Abstract
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) improves the motor symptoms of Parkinson’s disease (PD). The STN may represent an important relay station not only in the motor but also the associative cortico-striato-thalamocortical pathway. Therefore, STN stimulation may alter cognitive functions such as working memory (WM). We examined cortical effects of STN-DBS on WM in early PD patients using functional near-infrared spectroscopy. The effects of dopaminergic medication on WM were also examined. Lateral frontal activity during WM maintenance was greater when patients were taking dopaminergic medication. STN-DBS led to a trend-level worsening of WM performance, accompanied by increased lateral frontal activity during WM maintenance. These findings suggest that STN-DBS in PD might lead to functional modifications of the basal ganglia-thalamocortical pathway during WM maintenance.
Keywords: working memory, deep brain stimulation, Parkinson’s disease, functional near-infrared spectroscopy, frontal cortex
Introduction
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) has emerged as a highly effective treatment for improving the motor symptoms in patients with Parkinson’s disease (PD) (Okun et al., 2009; Follett et al., 2010; Odekerken et al., 2013; Schuepbach et al., 2013). A prominent hypothesis suggests a functional division of the STN into sensorimotor (dorsolateral), cognitive-associative (ventromedial), and limbic (orbital and medial) parts with distinct connectivity to cortical regions (Hamani et al., 2004; Parent & Hazrati, 1995; Temel et al., 2005). Therefore, the STN may represent an important relay station not only in the motor but also the associative cortico-striato-thalamocortical circuitry that connects the basal ganglia via thalamic projections with regions in the dorsolateral prefrontal cortex (DLPFC) (Alexander et al., 1986; Temel et al., 2005). However, a complete functional segregation within the STN suggested by the anatomical work has been challenged by several studies of STN-DBS in PD (Eisenstein et al., 2014; Hershey et al., 2010; Mallet et al., 2007; McNeely et al., 2011). Moreover, given the small size of the STN and the variability in the number and localization of its subdivisions (Keuken et al., 2012), the current applied during DBS to the dorsolateral sensorimotor part is likely to spread to adjacent regions involved in cognition. Therefore, STN stimulation, which has a beneficial effect on motor functions in PD may also lead to modifications of cognitive functions, such as working memory (WM).
WM allows us to actively hold and manipulate information, thus supporting complex cognitive functions such as reasoning, comprehension, and learning (Baddeley, 2012). WM deficits, associated with abnormal dorsolateral prefrontal cortex (DLPFC) activity, affect all aspects of behavior and quality of life (Cahn et al., 1998; Schrag, et al., 2000). The effects of STN stimulation on the DLPFC have been investigated via different approaches. First, cognitive functions that are known to be mediated by the DLPFC have been compared pre- and post-DBS surgery. These studies report a moderate decline in verbal fluency after DBS surgery (Saint-Cyr et al., 2000; Weaver et al., 2009; Witt et al., 2008; Zahodne et al., 2009). However, effects of DBS surgery on WM is less clear (Saint-Cyr et al., 2000; Witt et al., 2008; Weaver et al., 2009; Zahodne et al., 2009; Follett et al., 2010; Rothlind et al., 2015). Second, to isolate specific effects of STN-DBS on WM, patients who have already had DBS surgery were tested on and off DBS. So far, the findings are inconclusive. Some studies report no effect of DBS on WM (Selzler et al, 2013; Ventre-Dominey et al., 2014), while others report a beneficial effect (Pillon et al., 2000; Rivaud-Péchoux et al., 2000; Mollion et al., 2011). However, some studies report adverse effects of DBS on WM when task demands are high (Alberts et al., 2008; Hershey et al., 2004, 2008). Lastly, there is evidence to suggest that STN-DBS in PD increases WM-related activity in DLPFC (Ventre-Dominey et al., 2014), and such change in activity correlates with impaired WM performance (Campbell et al., 2008).
Impairments of WM and executive functions may stem from STN-DBS but they may also reflect the degenerative process of PD. More than half of PD patients develop MCI after five years (Broeders et al., 2013), reaching approximately 80% after eight years (Aarsland et al. 2003). Even among the non-demented PD patients, there may be cognitive impairments (Litvan, 2011). These deficits are correlated with changes in the associative cortico-striato-thalamocortical circuitry. However, the contributions of the sub-components of WM remain poorly understood. For instance, it is unclear whether WM deficits in PD are caused by abnormal processing within the basal ganglia (Dagher et al, 2001; Marklund et al., 2009; Owen, et al, 1998) or reflect a prefrontal deficit (Cools et al., 2002; Mattay et al. 2002). Hypo-activity of the striatum and DLPFC during WM tasks in PD patients points to a disruption of the normal information flow through the basal ganglia-prefrontal pathway (Ekman et al., 2012; Lewis et al., 2003) but direct evidence for a dysfunctional basal ganglia-PFC interplay sub-serving WM has not been demonstrated because past neuroimaging studies only report correlational information.
STN-DBS combined with functional imaging is a unique approach that can assess the involvement of frontostriatal pathways in WM, and identify dysfunctional mechanisms associated with them. In contrast to the majority of existing studies, we focused on the patients in the early stages of the disease because pathological neuroanatomical changes as well as WM deficits worsen over time (Hawkes et al., 2010; Owen et al., 1992). Thus specific effects of DBS on WM are more likely to be detected during the early than later stages. Additionally, early PD patients are often on lower doses of medication, thus reducing the potentially confounding effects of pharmaceutical therapies.
We used functional near-infrared spectroscopy (fNIRS) to measure prefrontal cortical activity during the delay period of a spatial delayed-response task in early PD patients under conditions on and off STN-DBS. FNIRS measures cortical activation based on blood oxygenation levels similar to fMRI. FNIRS is compatible with metal implants and provides a noninvasive, silent, and comfortable environment, making it especially suitable for DBS patients, and allows us to directly test the effect of STN stimulation during a WM task. The cortical effect of STN-DBS was compared to the effect of dopaminergic medication in PD. Finally, we assessed WM-related prefrontal activity in a group of demographically matched healthy controls.
Materials and Methods
Participants
Nine PD patients with bilateral STN stimulators were recruited from the Vanderbilt Clinic of Neurological Surgery from of a larger pool of patients who were participating in a randomized controlled study of DBS in early PD (FDA Investigational Device Exemption G050016, ClinicalTrials.gov NCT00282152). Detailed description of this clinical trial is found in Charles et al. (2012).
Inclusion criteria were: 50–75 years old, no focal pathology or mass lesion on MRI, and no evidence of dementia on pre-surgical testing. Patients with a history of neurological events or diagnoses other than PD were excluded. At the time of testing, all patients met criteria for Hoehn and Yahr stage II (Goetz et al., 2004) and had been medicated with dopaminergic drug for more than 6 months but less than 4 years.
Eight healthy participants with no history of neuropsychiatric disorder were recruited through advertisement. The two groups were matched for age [t(15) = 0.14, p = .89], education [t(15) = −0.97, p = .35], and IQ [t(15) = −.32, p = .76]. Due to technical problems WM performance data were lost for 3 patients, but fNIRS were recorded from everybody. The sample of the remaining 6 PD patients with recorded performance data did not differ from healthy controls in age [t(12) = −0.06, p = .95], education [t(12) = −0.69, p = .50], and IQ [t(15) = 0.40, p = .70].
Demographic, clinical, and medication data are reported in Tables 1 and 2. Written informed consent was obtained after the participants were given a complete description of the study. The Vanderbilt University Institutional Review Board approved the protocol and consent procedure, in accordance with the Declaration of Helsinki.
Table 1.
Demographic and clinical information.
| PD patients Mean (SD) |
Healthy controls Mean (SD) |
|
|---|---|---|
| Male/Female | 8/1 (all right-handed) | 6/2 (all right-handed) |
| Age (years) | 63.8 (6.8) | 63.3 (8.6) |
| Years of education | 14.7 (2.4) | 15.9 (2.7) |
| IQa | 110.3 (9.7) | 111.9 (10.4) |
| MMSE | n/a | 29.1 (1.4) |
| DRS-IIb | 13.89 (2.26) | n/a |
| Years of illness | 4.0 (1.66) | n/a |
| Hoehn & Yahr stage | 2.0 (0.0) | n/a |
| UPDRS motor scorec: DBS on/MED on | 25.6 (13.6) (range: 3–45) | n/a |
| UPDRS motor score: DBS off/MED off | 38.2 (16.5) (range:12–66) | n/a |
| Time since surgery/first visit (months) | 17.3 (4.4) | n/a |
NART
Age-corrected total scaled scores were calculated (maximum score = 18).
The maximum UPDRS motor score with rigidity scores excluded was 88.
SD = standard deviation, MMSE = Mini-mental State Examination, DSR-II = Dementia Rating scale II.
Table 2.
Medication information.
| Subject | Medication | LED at time of testinga |
|---|---|---|
| 1 | levodopa/carbidopa, pramipexole | 600 |
| 2 | ropinirole | 480 |
| 3 | levodopa/carbidopa, entacapone, pramipexole | 549 |
| 4 | pramipexole, selegiline | 325 |
| 5 | ropinirole | 160 |
| 6 | levodopa, selegiline | 300 |
| 7 | levodopa/carbidopa, entacapone | 399 |
| 8 | levodopa/carbidopa, ropinirole | 850 |
| 9 | levodopa/carbidopa, entacapone | 399 |
| Mean (SD): 451.33 (200.19) |
Levodopa equivalent dose (LED) based on conversion formulae reported by Tomlinson et al. (2010).
SD = standard deviation
For patients, Unified Parkinson’s Disease Rating Scale motor scores (UPDRS III; Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease, 2003) were obtained during a week-long period of observation as follows: 1. at the beginning of the week when the patients were on medication and DBS was on; 2. at the end of the week when the patient had been off medication for a week and DBS was off. UPDRS assessment was video-recorded and evaluated blind to treatment, medication, and stimulation states (Charles et al., 2012).
Protocol for WM fNIRS
PD patients were tested in three experimental conditions on consecutive days: (1) DBS and medication on (DBS-on/MED-on), (2) DBS off and medication on (DBS-off/MED-on), (3) DBS and medication off (DBS-off/MED-off). DBS was turned off at least 30 minutes before testing. Medication was discontinued at least 24 hours before testing.
DBS Surgery and Contact Location
Surgical targeting of bilateral STN chronic DBS electrodes (Medtronic 3389, four contacts) involved preoperative imaging (T1 and T2 weighted MRI, CT), microelectrode mapping, and stimulation mapping for placement of optimal effect (for details, see Kahn et al., 2012). Following surgery, CT scans were obtained on the same day to confirm electrode location. We subsequently identified the contact of stimulation after a period of clinical optimization. The electrode positioning and the active contacts in AC-PC space are given in Table 3. Participants received monopolar stimulation with mean pulse amplitude of 1.8V (SD =0.59) in the left STN and 1.7V (SD =0.31) in the right STN. Pulse width and frequency were set to 60 Hz and 130 Hz, respectively.
Table 3.
Subthalamic nucleus electrode positioning and stimulation parameters.
| Subject | Left side | Right side | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Active contacts | X | Y | Z | V | Active contacts | X | Y | Z | V | |
| 1 | C+, 2− | 13.63 | 0.36 | −2.49 | 1.3 | C+, 0− | −13.99 | −2.42 | −4.43 | 1.3 |
| 2 | C+, 2− | 8.52 | −4.89 | −3.02 | 1.8 | C+, 2− | −11.0 | −3,50 | −2,00 | 1.8 |
| 3 | C+, 2− | 9.43 | −0.87 | −3,58 | 3,2 | C+, 2− | −12.20 | −0.84 | −4,57 | 2.2 |
| 4 | C+, 1− | 11.64 | −3.51 | −6.17 | 1.4 | C+, 0− | −12.50 | −4.00 | −3.50 | 1.4 |
| 5 | C+, 3− | 10.50 | −0.90 | −6.21 | 1.7 | C+, 2− | −11.56 | 1.57 | −5.96 | 1.7 |
| 6 | C+, 2− | 10.69 | −0.37 | −1.88 | 1.3 | C+, 2− | −10.60 | 1.59 | −2.52 | 1.3 |
| 7 | C+, 2− | 11.82 | −2.00 | −4.25 | 1.7 | C+, 1− | −12.27 | −2.50 | −4.57 | 1.7 |
| 8 | C+, 3− | 12.51 | −1.23 | −3.06 | 2.2 | C+, 0− | −12.39 | −1.58 | −1.26 | 1.7 |
| 9 | C+, 2− | 11.81 | −0.75 | −0.41 | 2 | C+, 3− | −10.32 | −0.95 | 0.38 | 2 |
All values are in millimeters and relative to the midpoint between the anterior and posterior commissures. Positive/negative x-value = right/left; positive/negative y-value = anterior/posterior; positive/negative z-value = superior/inferior. V = voltage. The coordinates indicate the position of the center of the 4 contact array.
Stimuli and Spatial Delayed-Response Task
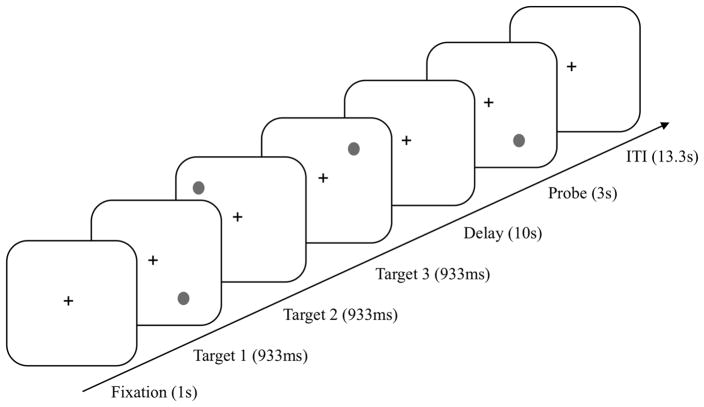
We used a spatial delayed-response task to assess WM (Goldman-Rakic, 1999) (Figure 1). Participants sat approximately 60cm from a 17-inch monitor in a completely darkened room. Each trial began with a fixation point for 1s, followed by 3 targets (identical black circles) sequentially, in different locations for 933ms each. During the 10s delay, participants fixated on a central cross. Then a probe was presented for 3s and participants decided whether the location of the probe matched one of the remembered target locations or not. They responded verbally (yes or no). The experimenter pressed the corresponding key, as tremor severity may prevent reliable manual responses in the DBS-off condition. On each day (DBS-on/MED-on, DBS-off/MED-on, DBS-off/MED-off), participants performed 4 blocks of 7 trials each. The trial order was randomized across blocks. Practice trials were given until participants were completely familiarized and accurate.
Figure 1.
Stimuli and trial design in the spatial delayed-response task.
FNIRS
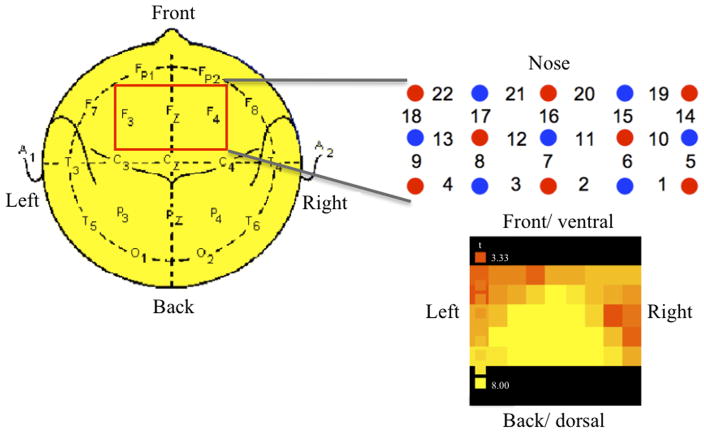
Changes in oxyhemoglobin and deoxyhemoglobin were recorded on a continuous wave system (ETG-4000 Hitachi Medical Co.) using a 3×5 optode probe set (8 light emitters, 7 detectors) resulting in 22 channels (Figure 2). Each emitter consisted of two continuous laser diodes (3mW±0.15mW on ‘high power’) with two wavelengths, 695±20nm, and 830±20nm, that are most sensitive to deoxyhemoglobin and oxyhemoglobin, respectively. Wavelengths were amplitude modulated (0.6 and 1.5KHz). The signal was amplified, demodulated, and digitized (10Hz). The inter-optode distance was 30mm, producing a light penetration close to 20mm. The probeset was placed centrally over the forehead in accordance with the international 10–20 system for EEG electrode placement. The middle extended over the F3 and F4 points such that the probeset covered bilaterally the lateral and medial frontal cortex.
Figure 2.
FNIRS setup. A 3 × 5 probe optode set [8 emitters (red) and 7 detectors (blue) resulting in 22 channels] was placed centrally over the forehead with the middle row extending over the F3 and F4 points according to the international EEG 10–20 system. The statistical map indicates changes in oxyhemoglobin interpolated across channels.
Data Analysis
FNIRS data were analyzed using Matlab (MathWorks MA) and BrainVoyager QX (BrainInnovation, Maastricht). Raw data were preprocessed by applying a bandpass filter (range 0.01–0.5Hz) to remove artifacts due to respiration and cardiac variation. After temporal downsampling (from 10–1Hz), data were converted to the measurement of oxyhemoglobin, deoxyhemoglobin, and total hemoglobin levels according to the modified Beer-Lambert Law for scattering media (Obrig et al., 2000). We focused on oxyhemoglobin level because it has been suggested to be the most robust indicator for changes in regional cerebral blood flow in cognitive fNIRS studies (Hoshi et al., 2001). Data preprocessing included linear detrending to remove overall linear drifts. A multi-subject statistical analysis was performed by multiple linear regression of the oxyhemoglobin time series for each sensor (Schroeter et al., 2004). The general linear model of the experiment was computed from 138 z-normalized functional time courses with 4 regressors for each experimental condition representing the different time periods of the task (encoding, delay, probe, inter-trial interval) and one error regressor. The analysis focused on the delay period, which was modeled by a regressor of 10-s duration. The inter-trial interval served as a baseline condition and was modeled by a regressor spanning the last 10s. The corresponding regressors were obtained by convolution of an ideal boxcar response with a gamma function model of the hemodynamic response (Zhang et al., 2005). The statistical maps were generated by associating each sensor with the F-value corresponding to the specific set of regressors and calculated on the basis of the least mean squares solution of the general linear model with a fixed-effects model. To compare activations between groups (controls vs. DBS-off/MED-off) and between experimental conditions within the delay phase (DBS-on/MED-on vs. DBS-off/MED-on; DBS-off/MED-on vs. DBS-off/MED-off), linear contrasts were performed using t-statistics. Multi-subject statistical maps were thresholded at q< 0.05, corrected for false discovery rate.
To account for small sample size (8 healthy controls, 6 patients) of the behavioral WM data, non-parametric tests were used. The Mann-Whitney-U test was used to compare performance between controls and PD patients in the DBS-off/MED-off condition. The Wilcoxon test was used to assess the effects of DBS (DBS-on/MED-on vs. DBS-off/MED-on), and medication (DBS-off/MED-on vs. DBS-off/MED-off) on WM performance. Effect sizes are given in terms of the common language effect size indicator (CL; McGraw & Wong, 1992).
Results
UPDRS scores
Blinded UPDRS motor scores were significantly worse in the DBS-on/medication-on state than the DBS-off/medication-off state [t(8) = −2.45, p < .05].
Effect of PD
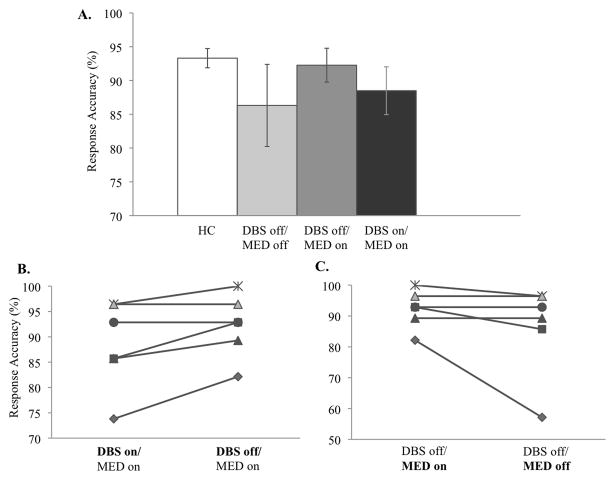
We compared healthy controls and PD patients in the condition DBS-off/MED-off. There was no group difference in WM accuracy (z = −0.86, p = .41, CL = 0.6351) (Figure 3A). Thus, these early PD patients had intact WM performance.
Figure 3.
Behavioral performance. (A) Response accuracy in healthy controls (= HC, N = 8) and PD patients (N = 6) who were tested in three conditions. (B) Individual performance in conditions DBS-on/MED-on and DBS-off/MED-on. (C) Individual performance in conditions DBS-off/MED-on and DBS-off/MED-off. Each line represents data of one patient. The same symbols are used for a given patient in Figures 3B and 3C. Error bars indicate standard error of the mean.
Comparing fNIRS across groups
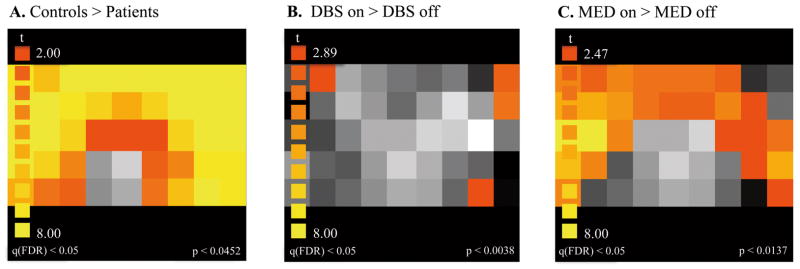
Statistical maps for oxyhemoglobin are shown in Figure 4. There was a significantly greater delay-related activation in bilateral frontal regions in controls compared with patients in the DBS-off/MED-off condition (Figure 4A) indicating hypofrontality in PD (when they are free of medication and stimulation) compared to controls.
Figure 4.
Statistical (t) oxyhemoglobin maps for WM maintenance. (A) PD effect: Controls > patients (DBS-off/MED-off), (B) DBS effect: DBS-on/MED-on > DBS-off/MED-on, (C) Medication effect: DBS-off/MED-on > DBS-off/MED-off.
Effect of bilateral STN stimulation on WM maintenance in PD
There was a trend for lower WM accuracy when the DBS was turned on compared to off in PD patients (z=−1.84, p=0.066, CL=0.833) (Figures 3A,B). To assess the cortical effect of STN-DBS on delay-related activity (i.e. storage component of WM), we compared the oxyhemoglobin time-series during the delay between DBS-on/MED-on and DBS-off/MED-on. Frontal oxyhemoglobin levels were higher in the DBS-on than in the DBS-off condition (Figure 4B).
Effect of dopaminergic medication on WM in PD
To assess the medication effect without the DBS, we compared WM performance and frontal activity between conditions DBS-off/MED-on and DBS-off/MED-off. WM accuracy did not differ between medication-on and medication-off conditions (z=−1.60, p=0.11, CL=0.753) (Figures 3A,C). Although on average, medication did not exert a significant effect on WM performance, three patients performed better on medication than off, and these were the same patients whose WM performance deteriorated when DBS was switched on (Figures 3A,C). However, due to the small sample size, it remains a clinical observation. Medication effects on frontal activity during WM maintenance were assessed by comparing the oxyhemoglobin time-series during the delay between conditions DBS-off/MED-on and DBS-off/MED-off. Frontal oxyhemoglobin levels were higher in MED-on compared to MED-off condition (Figure 4C).
Discussion
The primary goal of this study was to investigate the effect of STN-DBS in PD patients on WM-related activity in the frontal cortex. To elucidate the specificity of the effects of STN-DBS and to reduce potential confounds of cognitive deficits that emerge in later stages of PD, we recruited early PD patients. The effect of STN-DBS was compared to the effect of dopaminergic medication in the same patients. Finally, we assessed WM delay-related frontal activity in demographically-matched healthy controls.
Effect of Parkinson’s disease
Overall, WM was intact in early PD patients even when they were tested without medication and DBS. WM is critical to all cognitive processes. WM deficits contribute significantly to MCI. Although MCI is common even in early PD (Litvan et al., 2011; Yarnall et al., 2014), our early PD patients were unlikely to have MCI because they had normal WM and excellent IQ scores (mean =110.3, s.d.=9.7) well above the population mean. Therefore, any changes in neural activity observed in the DBS-on or medication-on conditions could be attributed to the therapies (stimulation or medication) rather than the degenerative process of PD. However, to rule out any possibilities for the existence of MCI in early PD patients, future studies should address all of the MCI criteria, as outlined in Litvan et al. (2011).
Intact WM performance observed in our patients suggests that different cognitive domains (i.e., amnestic and non-amnestic) and different WM components (i.e, maintenance and manipulation) may be compromised during different stages of PD. Whereas deficits in the manipulation of information in WM is observed in the early course of PD (Lewis et al., 2003; Owen, 2004), deficits in WM maintenance emerge during later stages(Owen et al., 1992, 1993). Our delayed-response task emphasized WM maintenance and did not require the manipulation of WM representations. Moreover, in early PD patients, if there are any cognitive deficits, they are often found in non-amnestic functions, such as executive control (Caviness et al., 2007; Litvan et al., 2011).
Although WM maintenance seems to be intact in our patients especially on medication, they still showed lower delay-related activation in the DLPFC compared to controls. It is possible that the delay-related hypo-frontality observed in our patients signals the beginning of a prefrontal dysfunction, which may eventually lead to behavioral impairments with further disease progression and/or higher task demands. This hypothesis remains to be tested as the PD progresses to later stages.
Effect of bilateral STN stimulation on WM
We observed a trend towards reduced WM accuracy under bilateral STN-DBS in a small group of early PD patients. Because we compared WM performance within-subjects on and off DBS, it seems unlikely that this trend-level reduction was due to effects of the surgery itself and/or factors that can confound pre- and postsurgical comparisons (e.g. effects of time). If the storage of multiple locations in WM is slightly compromised when the STN-DBS is switched on, it may suggest a possible cognitive side effect of STN-DBS (Alberts et al., 2008; Hershey et al., 2004, 2008). However, our sample size was small and cannot conclude that STN-DBS led to worsening of WM accuracy. The trend-level difference in WM accuracy on- and off-STN DBS suggests that a larger sample size may be able to address this question.
We did observe an effect of STN-DBS on PFC activity during a WM task in these patients. Although increased frontal activity did not lead to better WM performance, half of the patients were performing at 90% accuracy, so there is a possibility of a ceiling effect.
Mechanisms of action of STN stimulation
Although the leads targeted the dorsolateral sensorimotor part of the STN, the current could spread to adjacent regions associated with cognition (Butson et al, 2007). Considerable evidence suggests that STN stimulation activates afferent and efferent axons surrounding the electrode (Hashimoto et al. 2003). Because axonal fibers have lower stimulation thresholds than cell bodies, the local stimulation effect is not necessarily limited to the sphere of efficient current field. Furthermore, the modulation threshold may be lower in the associative circuit than the motor circuit due to unique electrophysiological characteristics (Temel et al., 2005). Considering these “spatial” and “threshold” effects of stimulation, it is likely that the stimulation in the STN region result in functional modifications not only within the motor but also within parts of the associative pathway supporting WM. However, the exact mechanisms on the local and system level that underlie the functional modifications still need to be determined.
The loss of dopaminergic transmission in the basal ganglia results in irregular patterns of neuronal discharges and oscillatory activity in the STN and the basal ganglia output nuclei in PD (Deniau et al., 2010). Subsequently, noisy signals in the motor cortico-striato-thalamocortical circuitry may lead to the characteristic motor symptoms of PD. Although the mechanisms of action of STN-DBS are uncertain, there is increasing evidence that the therapeutic effect of STN stimulation in PD may stem from the regularization of the activity pattern rather than changes in firing rates per se (Garcia et al, 2005). The more regular pattern of activity is then transmitted throughout the motor cortico-striato-thalamocortical circuitry and might mimic an “informational lesion” that prevents the transmission of pathological information (Grill et al., 2004). Such informational lesions might explain reduced activity in the motor cortex under STN-DBS observed in neuroimaging studies (Karimi et al., 2008; Payoux et al., 2004).
In our study, STN-DBS led to increased prefrontal activity during a WM task without a statistically significant effect on the behavioral WM performance. Typically, in the neuroimaging literature, greater activity of recruitment of brain regions has been interpreted to reflect ‘better’ performance. However, under pathological conditions, greater activity could indicate more effort or resources needed to achieve a certain level of performance (e.g. correctly remembering a target position), and/or the presence of more ‘noise’ in the signal. The regularization of STN output through DBS might decrease the transmission of pathological signals within the motor cortico-striato-thalamocortical circuitry, whereas the opposite effect could occur in non-motor pathways. The forced regular rate of firing induced by DBS spreading to associative regions of the STN may override or interfere with the characteristic firing patterns that are optimal for WM storage (Hikosaka et al., 1989). Thus, increased frontal activity under STN-DBS observed in our study might be a consequence of increased pathological (noisy) signals within the associative pathway.
It has been proposed that the basal ganglia performs a gating function and thus controls the thalamocortical pathways in the motor domain (Lidsky et al., 1985). Our findings hint at a similar mechanism in the cognitive domain. In the context of WM, the gating mechanism might control the transfer of information into WM, thereby limiting interference or distractibility (Cools, 2006; Marklund et al., 2009; McNab & Klingberg, 2008). Such mechanism overlaps with key components of inhibitory control (McNab et al. 2008), which is impaired under STN-DBS in PD (Hershey et al., 2010; Ray et al., 2009), and regulated by the STN and the inferior PFC (Aron & Poldrack, 2006; Aron et al., 2007). However, our study was not designed to test the specific inhibitory mechanisms in the service of WM, and therefore, future studies are needed to test whether STN-DBS specifically impairs this process. It is possible that while STN stimulation in PD might restore the gating mechanism in the motor cortico-striato-thalamocortical circuitry, it may also lead to dysfunctional gating mediated by the associative circuitry.
Alternatively, the STN stimulation-induced increase in prefrontal activity during a WM task could reflect a compensatory mechanism. Such a mechanism has been debated in the schizophrenia literature to explain hyper-frontality in low-demand conditions of WM tasks where the performance of schizophrenic patients is close to that of healthy controls (Callicott et al., 2003). The compensatory hypothesis suggests that when the task is easy, healthy participants do not need to exert effort in order to achieve a certain level of accuracy but those with compromised WM need to rely on greater cortical activity to reach the same level of performance. Our WM task was easy, as can be seen in the high accuracy scores (Figure 3). To test for the compensatory hypothesis, the neural activity across the two groups must be compared while matching for performance accuracy. Therefore, we examined the frontal activity when the participants correctly remembered the target locations (correct trials). Because the two groups did not significantly differ on WM performance overall, the correct-trial analysis did not affect the overall fNIRS results. Thus, even after controlling for possible performance difference by analyzing neural activity for correct trials only, we still observed greater WM-related frontal activity in the DBS-on condition. This finding might reflect abnormal compensation for the disruption of information flow through the basal ganglia-prefrontal pathway but our study cannot directly test this conjecture. To better understand the mechanisms underlying the STN-DBS, future studies need to directly test gating/decreased efficiency hypothesis vs. dynamic compensation.
Effect of dopaminergic medication on WM
Dopaminergic medication increased prefrontal activity but did not significantly change WM performance overall. Three patients performed better on medication and these were the same patients who showed worsened performance with DBS-on. Although the sample size is small, these preliminary findings suggest a differential effect of STN stimulation and dopaminergic medication on WM in our early-PD patients. Whereas increased prefrontal activity through dopaminergic medication might be beneficial for WM, an additional boost through STN-DBS might counteract this effect, a hypothesis that needs to be tested in the future. How can we explain this effect? First, it is important to note that the effect of STN-DBS on frontal activity was qualitatively similar to the effect of dopaminergic medication. Both medication and DBS increased frontal activity even if they did not significantly change behavioral performance (perhaps due to a ceiling effect). This raises the possibility that the DBS may work by influencing the activity of dopaminergic neurons. Animal studies have shown that STN stimulation increases striatal dopamine release (Meissner et al., 2003). Second, it must be noted that the effects of dopaminergic medication on WM can be beneficial or detrimental following an inverted U shape, depending on task demands and dopamine levels in distinct parts of the striatum (Cools, 2006). For instance, the Levodopa equivalent dose that is sufficient to treat motor symptoms may be too much for other regions that are relatively intact (e.g., frontal cortex), resulting in WM impairments (Cools, 2006). Although we found no evidence for a medication-induced dopaminergic ‘overdose’ when the STN stimulation is added on top of the medication, such an ‘overdose’ effect might be functionally expressed. However, it is also possible that DBS affects motor functions without any clear influence on dopamine release (de Hemptinne et al. 2013; Hirschmann et al. 2013)
There are several limitations to our study. First, our sample size was small and accordingly nonparametric tests were used. The behavioral data from three patients were not recorded, which reduced the sample size for the WM performance analysis. However, fNIRS data were available for all participants and thus, neuroimaging data were more robust. Second, to ensure that the total duration of the experiment was acceptable for our patients we used a small number of trials. Third, WM performance was intact in our early PD patients when they were off stimulation and off medication. Therefore, we do not know if our results generalize to other PD patients who do suffer from MCI (Litvan et al., 2011). Fourth, we chose 30 minutes washout period based on past studies that have shown that after 30 minutes, the motor symptoms reappear (Temperli et al., 2003), and cognitive and cortical functions deteriorate (Hershey et al., 2004; Selzler et al., 2013) in PD patients. However, it is unclear whether this applies to early PD patients as well. If chronic effects of STN-DBS occurred in our patients, we may have underestimated the effect of STN-DBS on WM. In addition, our patients had been medicated for at least 6 months, so we cannot exclude the potential effects of medication. However, increased frontal activity during WM maintenance in the DBS-on condition suggests that DBS effect on cortical activity goes beyond the effect of medication. Patients were always tested in the on-condition before the off-condition because we recruited them from an ongoing clinical trial with a fixed sequence of protocol. Therefore, practice effects cannot be excluded, but this seems unlikely because in general, practice effects for WM occur only with hundreds of trials (Olesen et al, 2004). More importantly, WM performance was worst in the DBS-off/MED-off condition when the task had been practiced the most. Behavioral performance and frontal activity could not be compared between the conditions DBS-on/MED-on and DBS-on/MED-off (patients were not tested under the latter condition), and therefore it was not possible to further assess the hypothesis that adding dopaminergic medication on top of STN-DBS caused an medication-induced dopaminergic ‘overdose’ in our patients.
Another caveat is the possibility of task-unrelated, general effects of DBS, but if this was the case STN-DBS should increase frontal activity not only during the delay period but also during the inter-trial interval. To test this hypothesis we compared the oxyhemoglobin time series during the inter-trial interval between conditions DBS-on/MED-on and DBS-off/MED-on. We observed no frontal region that showed higher levels of oxyhemoglobin under STN stimulation. Furthermore, specific effects of STN-DBS were demonstrated in a previous study of WM; increased DLPFC but not supplementary motor cortex activity under STN correlated with poor WM (Campbell et al., 2008).
Conclusion
Bilateral STN-DBS in early PD patients led to increased frontal cortical activity during a WM task without significantly changing WM accuracy possibly due to behavioral ceiling effect. This finding suggests functional modifications of the basal-ganglia prefrontal circuits upon STN stimulation.
Acknowledgments
We thank Kristen Merkle, Chris Cannistraci, and Aaron Albritton for help with data collection, Jejoong Kim for his help with data analysis, and Corrie Camalier for valuable comments on a former version of the manuscript.
Funding
This work was supported by a Discovery Grant, MH-073028, NS-070136, and HD-015052, the Vanderbilt CTSA grant UL1 RR024975/UL1 TR000445 from the National Center for Research Resources, NIH-NIBIB 1 R01-EB006136, and by gifts from private donors.
Footnotes
The interpretation of the value .63 is that if you were randomly to select pairs of scores (i.e., one from the controls and one from the patients), 63% of the time the WM performance of the controls would exceed the WM performance of the patients.
The interpretation of the value .833 is that if you were randomly to select one case (matched pairs), the probability that the WM performance in the DBS-off condition would exceed the WM performance in the DBS-on condition is 83.3%.
The interpretation of the value .75 is that if you were randomly to select one case (matched pairs), the probability that the WM performance in the MED-on condition would exceed the WM performance in the MED-off condition is 75%.
Conflicts of interest
Vanderbilt University has received income in excess of $10,000 from grants or contracts with Medtronic, Allergan, Ipsen, Merz, UCB, and Teva for educational or research programs led by David Charles. David Charles receives income in excess of $10,000 from Medtronic, Allergan, Ipsen, and the Alliance for Patient Access for education and consulting services.
Contributor Information
Jutta S. Mayer, Email: mayer@psych.uni-frankfurt.de.
Joseph Neimat, Email: joseph.neimat@vanderbilt.edu.
Bradley S. Folley, Email: bradfolley@gmail.com.
Sarah K. Bourne, Email: sarah.k.bourne@gmail.com.
Peter E. Konrad, Email: peter.konrad@vanderbilt.edu.
David Charles, Email: david.charles@vanderbilt.edu.
Sohee Park, Email: sohee.park@vanderbilt.edu.
References
- Alberts JL, Voelcker-Rehage C, Hallahan K, et al. Bilateral subthalamic stimulation impairs cognitive-motor performance in Parkinson’s disease patients. Brain. 2008;131:3348–3360. doi: 10.1093/brain/awn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Anderson VC, Burchiel KJ, Hogarth P, et al. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol. 2005;62:554–560. doi: 10.1001/archneur.62.4.554. [DOI] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DM, et al. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci. 2007;27:11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD. Working memory: theories, models, and controversies. Ann Rev Psychology. 2012;63:1–29. doi: 10.1146/annurev-psych-120710-100422. [DOI] [PubMed] [Google Scholar]
- Butson CR, Cooper SE, Henderson JM, McIntyre CC. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage. 2007;34:661–670. doi: 10.1016/j.neuroimage.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn DA, Sullivan EV, Shear PK, et al. Differential contributions of cognitive and motor component processes to physical and instrumental activities of daily living in Parkinson’s disease. Arch Clinical Neuropsychology. 1998;13:575–583. [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, et al. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. American J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Campbell MC, Karimi M, Weaver PM, et al. Neural correlates of STN DBS-induced cognitive variability in Parkinson disease. Neuropsychologia. 2008;46:3162–3169. doi: 10.1016/j.neuropsychologia.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness JN, Driver-Dunckley E, Connor DJ, et al. Defining mild cognitive impairment in Parkinson’s disease. Mov Disord. 2007;22:1272–1277. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- Charles PD, Dolhun RM, Gill CE, et al. Deep brain stimulation in early Parkinson’s disease: Enrollment experience from a pilot trial. Parkinsonism & Related Disorders. 2012;18:268–273. doi: 10.1016/j.parkreldis.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobeh Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cools R, Gibbs SE, Miyakawa A, et al. Working memory capacity predicts dopamine synthesis capacity in the human striatum. J Neuroscience. 2008;28:1208–1212. doi: 10.1523/JNEUROSCI.4475-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Stefanova E, Barker RA, et al. Dopaminergic modulation of high-level cognition in Parkinson’s disease: the role of the prefrontal cortex revealed by PET. Brain. 2002;125:584–594. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- Dagher A, Owen AM, Boecker H, Brooks DJ. The role of the striatum and hippocampus in planning: a PET activation study in Parkinson’s disease. Brain. 2001;124:1020–1032. doi: 10.1093/brain/124.5.1020. [DOI] [PubMed] [Google Scholar]
- de Hemptinne C, Ryapolova-Webb ES, Air EL, et al. Exaggerated phase–amplitude coupling in the primary motor cortex in Parkinson disease. PNAS. 2013;110(12):4780–4785. doi: 10.1073/pnas.1214546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniau JM, Degos B, Bosch C, Maurice N. Deep brain stimulation mechanisms: beyond the concept of local functional inhibition. European J Neuroscience. 2010;32:1080–1091. doi: 10.1111/j.1460-9568.2010.07413.x. [DOI] [PubMed] [Google Scholar]
- Eisenstein SA, Koller JM, Black KD, et al. Functional anatomy of subthalamic nucleus stimulation in Parkinson disease. Ann Neurol. 2014;76:279–295. doi: 10.1002/ana.24204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman U, Eriksson J, Forsgren L, et al. Functional brain activity and presynaptic dopamine uptake in patients with Parkinson’s disease and mild cognitive impairment: a cross-sectional study. Lancet Neurology. 2012;11:679–687. doi: 10.1016/S1474-4422(12)70138-2. [DOI] [PubMed] [Google Scholar]
- Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. NEJM. 2010;362:2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- Garcia L, D’Alessandro G, Bioulac B, Hammond C. High-frequency stimulation in Parkinson’s disease: more or less? TINS. 2005;28:209–216. doi: 10.1016/j.tins.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force Report on the Hoehn and Yahr Staging Scale: Status and Recommendations. Movement Disorders. 2004;19:1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- Grill WM, Snyder AN, Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport. 2004;15:1137–1140. doi: 10.1097/00001756-200405190-00011. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, et al. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neuroscience. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Saint-Cyr JA, Fraser J, et al. The subthalamic nucleus in the context of movement disorders. Brain. 2004;127:4–20. doi: 10.1093/brain/awh029. [DOI] [PubMed] [Google Scholar]
- Hawkes CH, Del Tredici K, Braak H. A timeline for Parkinson’s disease. Parkinsonism & Related Disorders. 2010;16:79–84. doi: 10.1016/j.parkreldis.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Hershey T, Campbell MC, Videen TO, et al. Mapping Go-No-Go performance within the subthalamic nucleus region. Brain. 2010;133:3625–3634. doi: 10.1093/brain/awq256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey T, Revilla FJ, Wernle A, et al. Stimulation of STN impairs aspects of cognitive control in PD. Neurology. 2004;62:1110–1114. doi: 10.1212/01.wnl.0000118202.19098.10. [DOI] [PubMed] [Google Scholar]
- Hershey T, Wu J, Weaver PM, et al. Unilateral vs. bilateral STN DBS effects on working memory and motor function in Parkinson disease. Experimetal Neurology. 2008;210:402–408. doi: 10.1016/j.expneurol.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. III. Activities related to expectation of target and reward. J Neurophysiology. 1989;61:814–832. doi: 10.1152/jn.1989.61.4.814. [DOI] [PubMed] [Google Scholar]
- Hirschmann J, Özkurt TE, Butz M, et al. Differential modulation of STN-cortical and cortico-muscular coherence by movement and levodopa in Parkinson’s disease. Neuroimage. 2013;68:203–213. doi: 10.1016/j.neuroimage.2012.11.036. [DOI] [PubMed] [Google Scholar]
- Hoshi Y, Kobayashi N, Tamura M. Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model. J Applied Physiology. 2001;90:1657–1662. doi: 10.1152/jappl.2001.90.5.1657. [DOI] [PubMed] [Google Scholar]
- Kahn E, D’Haese PF, Dawant B, et al. Deep brain stimulation in early stage Parkinson’s disease: operative experience from a prospective randomised clinical trial. J Neurology, Neurosurgery, Psychiatry. 2012;83:164–170. doi: 10.1136/jnnp-2011-300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Golchin N, Tabbal SD, et al. Subthalamic nucleus stimulation-induced regional blood flow responses correlate with improvement of motor signs in Parkinson disease. Brain. 2008;131:2710–2719. doi: 10.1093/brain/awn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuken MC, Uylings HB, Geyer S, et al. Are there three subdivisions in the primate subthalamic nucleus? Frontiers Neuroanatomy. 2012;6:14. doi: 10.3389/fnana.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Lal R, O’Neil JP, et al. Striatal dopamine and working memory. Cerebral Cortex. 2009;19:445–454. doi: 10.1093/cercor/bhn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Friedman HR, Davachi L, Goldman-Rakic PS. Differential activation of the caudate nucleus in primates performing spatial and nonspatial working memory tasks. J Neuroscience. 1997;17:3870–3882. doi: 10.1523/JNEUROSCI.17-10-03870.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJ, Cools R, Robbins TW, et al. Using executive heterogeneity to explore the nature of working memory deficits in Parkinson’s disease. Neuropsychologia. 2003;41:645–654. doi: 10.1016/s0028-3932(02)00257-9. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, et al. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neuroscience. 2003;23:6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Aarsland D, Adler CH, et al. MDS Task Force on mild cognitive impairment in Parkinson’s disease: critical review of PD-MCI. Mov Disord. 2011;26:1814–1824. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidsky TI, Manetto C, Schneider JS. A consideration of sensory factors involved in motor functions of the basal ganglia. Brain Research. 1985;356:133–146. doi: 10.1016/0165-0173(85)90010-4. [DOI] [PubMed] [Google Scholar]
- Limousin P, Pollak P, Benazzouz A, et al. Effect on parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995;345:91–95. doi: 10.1016/s0140-6736(95)90062-4. [DOI] [PubMed] [Google Scholar]
- Mallet L, Schupbach M, N’Diaye K, et al. Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. PNAS. 2007;104:10661–1066. doi: 10.1073/pnas.0610849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund P, Larsson A, Elgh E, et al. Temporal dynamics of basal ganglia under-recruitment in Parkinson’s disease: transient caudate abnormalities during updating of working memory. Brain. 2009;132:336–346. doi: 10.1093/brain/awn309. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Tessitore A, Callicott JH, et al. Dopaminergic modulation of cortical function in patients with Parkinson’s disease. Ann Neurology. 2002;51:156–164. doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- McGraw KO, Wong SO. A common language effect size statistic. Psychol Bull. 1992;111:361–365. [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to WM. Nature Neuroscience. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- McNab F, Leroux G, Strand F, et al. Common and unique components of inhibition and working memory: an fMRI, within-subjects investigation. Neuropsychologia. 2008;46:2668–2682. doi: 10.1016/j.neuropsychologia.2008.04.023. [DOI] [PubMed] [Google Scholar]
- McNeely ME, Hershey T, Campbell MC, et al. Effects of deep brain stimulation of dorsal versus ventral subthalamic nucleus regions on gait and balance in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2011;82:1250–1255. doi: 10.1136/jnnp.2010.232900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner W, Harnack D, Reese R, et al. High-frequency stimulation of the subthalamic nucleus enhances striatal dopamine release and metabolism in rats. J Neurochemistry. 2003;85:601–609. doi: 10.1046/j.1471-4159.2003.01665.x. [DOI] [PubMed] [Google Scholar]
- Mollion H, Dominey PF, Broussolle E, Ventre-Dominey J. Subthalamic nucleus stimulation selectively improves motor and visual memory performance in Parkinson’s disease. Mov Disord. 2011;26:2019–2025. doi: 10.1002/mds.23769. [DOI] [PubMed] [Google Scholar]
- Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and Recommendations. Movement Disorders. 2003;18:738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- Obrig H, Wenzel R, Kohl M, et al. Near-infrared spectroscopy: does it function in functional activation studies of the adult brain? International J Psychophysiology. 2000;35:125–142. doi: 10.1016/s0167-8760(99)00048-3. [DOI] [PubMed] [Google Scholar]
- Odekerken VJ, van Laar T, Staal MJ, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurology. 2013;12(1):37–44. doi: 10.1016/S1474-4422(12)70264-8. [DOI] [PubMed] [Google Scholar]
- Okun MS, Fernandez HH, Wu SS, et al. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurology. 2009;65(5):586–595. doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nature Neuroscience. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Owen AM. Cognitive dysfunction in Parkinson’s disease: the role of frontostriatal circuitry. Neuroscientist. 2004;10:525–537. doi: 10.1177/1073858404266776. [DOI] [PubMed] [Google Scholar]
- Owen AM, Beksinska M, James M, et al. Visuospatial memory deficits at different stages of Parkinson’s disease. Neuropsychologia. 1993;31:627–644. doi: 10.1016/0028-3932(93)90135-m. [DOI] [PubMed] [Google Scholar]
- Owen AM, Doyon J, Dagher A, et al. Abnormal basal ganglia outflow in Parkinson’s disease identified with PET. Implications for higher cortical functions. Brain. 1998;121:949–965. doi: 10.1093/brain/121.5.949. [DOI] [PubMed] [Google Scholar]
- Owen AM, James M, Leigh PN, et al. Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain. 1992;115:1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Research Reviews. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- Payoux P, Remy P, Damier P, et al. Subthalamic nucleus stimulation reduces abnormal motor cortical overactivity in Parkinson disease. Arch Neurology. 2004;61:1307–1313. doi: 10.1001/archneur.61.8.1307. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Gutierrez E, Bandettini P, Ungerleider L. Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron. 2002;35:975–987. doi: 10.1016/s0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- Pillon B, Ardouin C, Damier P, et al. Neuropsychological changes between “off” and “on” STN or GPi stimulation in Parkinson’s disease. Neurology. 2000;55:411–418. doi: 10.1212/wnl.55.3.411. [DOI] [PubMed] [Google Scholar]
- Postle BR, D’Esposito M. Dissociation of human caudate nucleus activity in spatial and nonspatial WM: an event-related fMRI study. Cognitive Brain Research. 1999;8:107–115. doi: 10.1016/s0926-6410(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Ray NJ, Jenkinson N, Brittain J, et al. The role of the subthalamic nucleus in response inhibition: evidence from deep brain stimulation for Parkinson’s disease. Neuropsychologia. 2009;47:2828–2834. doi: 10.1016/j.neuropsychologia.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Rivaud-Péchoux S, Vermersch AI, Gaymard B, et al. Improvement of memory guided saccades in parkinsonian patients by high frequency subthalamic nucleus stimulation. J Neurology, Neurosurgery, Psychiatry. 2000;68:381–384. doi: 10.1136/jnnp.68.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlind JC, York MK, Carlson K, et al. Neuropsychological changes following deep brain stimulation surgery for Parkinson’s disease: comparisons of treatment at pallidal and subthalamic targets versus best medical therapy. J Neurol Neurosurg Psychiatry. 2015;86:622–629. doi: 10.1136/jnnp-2014-308119. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Trepanier LL, Kumar R, et al. Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson’s disease. Brain. 2000;123:2091–2108. doi: 10.1093/brain/123.10.2091. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Piccini P, Hotton G, et al. Cognitive deficits and striato-frontal dopamine release in Parkinson’s disease. Brain. 2008;131:1294–1302. doi: 10.1093/brain/awn054. [DOI] [PubMed] [Google Scholar]
- Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson’s disease? J Neurology, Neurosurgery, Psychiatry. 2000;69:308–312. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Bücheler MM, Müller K, et al. Towards a standard analysis for functional near-infrared imaging. Neuroimage. 2004;21:283–290. doi: 10.1016/j.neuroimage.2003.09.054. [DOI] [PubMed] [Google Scholar]
- Schuepbach WMM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson’s disease with early motor complications. NEJM. 2013;368(7):610–622. doi: 10.1056/NEJMoa1205158. [DOI] [PubMed] [Google Scholar]
- Selzler K, Burack M, Bender R, Mapstone M. Neurophysiological correlates of motor and working memory performance following subthalamic nucleus stimulation. J Cognitive Neuroscience. 2012;25:37–48. doi: 10.1162/jocn_a_00306. [DOI] [PubMed] [Google Scholar]
- Temel Y, Blokland A, Steinbusch HW, Visser-Vandewalle V. The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog Neurobiology. 2005;76:393–413. doi: 10.1016/j.pneurobio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Temperli P, Ghika J, Villemure JG, et al. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Ventre-Dominey J, Bourret S, Mollion H, et al. Dissociable dorsal and ventral frontostriatal working memory circuits: Evidence from subthalamic stimulation in Parkinson’s disease. Human Brain Mapping. 2014;35:552–566. doi: 10.1002/hbm.22205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt K, Daniels C, Reiff J, Krack P, et al. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study. Lancet Neurol. 2008;7:605–614. doi: 10.1016/S1474-4422(08)70114-5. [DOI] [PubMed] [Google Scholar]
- Yarnall AJ, Breen DP, Duncan GW, et al. Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE-PD study. Neurology. 2014;82:308–316. doi: 10.1212/WNL.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Okun MS, Foote KD, et al. Cognitive declines one year after unilateral deep brain stimulation surgery in Parkinson’s disease: a controlled study using reliable change. Clin Neuropsychol. 2009;23:385–405. doi: 10.1080/13854040802360582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brooks DH, Boas DA. A haemodynamic response function model in spatio-temporal diffuse optical tomography. Physics in Med Biol. 2005;50:4625–4644. doi: 10.1088/0031-9155/50/19/014. [DOI] [PubMed] [Google Scholar]