI] [PubMed] [Google Scholar]
- Brooks L, McCabe P, Schneiderman N. Stress and the cardiometabolic syndrome. In: Contrada RJ, Baum A, editors. The Handbook of Stress Science. New York: Springer; 2011. pp. 1–9. [Google Scholar]
- Brown TA. Confirmatory Factor Analysis for Applied Research. New York: Guilford Press; 2006. [Google Scholar]
- Browne MW, Cudeck R. In: Alternative ways of assessing model fit. KA, editor. 1993. [Google Scholar]
- Burnham KP, Anderson DR. Multimodel inference understanding AIC and BIC in model selection. Socio/Methods Res. 2004;33(2):261–304. [Google Scholar]
- Bryan J, Luszcz MA. Measurement of executive function: Considerations for detecting adult age differences. J Clin Exp Neuropsychol. 2000;22(1):40–55. doi: 10.1076/1380-3395(200002)22:1;1-8;FT040. [DOI] [PubMed] [Google Scholar]
- Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF, Czajkowski SM, O’Connor C, Stone PH, Freedland KE. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104(17):2024–2028. doi: 10.1161/hc4201.097834. [DOI] [PubMed] [Google Scholar]
- Cesi S. How much does schooling influence general intelligence and its cognitive components? A reassessment of the evidence. Dev Psychol. 1991;27(5):703–722. [Google Scholar]
- Choi JB, Hong S, Nelesen R, Bardwell WA, Natarajan L, Schubert C, Dimsdale JE. Age and ethnicity differences in short-term heart-rate variability. Psychosom Med. 2006;68(3):421–426. doi: 10.1097/01.psy.0000221378.09239.6a. [DOI] [PubMed] [Google Scholar]
- Clark DM. Anxiety disorders: Why they persist and how to treat them. Behav Res Ther. 1999;37:S5–S27. doi: 10.1016/s0005-7967(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Cocia IR, Uscătescu LC, Rusu AS. Attention bias to threat in anxiety-prone individuals. Evidence from disengagement, but not engagement bias using cardiac vagal tone. J Psychophysiol. 2012;26(2):74–82. [Google Scholar]
- Cohen J. The cost of dichotomization. Appl Psychol Meas. 1983;7(3):249–253. [Google Scholar]
- Consonni D, Bertazzi PA, Zocchetti C. Why and how to control for age in occupational epidemiology. Occup Environ Med. 1997;54(11):772–776. doi: 10.1136/oem.54.11.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contrada RJ, Boulifard DA, Idler EL, Krause TJ, Labouvie EW. Course of depressive symptoms in patients undergoing heart surgery: Confirmatory analysis of the factor pattern and latent mean structure of the Center for Epidemiologic Studies Depression Scale. Psychosom Med. 2006;68(6):922–930. doi: 10.1097/01.psy.0000244391.56598.10. [DOI] [PubMed] [Google Scholar]
- Cook EW, Davis TL, Hawk LW, Spence EL, Gautier CH. Fearfulness and startle potentiation during aversive visual stimuli. Psychophysiology. 29:633–645. doi: 10.1111/j.1469-8986.1992.tb02038.x. [DOI] [PubMed] [Google Scholar]
- Davis JW, Chung R, Juarez DT. Prevalence of comorbid conditions with aging among patients with diabetes and cardiovascular disease. Hawaii Med J. 2011;70(10):209–213. [PMC free article] [PubMed] [Google Scholar]
- Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer RW, Karemaker JM, Strackee J. Comparing spectra of a series of 37 point events, particularly for heart rate variability spectra. IEEE Trans Biomed Eng, BME-31. 1984:384–387. doi: 10.1109/TBME.1984.325351. [DOI] [PubMed] [Google Scholar]
- Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, Schouten EG. Low heart rate variability in a 2-min rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC study. Circulation. 2000;102:1239–1244. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men: The Zutphen study. Am J Epidemiol. 1997;145:899–908. doi: 10.1093/oxfordjournals.aje.a009049. [DOI] [PubMed] [Google Scholar]
- Denver JW, Reed SF, Porges SW. Methodological issues in the quantification of respiratory sinus arrhythmia. Biol Psychology. 2007;74(2):286–294. doi: 10.1016/j.biopsycho.2005.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman RK, Nakamura Y, Garcia ME, Thompson RW, Dunn AL, Blair SN. Heart rate variability, trait anxiety, and perceived stress among physically fit men and women. Int J Psychophysiol. 2000;37:121–133. doi: 10.1016/s0167-8760(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Dütsch M, Burger M, Dörfler C, Schwab S, Hilz MJ. Cardiovascular autonomic function in poststroke patients. Neurology. 2007;69(24):2249–2255. doi: 10.1212/01.wnl.0000286946.06639.a7. [DOI] [PubMed] [Google Scholar]
- Engle RW. Working memory capacity as executive attention. Curr Dir Psychol Sci. 2002;11(1):19–23. [Google Scholar]
- Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol Psychol. 2007;74(2):185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Fuller BF. The effects of stress–anxiety and coping styles on heart rate variability. Int J Psychophysiol. 1992;12:81–86. doi: 10.1016/0167-8760(92)90045-d. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: Emerging evidence for a brain-body pathway to coronary heart disease risk. Neurolmage. 2009;47(3):922–936. doi: 10.1016/j.neuroimage.2009.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick G, Braunwald E, Lewis RM. Relative roles of the sympathetic and parasympathetic nervous systems in the reflex control of heart rate. Circ Res. 1965;16(4):363–375. doi: 10.1161/01.res.16.4.363. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Foot M, Davis M. Fear-potentiated startle: Relationship to the level of state/trait anxiety in healthy subjects. Biol Psychiatry. 1993;34:566–574. doi: 10.1016/0006-3223(93)90094-t. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Hansen AL, Johnsen BH, Sollers JJ, Stenvik K, Thayer JF. Heart rate variability and its relation to prefrontal cognitive function: The effects of training and detraining. Eur J Appl Physiol. 2004;93:263–272. doi: 10.1007/s00421-004-1208-0. [DOI] [PubMed] [Google Scholar]
- Hansen AL, Johnsen BH, Thayer JF. Vagal influence in the regulation of attention and working memory. Int J Psychophysiol. 2003;48:263–274. doi: 10.1016/s0167-8760(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Hansen AL, Johnsen BH, Thayer JF. Relationship between heart rate variability and cognitive function during threat of shock. Anxiety Stress Copin. 2009;22:77–89. doi: 10.1080/10615800802272251. [DOI] [PubMed] [Google Scholar]
- Harris FJ. On the use of windows for harmonic analysis with the discrete Fourier transform. Proceedings of the IEEE. 1978;66:51–83. [Google Scholar]
- Harrison JE, Buxton P, Husain M, Wise R. Short test of semantic and phonological fluency: Normal performance, validity, and test–retest reliability. Br J Clin Psychol. 2000;39:181–191. doi: 10.1348/014466500163202. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Van Alstine J, Usala PD, Hultsch DF, Dixon R. Measurement properties of the Center for Epidemiological Studies Depression Scale (CES-D) in older populations. Psychol Assess. 1990;2(1):64–72. [Google Scholar]
- Hofmann W, Schmeichel BJ, Baddeley AD. Executive functions and self-regulation. Trends Cognit Sci. 2012;16(3):174–180. doi: 10.1016/j.tics.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Hovland A, Pallesen S, Hammar Å, Hansen AL, Thayer JF, Tarvainen MP, Nordhus IH. The relationships among heart rate variability, executive functions, and clinical variables in patients with panic disorder. Int J Psychophysiol. 2012;86(3):269–275. doi: 10.1016/j.ijpsycho.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Hoyle RH, editor. Handbook of Structural Equation Modeling. New York: Guilford Press; 2012. [Google Scholar]
- Houtveen JH, Rietveld S, Geus EJ. Contribution of tonic vagal modulation of heart rate, central respiratory drive, respiratory depth, and respiratory frequency to respiratory sinus arrhythmia during mental stress and physical exercise. Psychophysiology. 2002;39(4):427–436. doi: 10.1017.S0048577202394022. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional versus new alternatives. Strut Eq Modeling. 1999;6:1–55. [Google Scholar]
- IBM Corporation. IBM SPSS Statistics (Version 21) [Computer Program] Armonk, NY: IBM Corp; 2012. [Google Scholar]
- Jennings RJ, Allen B, Gianaros PJ, Thayer JF, Manuck SB. Focusing neurovisceral integration: Cognition, heart rate variability, and cerebral blood flow. Psychophysiology. 2014 doi: 10.1111/psyp.12319. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- Jorm AF. Does old age reduce the risk of anxiety and depression? A review of epidemiological studies across the adult life span. Psychol Med. 2000;30:11–22. doi: 10.1017/s0033291799001452. [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Spielberg JM, Warren SL, Sutton BP, Miller GA, Heller W, Banich MT. Distracted and down: neural mechanisms of affective interference in subclinical depression. Social cognitive and affective neuroscience. 2014 doi: 10.1093/scan/nsu100. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H, Yoshikawa T, Nomura K, Ito H, Yamauchi H, Ogura M, Honjo S. Hemodynamic changes in the prefrontal cortex during digit span task: a near-infrared spectroscopy study. Neuropsychobiology. 2011;63(2):59–65. doi: 10.1159/000323446. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: A reviewand meta-analysis. Biol Psychiatry. 2010;67:1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Felmingham KL, Matthews S, Jelinek HF. Depression, comorbid anxiety disorders, and heart rate variability in physically healthy, unmedicated patients: Implications for cardiovascular risk. PLoS One. 2012;7(2):e30777. doi: 10.1371/journal.pone.0030777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhy D, Crowley OV, McKinley PS, Burg MM, Lachman ME, Tun PA, Ryff CD, Seeman TE, Sloan RP. The association of cardiac vagal control and executive functioning—Findings from the MIDUS study. J of Psychiatr Res. 2013;47(5):628–635. doi: 10.1016/j.jpsychires.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivimäki M, Shipley MJ, Allan CL, Sexton CE, Jokela M, Virtanen M, Tiemeier H, Ebmeier KP, Singh-Manoux A. Vascular risk status as a predictor of later-life depressive symptoms: a cohort study. Biol Psychiatry. 2012;72(4):324–330. doi: 10.1016/j.biopsych.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiger RE, Bigger JT, Bosner MS, Chung MK, Cook JR, Rolnitzky LM, Steinman R, Fleiss JL. Stability over time of variables measuring heart rate variability in normal subjects. Am J Cardiol. 1991;68:626–630. doi: 10.1016/0002-9149(91)90355-o. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 2. New York: Guilford Press; 2005. [Google Scholar]
- Kogan AV, Allena JJB, Weihs KL. Cardiac vagal control as a prospective predictor of anxiety in women diagnosed with breast cancer. Biol Psychol. 2012;90(1):105–111. doi: 10.1016/j.biopsycho.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Koster EH, Crombez G, Verschuere B, Van Damme S, Wiersema JR. Components of attentional bias to threat in high trait anxiety: Facilitated engagement, impaired disengagement, and attentional avoidance. Behav Res Ther. 2006;44(12):1757–1771. doi: 10.1016/j.brat.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Kovacic JC, Moreno P, Nabel EG, Hachinski V, Fuster V. Cellular senescence, vascular disease, and aging; part 2 of a 2-part review: clinical vascular disease in the elderly. Circulation. 2011;123(17):1900–1910. doi: 10.1161/CIRCULATIONAHA.110.009118. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? Am J Psychiatry. 2000;157:163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Kramer A, Hahn S, Gopher D. Task coordination and aging: Explorations of executive control processes in the task switching paradigm. Acta Psychol. 1999;101:339–378. doi: 10.1016/s0001-6918(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Kreibig SD. Autonomic nervous system activity in emotion: A review. Biol Psychol. 2010;84(3):394–421. doi: 10.1016/j.biopsycho.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Kuo TB, Lin T, Yang CC, Li CL, Chen CF, Chou P. Effect of aging on gender differences in neural control of heart rate. Am J Physiol. 1999;277(6 Pt 2):H2233–H2239. doi: 10.1152/ajpheart.1999.277.6.H2233. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Tun PA. Cognitive testing in large scale surveys. Assessment by telephone. In: Hofer S, Alwin A, editors. Handbook of Cognitive Aging: Interdisciplinary Perspective. Thousand Oaks, CA: Sage; 2008. pp. 506–522. [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107(1):139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lampert R, Ickovics JR, Viscoli CJ, Horwitz RI, Lee FA. Effects of propranolol on recovery of heart rate variability following acute myocardial infarction and relation to outcome in the Beta-Blocker Heart Attack Trial. Am J Cardiol. 2003;91:137–142. doi: 10.1016/s0002-9149(02)03098-9. [DOI] [PubMed] [Google Scholar]
- Levy MN. Autonomic interactions in cardiac control. Am N Y Acad Sci. 1990;601:209–221. doi: 10.1111/j.1749-6632.1990.tb37302.x. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, editors. Neuropsychological Assessment. 4. New York: Oxford University Press; 2004. [Google Scholar]
- Liao D, Barnes RW, Chambless LE, Simpson RJ, Jr, Sorlie P, Heiss G. Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability—the ARIC study. Atherosclerosis Risk in Communities. Am J Cardiol. 1995;76(12):906–912. doi: 10.1016/s0002-9149(99)80260-4. [DOI] [PubMed] [Google Scholar]
- Licht CM, de Geus EJ, van Dyck R, Penninx BW. Association between anxiety disorders and heart rate variability in The Netherlands Study of Depression and Anxiety (NESDA) Psychosom Med. 2009;71(5):508–518. doi: 10.1097/PSY.0b013e3181a292a6. [DOI] [PubMed] [Google Scholar]
- Licht CM, de Geus EJ, Zitrnan FG, Hoogendijk WJ, van Dyck R, Penninx BW. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA) Arch Gen Psychiatr. 2008;65(12):1358–1367. doi: 10.1001/archpsyc.65.12.1358. [DOI] [PubMed] [Google Scholar]
- Lindström J, Tuomilehto J. The Diabetes Risk Score: A practical tool to predict type 2 diabetes risk. Diabetes Care. 2003;26(3):725–731. doi: 10.2337/diacare.26.3.725. [DOI] [PubMed] [Google Scholar]
- Luszcz M. Executive function and cognitive aging. In: Schaie KW, Willis SL, editors. Handbook of the Psychology of Aging. 7. London: Academic Press; 2011. pp. 59–72.pp. 59–72. [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychol Meth. 2002;7(1):19. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Onodera Y, Hamamoto T, Muraki K, Kato N, Kato T. Hypofrontality and microvascular dysregulation in remitted late-onset depression assessed by functional near-infrared spectroscopy. Neurolmage. 2005;26(1):234–242. doi: 10.1016/j.neuroimage.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Melzig CA, Weike AI, Hamm AO, Thayer JF. Individual differences in fear-potentiated startle as a function of resting heart rate variability: implications for panic disorder. Int J Psychophysiol. 2009;71(2):109–117. doi: 10.1016/j.ijpsycho.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Mid-life in the United States (MIDUS) Mid-life in the United States: A national study of health and well-being. 2007 Retrieved January 8, 2014 from http://www.midus.wisc.edu/newsletter/MIDUS_Final.pdf.
- Miller EK, Cohen JD. Integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miskovic V, Schmidt LA. Frontal brain electrical asymmetry and cardiac vagal tone predict biased attention to social threat. Int J Psychophysiol. 2010;75:332–338. doi: 10.1016/j.ijpsycho.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Mitchell RL, Phillips LH. The psychological, neurochemical and functional neuroanatomical mediators of the effects of positive and negative mood on executive functions. Neuropsychologic. 2007;45(4):617–629. doi: 10.1016/j.neuropsychologia.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognit Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute (NHLBI) [Accessed 1/3/13];Lowering heart disease risk: What are the risk factors for heart disease? 2012 Feb 29; at http://www.nhlbi.nih.gov/educational/hearttruth/lower-risk/risk-factors.htm.
- O’Brien IAD, O’Hare P, Corrall RJM. Heart rate variability in healthy subjects: Effect of age and the derivation of normal ranges for tests of autonomic function. Brit Heart J. 1986;55:348–354. doi: 10.1136/hrt.55.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41:673–690. [Google Scholar]
- Pittig A, Arch JJ, Lam CW, Craske MG. Heart rate and heart rate variability in panic, social anxiety, obsessive–compulsive, and generalized anxiety disorders at baseline and in response to relaxation and hyperventilation. Int J Psychophysiol. 2013;87(1):19–27. doi: 10.1016/j.ijpsycho.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Porges SJ. The Polyvagal Theory. New York: W. W. Norton; 2011. [Google Scholar]
- Radler BT, Ryff CD. Who participates? Longitudinal retention in the MIDUS National Study of Health and Well-Being. J Aging Health. 2010;22(3):307–331. doi: 10.1177/0898264309358617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radioff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cerebr Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Roderigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences, and modifiers. Cerebr Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003;117(6):1169. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Vernon SW. The center for epidemiologic studies depression scale: Its use in a community sample. Am J Psychiatry. 1983;140(1):41–46. doi: 10.1176/ajp.140.1.41. [DOI] [PubMed] [Google Scholar]
- Robinson RG. The Clinical Neuropsychiatry of Stroke. 2. New York: Cambridge University Press; 2006. [Google Scholar]
- Rottenberg J. Cardiac vagal control in depression: A critical analysis. Biological Psychol. 2007;74(2):200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- Ruiz-Padial E, Sollers JJ, 3rd, Vila J, Thayer JF. The rhythm of the heart in the blink of an eye: Emotion-modulated startle magnitude covaries with heart rate variability. Psychophysiology. 2003;40(2):306–13. doi: 10.1111/1469-8986.00032. [DOI] [PubMed] [Google Scholar]
- Ryff CD, Seeman T, Weinstein M. National Survey of Midlife Development in the United States (MIDUS II): Biomarker Project, 2004–2009 ICPSR 29282. Ann Arbor, MI: 2010a. Documentation for psychophysiology protocol in MIDUS II Biomarker Project (P4) [Google Scholar]
- Ryff CD, Seeman T, Weinstein M. National Survey of Midlife Development in the United States (MIDUS II): Biomarker Project, 2004–2009. Ann Arbor, MI: 2010b. Summary of the MIDUS II Biomarker Project (P4) ICPSR 29282. [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Savalei V, Bentler PM. A statistically justified pairwise ML method for incomplete nonnormal data: A comparison with direct ML and pairwise ADF. Struct Equ Modeling. 2005;12:183–214. [Google Scholar]
- Shcheslavskaya OV, Burg MM, McKinley PS, Schwartz JE, Gerin W, Ryff CD, Weinstein M, Seeman TE, Sloan RP. Heart rate recovery after cognitive challenge is preserved with age. Psycbosom Med. 2010;72(2):128–133. doi: 10.1097/PSY.0b013e3181c94ca0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnreich R, Kark JD, Friedlander Y, Sapoznikov D, Luria MH. Five minute recordings of heart rate variability for population studies: repeatability and age–sex characteristics. Heart. 1998;80:156–162. doi: 10.1136/hrt.80.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow WG, Tierney MC, Zorzitto ML, Fisher RH, Reid DW. One-year test-retest reliability of selected neuropsychological tests in older adults. J Clin Exp Neuropsychol. 1988;10:60. doi: 10.1080/01688638908400903. (Abstract) [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State–Trait Anxiety Inventory (Form Y) (“Self-evaluation questionnaire”) Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Spielberger CD. State–Trait Anxiety Inventory: A Comprehensive Bibliography. Consulting Psychologists Press; Palo Alto, CA: 1989. [Google Scholar]
- Spreng RN, Wojtowicz M, Grady C. Reliable differences in brain activity between young and old adults: A quantitative meta-analysis across multiple cognitive domains. Neurosci Biobehav Rev. 2010;34:1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Stein PK, Carney RM, Freedland KE, Skala JA, Jaffe AS, Kleiger RE, Rottman JN. Severe depression is associated with markedly reduced heart rate variability in patients with stable coronary heart disease. J Psycbosom Res. 2000;48(4):493–500. doi: 10.1016/s0022-3999(99)00085-9. [DOI] [PubMed] [Google Scholar]
- Stein PK, Kleiger RE, Rottman JN. Differing effects of age on heart rate variability in men and women. Am J Cardiol. 1997;80(3):302–305. doi: 10.1016/s0002-9149(97)00350-0. [DOI] [PubMed] [Google Scholar]
- Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 2009;53:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychiatry. 2007;74:224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Loerbroks A, Sternberg EM. Inflammation and cardiorespiratory control: the role of the vagus nerve. Respir Physiol Neurobiol. 2011;178(3):387–394. doi: 10.1016/j.resp.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Toivonen L. Influence of acute alpha 1-adrenergic antagonism on heart rate variability in patients with old myocardial infarction. J Cardiovasc Pharmacol. 1994;23(6):932–935. doi: 10.1097/00005344-199406000-00011. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Waller NG. Structural equation modeling: Strengths, limitations, and misconceptions. Annu Rev Clin Psychol. 2005;1:31–65. doi: 10.1146/annurev.clinpsy.1.102803.144239. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Larson MG, Venditti FJ, Manders ES, Evans JC, Feldman CL, Levy D. Electrophysiology/pacing: impact of reduced heart rate variability on risk for cardiac events: The Framingham Heart study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Venditti FJ, Jr, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90(2):878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- Tun PA, Lachman ME. Telephone assessment of cognitive function in adulthood: The Brief Test of Adult Cognition by Telephone (BTACT) Age Ageing. 2006;35:629–633. doi: 10.1093/ageing/afl095. [DOI] [PubMed] [Google Scholar]
- Tun PA, Lachman ME. Age differences in reaction time and attention in a national telephone sample of adults: Education, sex, and task complexity matter. Dev Psychol. 2008;44(5):1421–1429. doi: 10.1037/a0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twenge JM. The age of anxiety? Birth cohort change in anxiety and neuroticism, 1952–1993. J Pers Soc Psychol. 2000;79(6):1007–1021. doi: 10.1037//0022-3514.79.6.1007. [DOI] [PubMed] [Google Scholar]
- U.S. Bureau of the Census. Educational attainment in the United States: March 1977 and 1976. Washington, DC: U.S. Government Printing Office; 1977. Current population reports, series P-20, 314. [Google Scholar]
- U.S. Bureau of the Census. Educational attainment in the United States: March 1982 to 1985. Washington, DC: U.S. Government Printing Office; 1987. Current population reports, series P-20, 415. [PubMed] [Google Scholar]
- U.S. Department of Education. The condition of education 1996, NCES 96-304. Washington, DC: U.S. Government Printing Office; 1996. National Center for Education Statistics. [Google Scholar]
- van Hooren SAH, Valentijn AM, Bosma H, Ponds RWHM, van Boxtel MPJ, Jolles J. Cognitive functioning in healthy older adults aged 64–81: A cohort study into the effects of age, sex, and education. Aging Neuropsychol Cognit. 2007;14(1):40–54. doi: 10.1080/138255890969483. [DOI] [PubMed] [Google Scholar]
- Vicario A, Martinez CD, Baretto D, Diaz Casale A, Nicolosi L. Hypertension and cognitive decline: Impact on executive function. J Clin Hypertens. 2005;7(10):598–604. doi: 10.1111/j.1524-6175.2005.04498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen R, Jula A, Salminen JK, Voipio-Pulkki LM, Helenius H, Kuusela T, Airaksinen J. Anxiety and hostility are associated with reduced baroreflex sensitivity and increased beat-to-beat blood pressure variability. Psychosom Med. 2003;65(5):751–756. doi: 10.1097/01.psy.0000088760.65046.cf. [DOI] [PubMed] [Google Scholar]
- Voss A, Heitmann A, Schroeder R, Peters A, Perz S. Short-term heart rate variability—age dependence in healthy subjects. Physiol Meas. 2012;33(8):1289–1311. doi: 10.1088/0967-3334/33/8/1289. [DOI] [PubMed] [Google Scholar]
- Wade TJ, Cairney J. The effect of sociodemographics, social stressors, health status and psychosocial resources on the age-depression relationship. Can J Public Health. 2000;91(4):307–312. doi: 10.1007/BF03404295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LL, Grossman P, Krishnan R, Sherwood A. Anxiety and vagal control of heart rate. Psychosom Med. 1998;61:334–340. doi: 10.1097/00006842-199807000-00018. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R manual. New York: Psychological Corporation; 1981. [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–92. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- West R. In defense of the frontal lobe hypothesis of cognitive aging. J lnt Neuropsychol Soc. 2000;6:727–729. doi: 10.1017/s1355617700666109. [DOI] [PubMed] [Google Scholar]
- Yusuf HR, Giles WH, Croft JB, Anda RF, Casper ML. Impact of multiple risk factor profiles on determining cardiovascular disease risk. Prev Med. 1998;27(1):1–9. doi: 10.1006/pmed.1997.0268. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Cunningham WA. Executive function: Mechanisms underlying emotion regulation. In: Gross James J., editor. Handbook of Emotion Regulation. New York: Guilford Press; 2007. pp. 135–158. [Google Scholar]