Abstract
A major class of human birth defects arise from aberrations during neural tube closure (NTC). We report on a NTC signaling pathway requiring T-type calcium channels (TTCC) that is conserved between primitive chordates (Ciona) and Xenopus. With loss of TTCCs there is a failure to seal the anterior neural folds. Accompanying loss of TTCCs is an upregulation of EphrinA effectors. Ephrin signaling is known to be important in NTC, and ephrins can affect both cell adhesion and repulsion. In Ciona, ephrinA-d expression is down regulated at the end of neurulation, while with loss of TTCC ephrinA-d remains elevated. Accordingly, overexpression of ephrinA-d phenocopied TTCC loss of function, while overexpression of a dominant negative Ephrin receptor was able to rescue NTC in a Ciona TTCC mutant. We hypothesize that signaling through TTCCs is necessary for proper anterior NTC through down regulation of ephrins, and possibly elimination of a repulsive signal.
eTOC blurb
Abdul-Wajid et al. demonstrate a conserved requirement for T-type calcium channels in neural tube closure. The results describe how neural fold fusion is disrupted in T-type calcium channel mutants and morphants via misregulation of EphrinA effectors.
Introduction
Neurulation, the process that transforms the neural plate into the neural tube, is a hallmark of the chordates. Although the details of neural tube closure (NTC) vary between species (Harrington et al., 2009), broadly conserved cytoskeletal mechanisms bring together the two lateral edges of the neural plate followed by their fusion at the dorsal midline. Disruption of NTC cellular mechanisms, such as those generating apically polarized constrictions in the epithelial neural plate cells, causes severe and sometimes irreparable defects in the spinal cord and brain (Haigo et al., 2003, Ybot-Gonzalez and Copp, 1999). Similarly, mutations that disrupt the planar cell polarity pathway genes, such as dishevelled and vangl2, have been shown to disrupt convergent extension movements necessary for NTC (Murdoch et al., 2001, Wallingford and Harland, 2001). The final step of NTC is neural fold adhesion and fusion, and some key factors, such as the products of the grhl2 and N-cadherin genes, have been found through reverse and forward genetic approaches (Bronner-Fraser et al., 1992, Pyrgaki et al., 2011).
Despite the wealth of studies, our understanding of NTC remains incomplete and insufficient to explain the spectrum of human NTC defects (Greene and Copp, 2014, Wallingford et al., 2013). We describe a regulatory step in NTC that requires signaling through T-type calcium (Ca2+) channels (TTCCs) for proper fusion of the closing neural tube. The requirement for T-type Ca2+ signaling appears to be late in NTC, and is distinct from earlier Ca2+ signaling events that are required for neural plate induction (Hackley et al., 2013, Leclerc et al., 2012), as well as from mechanisms of cell polarity and cytoskeletal-driven neural plate folding.
The original observation of the requirement for TTCCs in chordate NTC came from mapping analysis of mutant, bugeye (bug) in the ascidian Ciona savignyi (Abdul-Wajid et al., 2014). Here, we extend these observations from C. savignyi, and show conservation of this gene function during NTC in the amphibian Xenopus laevis.
Results
Loss of CAV3 results in an open anterior neural tube
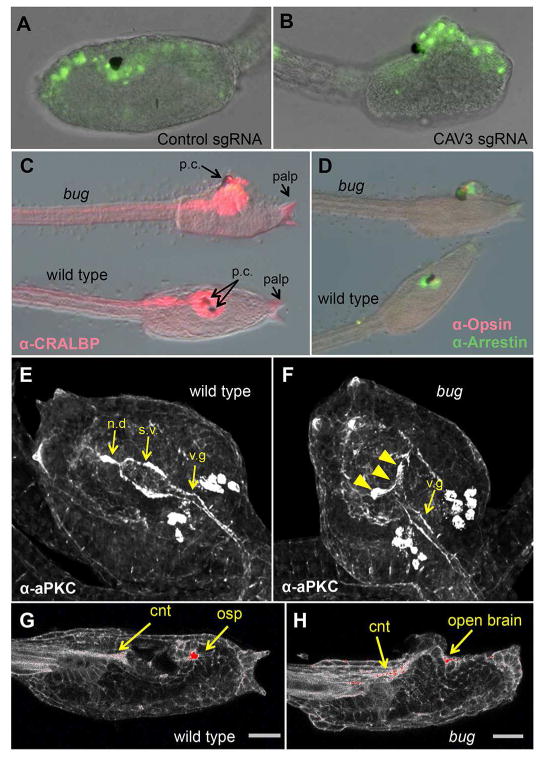
In a screen for spontaneous mutants in wild-collected Ciona savignyi (Veeman et al., 2011) we identified a recessive line (bugeye) with an open brain phenotype, and mapped the causative mutation to the cis-regulatory region of the single TTCC gene (CAV3) of the C. savignyi genome (Abdul-Wajid et al., 2014). Further experiments showed that the expression level of CAV3 was greatly reduced in homozygous bug mutants relative to their wild type siblings, suggesting that deficient Ca2+ signaling through the CAV3 channel was responsible for the open brain phenotype. To investigate this possible causative link further, the CAV3 gene in the closely related species Ciona intestinalis was targeted using CRISPR/Cas9 genome editing (Ran et al., 2013). Like C. savignyi, C. intestinalis has only a single CAV3 gene (Okamura et al., 2005). We targeted the C. intestinalis CAV3 gene using two guide RNAs complimentary to exon 3 and 49. Nuclear Cas9 was expressed using the ubiquitous EF1alpha promoter (Stolfi et al., 2014). The eggs also received the plasmid ETR1>H2B:GFP to mark cells of the central nervous system (CNS) (Veeman et al., 2010). At the late tailbud stage a bugeye(bug)-like phenotype (i.e., open brain) was observed in 17% of the electroporated embryos (n=362), versus 3% (n=374) of embryos receiving a control guide RNA (Figure 1A and B) (p=0.0001 Fisher Exact). If both CAV3 guide RNAs cleaved the target sequence successfully, an expected deletion of ~23Kb of DNA from C.intestinalis CAV3 gene would be produced. Using PCR primers spanning the outer edges of the target sites, a ~130bp PCR product was detected in the bug-like C. intestinalis embryos, but not in the control guide RNA electroporated embryos (Figure S1A). Sequences of several cloned PCR products confirmed they were generated from the targeted region, and that they contained large and variable deletions (Figure S1B).
Figure 1.
CAV3 is required for Ciona intestinalis neural tube closure. A and B. Representative C. intestinalis embryos expressing a control guide RNA (sgRNA) (A), or guide RNAs targeting exons 3 and 49 of the C. intestinalis CAV3 gene (ciCAV3 sgRNA). See also Figure S1. (B). Green fluorescence from a pan-neural ETR1 promoter driving nuclear GFP construct marks neural tube cells. C and D. Bug mutants differentiate and polarize the anterior neural tube. Bugeye (bug) and wild type siblings stained for the neural markers CRALBP, Opsin and Arrestin, as indicated. E and F. Immunostaining for the cell polarity marker aPKC in wild type and bug mutants. s.v. = sensory vesicle, n.d.= neurohypophyseal duct, v.g.= visceral ganglion. The staining intensity is consistent between bug and wild type, although the sensory vesicle in bug embryos is open (arrowheads in F). Views are dorsal. G and H. Phalloidin staining of bug and wild type sibling larvae. The most intense staining is in red. cnt= caudal neural tube, osp=oral siphon primordium. Scale bar = 24 μm.
CNS cells in the bug mutant are differentiated and polarized
NTC defects, whether genetic or environmental, can be placed into two broad, and non-mutually exclusive, categories: those that arise from failure of neural tube differentiation, and those that arise due to defects in the mechanics of neural tube folding and/or adhesion (Wallingford et al., 2013). In C. intestinalis we previously characterized the mutant frimousse, which causes severe defects to the anterior neural tube, including a failure in brain closure (Deschet and Smith, 2004, Hackley et al., 2013). This mutation, the product of a lesion in a connexin gene, results from a failure to maintain neural induction in the anterior neural plate, and is evident in the loss of expression of anterior CNS genes, and in the aberrant expression of an epidermal gene in the mis-specified brain. In contrast to frimousse, in bug we observed comparable immunostaining of the pan-neural protein CRALBP, and the brain-specific proteins Opsin and Arrestin (Tsuda et al., 2003) in wild type and mutant larvae (Figure 1C and D). In addition to the CNS, the adhesive palps in bug mutants were indistinguishable from wild type. In Ciona the palps are derived from the anterior-most region of the neural plate (Nishida, 1987, Veeman et al., 2010), and in frimousse embryos they are absent.
Mechanically, NTC is characterized by the formation of apically polarized actin filaments. The action of these polarized filaments results in the apical constriction of the cells and the folding of the neural plate inward. Disruption of this actin machinery and/or accessory proteins can cause neural tube closure defects (Wallingford, 2005). In C. savignyi we observed that antibody staining for the apically localized cell polarity protein aPKC (Ghosh et al., 2008, Solecki et al., 2006) labels the surface of the CNS lumen (Figure 1E). In the C. savignyi brain periluminal staining can be seen in both the sensory vesicle and visceral ganglion (Ciona orthologs of the fore- and hindbrains, respectively), as well as in the neurohypophysial duct, which joins the sensory vesicle to the stomodeum (Manni et al., 2005). In bug mutants we observed strong aPKC staining throughout the CNS, although anterior staining of the sensory vesicle was splayed outward in the open brain rather than forming a closed lumen (arrowheads Figure 1F). Similarly, phalloidin labeling of bug mutants showed strong polarization of actin to the apices of cells in the CNS, including cells in the open anterior brain, of comparable intensity to phalloidin labeling in wild type embryos (Figure 1G versus 1H, red staining and yellow arrows). In summary, the CNS of bug mutants has no conspicuous defects in neural induction and specification, or in the formation of apically polarized cells
Onset of bug phenotype
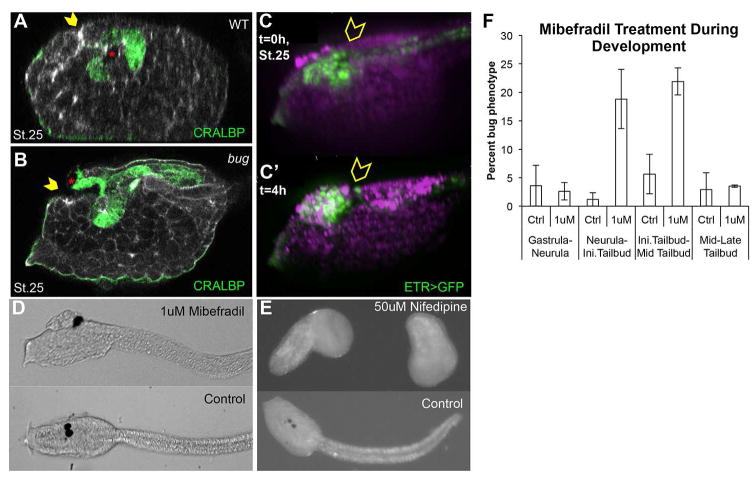
An important clue to the nature of the mechanism of the bug mutation comes from the observation that the phenotype, an open anterior neural tube, does not become apparent until after the late tailbud stage, when neural tube closure in wild type embryos is complete. At stage 25 (late tailbud) sagittal confocal sections of bug embryos show that the anterior neural tube closure is largely complete, with most of the brain covered in epidermis, but with an enlarged anterior neuropore (yellow arrowheads Figure 2A and B). Additionally, the two pigmented cells that comprise the ocellus and the otolith (Jiang et al., 2005) are misplaced in bug to the opening of the neuropore rather than in the brain vesicle (red asterisks Figure 2B). To better visualize the developing CNS, the bug line was crossed with a stable transgenic line expressing GFP under the control of the pan-neural ETR1 promoter. Time-lapse movies of bug embryos confirm that the brain is initially covered with epidermis (i.e., relatively normal brain closure and neurulation), and that, over the course of approximately 4 hours, the brain erupts from the anterior neuropore by hatching larva stage (Figure 2C and C′, and Movie S1). These data demonstrate that the late step of anterior neuropore sealing is defective in bug embryos (whereas earlier neurulation is normal), and that this results in the opening and protrusion of the initially epidermis-covered brain.
Figure 2.
Onset of open brain phenotype in Ciona. A and B. Mid-sagittal confocal sections of late tailbud (stage 25) bug and wild type (WT) sibling embryos showing immunostaining for CRALBP. Yellow arrowheads indicate the anterior neuropore. The pigmented cells of the sensory vesicle are indicated by red asterisks. C and C′. Still images from the start and end of Movie S1 of a bug embryo. Cells of the neural tube express GFP from the pan-neural ETR1 promoter. Magenta indicates FM464 stained membranes. Yellow arrowheads indicate sensory vesicle that, at the start of movie (C; t=0) is covered by non-neural tissue (i.e., epidermis), and then at four hours (C′; t=4h) is exposed on the surface. (A–C′: lateral view of head region with palps and anterior to the left). D. Treatment with mibefradil during neurulation phenocopies the bug phenotype. E. Treatment with nifedipine during neurulation stages causes an arrest in development. F. Mibefradil treatments. Dechorionated embryos at each developmental stage were subjected to control (water) or 1μM Mibefradil, washed after treatment, and then allowed to develop to swimming larvae stage. Percentage phenotype was calculated by counting the number of bugeye-like embryos over the total number of embryos treated. Error bars represent standard error of the mean from three independent experiments.
Temporal T-type Ca2+ channel function
To better understand the temporal requirement for CAV3 in neural tube closure, embryos were treated at various time points with the TTCC-blocking drug mibefradil (Martin et al., 2000). Preliminary experiments confirmed that mibefradil treatment of neurulating embryos closely phenocopies bug, while the L-type channel blocker, nifedipine, gives a much different phenotype (Figure 2D and 2E). Treatment of embryos with mibefradil during short temporal windows identified the period of sensitivity for anterior neural tube closure: between neurula and mid-tailbud stages (Figure 2F). Thus, there is a lag time of ~5hrs from when CAV3 function is required (between stage 16 and 21) and the stage at which the phenotype becomes fully evident (stage 25 onward) suggesting an early tailbud CAV3-dependent signal which plays a role in the later sealing of the anterior neuropore.
Xenopus requires a T-type Ca2+ channel to close the anterior neural tube
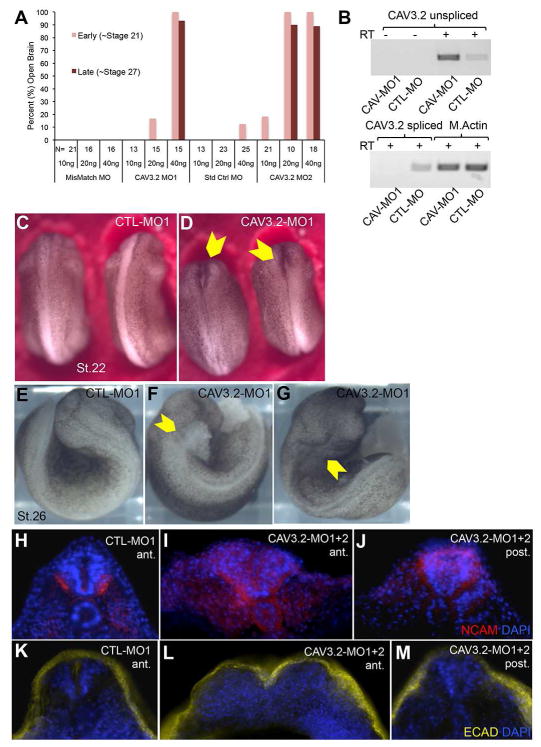
X. laevis has three orthologs of Ciona CAV3: CAV3.1, 3.2 and 3.3. CAV3.2 is the earliest of these to be expressed in the neurulating embryo (Lewis et al., 2009). To determine if this gene has a conserved role with its Ciona ortholog in neurulation, we used morpholino oligonucleotides (MO) to knock down its expression. Two splice-blocking MOs to X. laevis CAV3.2 were tested (CAV-MO1 and CAV-MO2), as well a two control MOs [standard control MO (Genetools), and custom mismatch MO]. Both CAV3.2 MOs produced open brain phenotypes in 80–100% of injected embryos (albeit at different dose efficacies), while no neurulation defects we observed with either control MO (Figure 3A). RT-PCR confirmed the efficacy of the MOs in blocking splicing and down regulation of spliced transcript (Figure 3B).
Figure 3.
X. laevis CAV3.2 morpholino (MO) knockdown. A. Quantification of MO knockdown phenotype at stages 21 and 27. Two MOs targeting X. laevis CAV3.2 (CAV3.2-MO1 and CAV3.2-MO2) and two control MOs [mismatch (CTL-MO1) and standard control (CTL-MO2); see Material and Methods)] were tested. The quantities of MO injected per embryo are indicated below the number (N) of embryos scored for each dose of MO. B. Disruption of CAV3.2 transcript splicing by CAV-MO1. RT-PCR was used to detect both the correct and expected splice-disrupted transcripts in cDNA samples from three pooled CTL-MO and three CAV-MO1 injected embryos. Muscle actin (M. Actin) RT-PCR was used to control for RNA load, and RT-minus controls are also shown. C and D. Early tailbud stage embryos (stage 22) injected at the one-cell stage with a CTL-MO1 (C), or a CAV3.2-MO1 (D). Yellow arrowheads in B indicate anterior open neural tube. E–G. Tailbud stage (stage 26) CTL-MO1 (E) and CAV3.2-MO1 (F and G) injected embryos. Anterior neural tube defects were observed in the CAV3.2-MO1 injections with either cellular matter erupting from the open neural tube (yellow arrowhead in F), or a with a malformed head and deep dimple in the hindbrain (yellow arrowhead in G). See also Movie S2. H–J. NCAM staining (red) in anterior or posterior coronal sections of CTL-MO1 (H) and CAV3.2-MO1+2 (I and J) injected embryos. K–M. E-cadherin staining (yellow) in anterior and posterior coronal sections of CTL-MO1 (K) or CAV3.2-MO1+2 (L and M) injected embryos. MO injected embryos used for sections were stage matched at ~stage 24 based on somite development. All sections were also stained for nuclei (DAPI, blue).
The defect caused by the CAV3.2 MOs can be seen in representative embryos at stage 22 as a failure of the anterior neural folds to fuse (Figure 3C and D). The defect is not simply a delay, as no fusion of the anterior folds is seen with extended culturing. In fact, by stage 26 we observed in 17% of 58 CAV-MO1 injected embryos that material (possibly brain) erupted from the open neural pore (Figure 3E and F; Movie S2). The remainder of the CAV3.2 MO1 injected embryos at stage 26 showed severe head malformations with a deep dimple at the hindbrain (Figure 3G).
As in the Ciona bug mutant, we observed no general defect in neural induction for X. laevis embryos injected with the CAV3.2 MOs based on NCAM antibody staining, although anterior closure was clearly defective (Figure 3H–J; stage 24 embryos). Immunostaining for E-cadherin, which marks the epidermis in X. laevis, showed that even where the anterior neural tube was splayed open in the CAV3.2 MO embryos, an abnormal covering of epidermis was found over and into the open neural folds (Figure 3K–M). These results demonstrate that X. laevis embryos also require a TTCC gene, CAV3.2, to effectively close, but apparently not to specify, the anterior neural tube.
CAV3 expression in Ciona
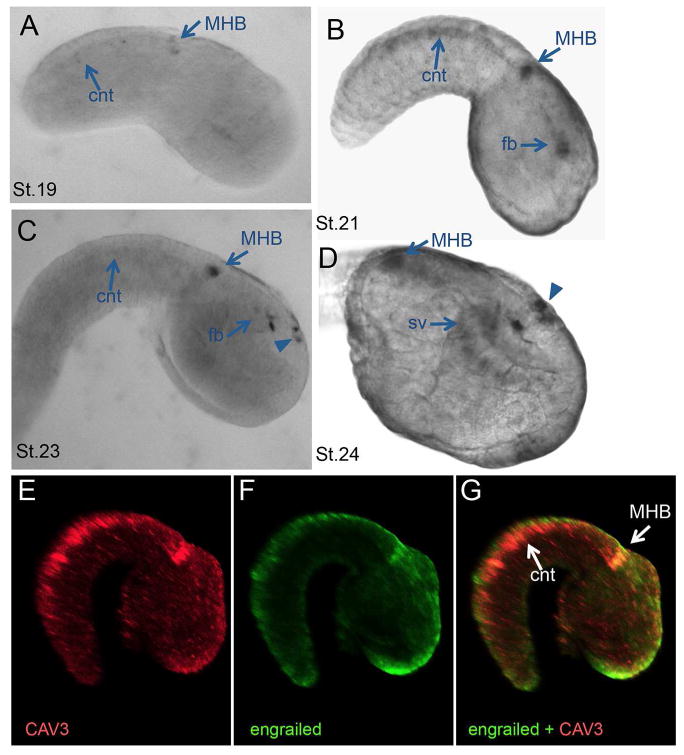
The results above suggest a conserved functional role for TTCCs in chordate neural tube closure. To further examine conservation between the two genes, their spatial expression was compared. The expression of the X. laevis CAV3.2 gene has already been described (Lewis et al., 2009). At developmental stages relevant for neural tube closure (as early as stage 20), X. laevis CAV3.2 expression is observed along the lateral edges of the developing neural folds and tube. This expression extends the length of the neural tube and increases into fore, mid and hindbrain regions at later stages (24–36) as well as into the presumptive heart tissue (Lewis et al., 2009). For C. savignyi the expression of the CAV3 gene was investigated by in situ hybridization. No specific signal could be detected until early tailbud stage [Ciona stage 19 (Hotta et al., 2007)]. At this stage the most conspicuous hybridization was observed in a cluster of cells that appear to correspond to the midbrain-hindbrain boundary (MHB) region and in the caudal nerve cord (Figure 4A). Based on the conserved expression patterns of otx, engrailed, pax2/5/8 and fgf8/17/18 in the brain, Ciona is thought to have a structure equivalent to the midbrain–hindbrain organizer (Raible and Brand, 2004). Expression of C. savignyi CAV3 increased in intensity in the MHB region at mid- and late-tailbud stages, and new expression was observed both in the forebrain (arrows in Figure 4B–D) and in a small patch of cells in the epidermis overlaying the sensory vesicle, posterior to the anterior neuropore (arrowheads in Figure 4C and D). Double fluorescent in situ hybridization confirms that the expression of engrailed and CAV3 overlap in the C. savignyi MHB region (Figure 4E–G).
Figure 4.
Expression of C. savignyi CAV3. A–D. In-situ hybridization of CAV3 expression in wild-type C. savignyi embryos. Expression is first detectable at early tailbud stage (A; stage 19) in the midbrain-hindbrain (MHB) region and faintly in the caudal nerve cord (cnt). This expression intensified by mid tailbud and new expression in the forebrain (fb) is observed (B; stage 21). By late tailbud (C and D; stage 23/24) new expression is seen in a cluster of cells in the epidermis (arrowheads) (sv=sensory vesicle). E–G. Double fluorescent in situ for CAV3 and engrailed yields overlapping expression at the MHB (but not in the cnt). Embryos are stage 22.
Ca2+ transients in neurulating embryos
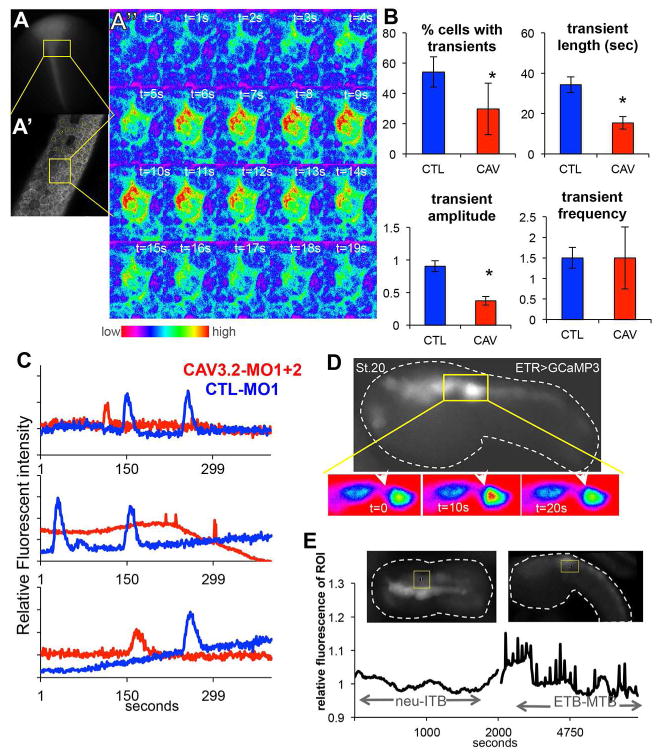
Calcium transients in the developing neural plate and tube of both Ciona and Xenopus have been well documented. We described the presence of gap junction-dependent Ca2+ transients in the neural plate of C. intestinalis (Hackley et al., 2013), and in Xenopus Ca2+ transients via the DHP-sensitive CAV1 family play a critical role in neural induction [reviewed in (Leclerc et al., 2012)]. However, TTCC transients in the neurulating chordate CNS have not been reported. To investigate this, one-cell stage X. laevis embryos were injected with the RNA encoding the Ca2+ indicator GCaMP3 (Tian et al., 2009) and either control or CAV3.2 MOs. Live imaging was centered to the anterior closing neural folds of neurula stage embryos, where neural tube closure defects were observed and CAV3.2 expressing cells are present (Lewis et al., 2009). In a single plane of view of control MO embryos we could detect Ca2+ transients in ~50% of the cells in the field of view, with each transient lasting on average 33 seconds (Figure 5A–C, Movie S3). The number of cells having transients, and the length and the intensity of the transients was reduced in CAV3.2 MO embryos (Figure 5B, asterisks indicate significance of p≤0.05, Movie S4). However, no difference was detected in the frequency of the transients (Figure 5B). Thus CAV3.2 is required for normal Ca2+ transients in the developing X. laevis anterior neural tube.
Figure 5.
Calcium (Ca2+) transients in the neural tubes of C.savignyi and X. laevis. A–A″. Xenopus embryo injected with GCaMP3 RNA shows Ca2+ transients during neurulation. Top left, a late neurula embryo expressing GCaMP3 in the closing neural tube (A). Low magnification view of X. laevis embryo showing typical area recorded for Ca2+ transients (yellow box). Anterior is up. A′, High magnification of the Z plane of the X. laevis anterior neural plate that was imaged. A″. GCaMP fluorescence for a single cell in the neural plate undergoing a Ca2+ transient ([Ca2+] indicated by color scale). See also Movies S3 and S4. B. CAV3.2 morpholino knockdown (CAV3.2-MO1, CAV) reduces the number of cells in the neural tube displaying Ca2+ transients, the length and amplitude of the transients, but not the frequency. The y-axis in the amplitude graph shows relative fluorescent intensity (see Materials and Methods). The frequency values are also described in Materials and Methods. Control embryos were injected with a non-specific MO (CTL-MO2, CTL). Data represent quantification of three embryos from three independent experiments and error bars represent standard error of the mean (*= p<0.05). C. Typical Ca2+ transients in CAV-MO knockdown and CTL-MO X. laevis neural tube cells. D. Ca2+ transients in C. savignyi embryos detected with GCaMP3 expressed from the pan-neural ETR1 promoter. Top panel shows one frame from a time-lapse movie. Lower panel is a close up of the midbrain hindbrain region (yellow box in top panel) showing a single Ca2+ transient with fluorescence intensity using the color scale from A″. See also Movie S5. E. Time-lapse of Ca2+ transients in the C. savignyi MHB region. Representative images from two time points (neurula and mid-tailbud) of an embryo expressing GCaMP3 with the MHB region of interest (ROI) outlined in yellow. Dashed white lines outline the embryo. Bottom panels show relative fluorescence intensity in the ROI over time. Neu-iTB = neurula to initial tailbud and ETB-MTB = early to mid tailbud.
At early tailbud stages in C. savignyi we observed a strong correlation between CAV3 expression and Ca2+ transients using an electroporated construct with the ETR1 driver for GCaMP3 (Hackley et al., 2013). Starting at early tailbud stage, GCaMP3 Ca2+ transients were observed in the MHB region (Figure 5D and 5E; Movie S5), and continued through the mid tailbud stage. The MHB transients diminish after mid tailbud stage (data not shown).
T-type Ca2+ channels and ephrin regulation
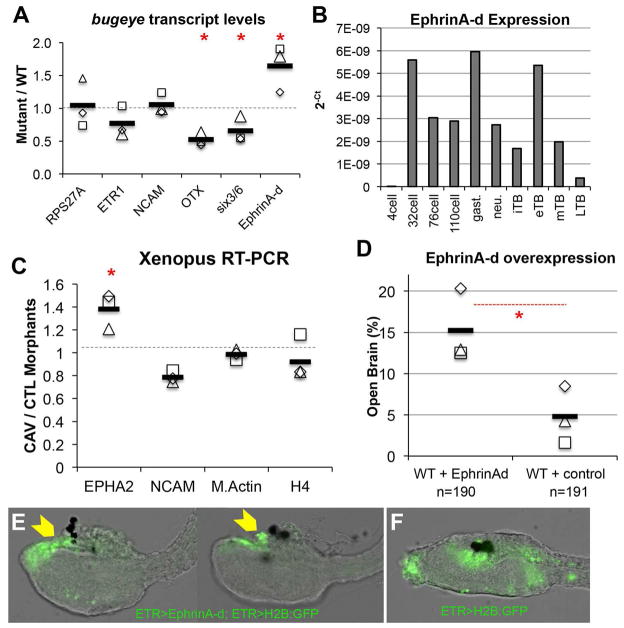
The characterization of the TTCC phenotypes in both Ciona and Xenopus suggest that the neural tube closure defects arises not from a deficiency in neural induction or neural tube folding, but rather from a later event, perhaps one involving cell adhesion and/or neuropore sealing. In mouse, EphrinA-EphA interactions have been shown to be important for neural fold fusion (Abdul-Aziz et al., 2009). We investigated expression levels of the C. savignyi ortholog of this gene, ephrinA-d (Picco et al., 2007), as well as Neural Cell Adhesion Molecule (NCAM), and the neural-specific genes otx, six3/6, and etr1 (Takamura et al., 2001) at late tailbud stages in wild type and bug mutants by quantitative RT-PCR. The ubiquitously expressed gene RPS27A was included as a control (Olinski et al., 2006). While there was no difference in expression of NCAM and ETR1 between wild type and bug, ephrinA-d was up-regulated approximately 1.6 fold in the mutant relative to wild type. In these same bug embryos we observed down regulation of the anterior neural genes otx and six3/6 (Figure 6A). Because of the role of EphrinA in mouse neural tube closure and the similar expression patterns of ephrinA-d and CAV3 (Imai et al., 2009), the C. savignyi ephrinA-d upregulation was of particular interest. In C. intestinalis, ephrinA-d has been shown to be necessary for the proper patterning of the neural plate, although a later role in neural tube closure has not been reported (Haupaix et al., 2014, Stolfi et al., 2011). We examined the normal developmental time-course of ephrinA-d expression by qRT-PCR. Several peaks in ephrinA-d expression were observed: at 32-cell, gastrula and early tailbud stages consistent with the previously published in situ data (Figure 6B) (Imai et al., 2009). Of potential significance is a dramatic down regulation of ephrinA-d expression from early through late tailbud stages in wild type embryos.
Figure 6.
T-type Ca2+ channel regulation of EphrinA signaling components. A. qRT-PCR assay for expression of the neural genes etr1, otx, six3/6, NCAM and ephrinA-d in bug and wild type (WT) larvae. ΔΔCT values were calculated by normalizing to the ubiquitously expressed gene RPS27A and then comparing to WT expression levels. The values for three independent biological repeats are indicated (open squares, etc.), as well as the average of the three (black bar). Red asterisks indicates significant difference between bug mutants and WT for given transcript; p≤0.05, T-test. RPS27a values represent ΔCT comparison to WT. B. C. savignyi EphrinA-d transcript levels quantified over developmental time. qRT-PCR results are represented as log2(X) changes in absolute cycle threshold values for each developmental time point sample. C. RT-PCR for expression of EPHA2, NCAM, M.Actin and histone 4(H4) in stage 24 X. laevis embryos injected with either CAV3.2 splice disrupting morpholinos (CAV-MO1+2, CAV), or a control MO (CTL-MO2, CTL). The values for three independent biological repeats are indicated (open squares, etc.), as well as the average of the three (black bar). EPHA2 values are significantly different between CAV and CTL embryos (Red asterisks, p=0.025, Standard T-test). D. Percent of open brain phenotype observed in WT embryos electroporated with either the EphrinA-d cDNA expression construct (WT+ EphrinAd), or H2B:GFP cDNA (WT+ control). Results from three independent trails are shown, as well as the average of the three (black bars). Red asterisks indicates significance, p=0.0006, Fisher exact test. E. EphrinA-d overexpression driven by pan-neural ETR promoter in C. intestinalis embryos phenocopies bug. Yellow arrowheads indicate the open brain. Two representative embryos are shown. The co-electroporated plasmid ETR>H2B:GFP labels the nervous system. F. Control embryo expressing only ETR>H2B:GFP.
We also examined ephrinA signaling in Xenopus embryos. The ephrinA receptor EPHA2 is known to be expressed in the developing X. laevis neural tube (Brandli and Kirschner, 1995). A significant increase in the transcript for EPHA2 was observed in X. laevis embryos injected with the CAV3.2 MO relative to controls (Figure 6C, EPHA2 significance p=0.025, T-test), while no change was observed for NCAM expression (nor for actin and histone 4). While there are several EphrinBs expressed at this stage, their effects on the neural tube appears to be on apical constriction rather than adhesion (Ji et al., 2014).
To investigate if mis-expressing EphrinA-d in Ciona in the late tailbud Ciona embryos could replicate the bug phenotype, 1-cell stage embryos were electroporated with a plasmid containing the ephrinA-d cDNA driven by the ETR promoter, which expresses through the late tailbud stage in C. savignyi (Tresser et al., 2010). A significant fraction of embryos electroporated with the EphrinA-d construct had an open brain phenotype compared to controls overexpressing nuclear GFP under the same promoter (15.2%, versus 4.8% in controls, p=0.0006 Fisher Exact) (Figure 6D and 6E versus 6F).
Rescue of bug embryos by ephrinA inhibition
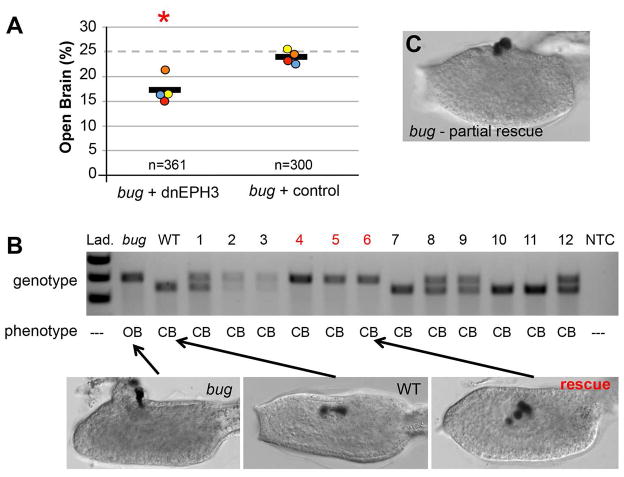
Based on the expression and over-expression data, we hypothesized that failure of bug embryos to properly down-regulate ephrinA-d late in neurulation could cause the open-brain phenotype. To test this hypothesis directly, we asked whether inhibition of EphrinA-d could restore anterior neural tube closure in bug embryos. For these experiments a previously characterized dominant negative version of the receptor for EphrinA-d, EPH3, (Picco et al., 2007, Haupaix et al., 2013) was expressed by electroporation in the CNS of Ciona bugeye embryos using the ETR1 promoter. Because the bug allele is lethal when homozygous (Abdul-Wajid et al., 2014) embryos are generated by crossing heterozygous adults. In clutches of bug embryos electroporated with the control plasmid, the bug phenotype was observed at close to the expected frequency of 25% (Figure 7A). In these experiments four independent clutches of embryos were electroporated, and the frequency ranged from 23.1% to 25.4% (average 23.9%, n=300, chi squared test p=0.689). In contrast, for embryos from the same clutches electroporated with the dominant negative EPH3 (dnEPH3) the frequency of observed bug phenotypes ranged from 15% to 21% (average 17.1%, n=361, chi-squared test p=0.002), indicating that anterior neural tube closure had been rescued in a portion of the bug embryos.
Figure 7.
Rescue of Ciona bugeye (bug) embryos with dominant negative EPH3 (dnEPH3). A. Embryos from heterozygous bug parents were electroporated with either dnEPH3 driven by the pan-neural promoter ETR1, or an empty ETR1 vector (control). The fraction of embryos with the bug phenotype (open brain) was determined for each group. Circles represent the percent of embryos showing the bug phenotype from four independent trials, and the black line is the average of the four trials (n is the combined number scored from the four trials). Red asterisks indicate significant P-value of 0.002 using a chi-squared test of deviation from the expected open brain frequency of 25%. B. Single tadpoles electroporated with dnEPH3 (lanes 1–12) and scored as having closed brains (CB) were genotyped with primers that distinguished the insertion-containing bug allele from the wild type allele. The bug and wild type (WT) lanes show the sizes the two alleles. Three of the twelve electroporated embryos (4–6, red) genotyped as homozygous for the bug allele despite having closed brains, and are considered as rescued. Representative images of wild type, bug, and rescued embryos are shown. See also Figure S2. C. Representative image of a partially rescued bug embryo.
To further investigate the possible bug rescue, individual embryos were genotyped. The bug allele contains an 80bp insertion that can readily be identified by gel electrophoresis of PCR-amplified genomic DNA from single embryos (Figure 7B). In a preliminary experiment, the link between the bug phenotype and the genomic insertion was confirmed (Figure S2). In forty-five embryos examined, the bug phenotype was only observed in embryos homozygous for the insertion. Importantly, closed anterior neural tubes were never observed in those embryos homozygous for the insertion, nor were open neural tubes observed in those embryos genotyping as heterozygous, or genetically homozygous wild type. For clutches electroporated with the dnEPH3 construct, twelve embryos scored as having closed anterior neural tubes were genotyped. In contrast to the results for control embryos, three of these embryos genotyped as homozygous for the bug allele (Figure 7B), indicating that they were rescued. Note also that the example rescued bug embryo (yellow arrow) has extra pigment cells, a previously described phenotype of dnEPH3 expression (Haupaix et al., 2014). Finally, we observed a number of embryos that appeared to be partially rescued by dnEPH3 (Figure 7C). These were scored as having open neural tubes for the data shown in Figure 7A.
Discussion
The present study shows a requirement for TTCC signaling in NTC for both ascidians and amphibians, suggesting that this is an ancient function that predates the split of the vertebrates and the tunicates. Zebrafish CAV3.2 is also expressed during neurulation with a partially overlapping pattern to Xenopus [zfin.org, probe cb648, (Lewis et al., 2009)]. The nature of the defects reported here for loss of T-type Ca2+ function in C. savignyi, C. intestinalis and X. laevis are consistent with it playing a role in the fusion/adhesion of neural folds, rather than in neural specification or neural fold generation. In C. savignyi the open anterior neuropore led to a dramatic eruption of the brain from the head of homozygous bug mutants. In X. laevis we observed a less frequent, but similar, expulsion of tissue from the open neuropore of CAV3.2 knockdown embryos.
The CAV3 family consists of three vertebrate members (CAV3.1, 3.2 and 3.3) and a single member in tunicates (Perez-Reyes and Lory, 2006, Okamura et al., 2005). This family of Ca2+ channels is characterized by rapid opening in response to small membrane depolarizations (Perez-Reyes, 2003). While TTCCs have most thoroughly been investigated in adult tissues such as the heart and neurons, these channels are expressed embryonically (Frischknecht and Randall, 1998, Gottmann et al., 1988). Recent studies have demonstrated that CAV3.2 in mice initiates differentiation cascades in early embryonic tissues including in neural progenitors, heart and skeletal muscle (Ferron et al., 2011, Louhivuori et al., 2013, Berthier et al., 2002). It has also been demonstrated that TTCC activity can directly initiate transcriptional programs in chondrocyte differentiation (Lin et al., 2014).
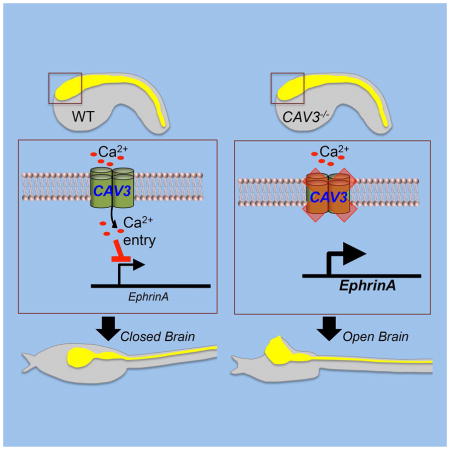
Results presented here show that loss of TTCC function aberrantly upregulates the expression of EphrinA-d and EPHA2, in C. savignyi and X. laevis, respectively. Although in mouse EphrinA signaling has been reported to promote neural tube fusion (Abdul-Aziz et al., 2009), and loss of ephrinA5 in mice leads to a failure of neural tube fusion (Holmberg et al., 2000), this same pathway in other contexts can lead to cell repulsion (Arvanitis and Davy, 2008). Moreover, results presented here show that overexpression of ephrinA-d can lead to an open brain phenotype in C. savignyi. Thus the actions of this pathway (adhesive or repulsive) may depend on the temporal context and/or the relative concentrations. Consistent with this, we observed in C. savignyi a spike in ephrinA-d expression in the early tailbud embryo, corresponding to the onset of neural fold fusion. However by late tailbud stage, ephrinA-d transcript levels fall dramatically, suggesting that any requirement for ephrinA-d in neural fold fusion is transient. Moreover, the ability of dnEPH3 to rescue bug embryos indicates that the down regulation of ephrinA-d is necessary for proper anterior neuropore closure.
The CAV3-EphrinA relationship is more complicated in X. laevis. While the temporal and spatial expression of all five EphrinA ligands, and ten EphrinA receptors, has not been described, two X. laevis EphrinA receptors, EPHA2 and EPHA4 have distinct expression patterns in the neural tube and partially overlap with CAV3.2 expression (Park et al., 2011, Brandli and Kirschner, 1995, Smith et al., 1997) (XDB3, http://Xenopus.nibb.ac.jp). However there remain many uncharacterized EphrinA ligands and receptors, and the two Xenopus TTCCs not examined here, CAV3.1 and CAV3.3, are also expressed at later stages of neural tube closure, and are partially redundant with CAV3.2. Redundancy may explain why no NTC defect is reported in the CAV3.2 knockout mouse (Chen et al., 2003). However, the present results suggest that neural fold fusion in these mice should be reexamined.
Finally, CAV3.2-dependent Ca2+ transients were observed in the X. laevis neural tube. In adult nerve, muscle, and cardiac cells TTCC activation, and the resulting Ca2+ entry, is tied to specific physiological/sensory stimuli (Nelson et al., 2006). However, the embryonic nervous system is characterized by early spontaneous ion channel activity [reviewed in (Leclerc et al., 2012, Rosenberg and Spitzer, 2011)]. Thus the CAV3.2-dependent Ca2+ transients in the closing Xenopus neural tube could be the products of either spontaneous activity, or possibly a sensory signaling event. One possible role of TTCCs in NTC could be to monitor the mechanical progress and/or quality of NTC. Interestingly, in adult proprioceptive nerves, CAV3.2 has been found to be essential for low-threshold mechanoreception (Francois et al., 2015). Future experiments will investigate the potential roles of spontaneous versus sensory activation of TTCCs in NTC.
Experimental Procedures
Ciona Methods
Animals
Adult Ciona were collected from the Santa Barbara Harbor and maintained in constant light in flowing sea water tables. Transgenic lines were maintained at a culturing facility at the University of California, Santa Barbara. Fertilized eggs used for electroporation and transgenesis were collected as previously described (Veeman et al., 2011).
Transgenics and plasmids
Electroporation methods of C. intestinalis and C. savignyi were done as previously described (Veeman et al., 2011). The Ciona CRISPR/Cas9 and U6 constructs were used as described (Stolfi et al., 2014). Guide RNA target sequences for C. intestinalis are listed in Table S2, as well as the primer sequences used to detect the CRISPR mediated large deletion (‘Mutdet’ primers). Construction of a plasmid expressing EphrinA-d under the control of the ETR1 promoter (ETR>EphrinA-d) is detailed in supplemental procedures. Overexpression of EphrinA-d experiments used 50μg of ETR>EphrinA-d plasmid and 15μg of ETR>H2B:GFP. The control experiment used 65μg of ETR>H2B:GFP. ETR>GCaMP3 was made by similar Gateway methods from an Addgene plasmid of GCaMP3. ETR>GCamP3 was used at 75ug in C. savignyi electroporations.
The dominant negative EPH3 construct (ETR>dnEPH3) was made using primers (Table S2) to amplify dnEPH3 from Ngn>dnEPH3 (Stolfi et al., 2011). This amplicon was TA cloned into PCR8 (Invitrogen) and then Gateway LR reacted with the ETR>rfa vector (Roure et al., 2007). The resulting plasmid (90 μg ) was electroporated into clutches of embryos from heterozygous bug parents, as above.
Immunohistochemistry and Imaging
Ciona embryos were fixed in 4% PFA and filtered seawater. CRALBP, Arrestin and Opsin Antibodies were used as previously described in (Tsuda et al., 2003). Anti-PKCzeta antibody was used at 1:300 (Santa Cruz Biotechnology). Washing, secondary and clearing are as previously described (Veeman et al., 2008). Imaging of fixed Ciona samples was either on an Olympus FV1000 confocal or Olympus IX81. Ciona ETR>GCamP3 imaging was on the Olympus IX81 under a 20X water objective. Images were captured every 10 seconds for 30min-1hr intervals starting from neurula stages and ending in late tailbud stages of C. savignyi. Calcium transients were analyzed and quantified on Image J. In situ hybridizations were imaged on an Olympus BX51 with DIC optics and 20X objective. Fluorescent in situ hybridizations were cleared in glycerol and imaged on an Olympus FV1000 confocal under a 20X objective.
RT-PCR
C. savignyi bug mutants and wild type siblings larvae were collected for RNA as previously described(Abdul-Wajid et al., 2014). For comparisons of mutant and wild type by qRT-PCR equal amounts of total RNA used in each PCR reaction, and run as biological and technical triplicates. For the qPCR developmental time course, equal amounts of RNA were used for cDNA synthesis at each time point (200ng). Primers for qPCR are listed in Table S2.
For single tadpole Ciona genomic PCR, genomic DNA was isolated as previously described (Veeman et al., 2011) and primers are listed in Table S2.
Drug Treatments
Mibefradil dihydrochloride (Sigma) was reconstituted at 4mM in filtered water, stored at 4 degrees. Nifedipine was used as previously described (Hackley et al., 2013) and equivalent amounts of DMSO were used as control in this experiment. Experimental concentrations were diluted in filtered seawater with antibiotics (Streptomycin and kanamycin) in a 3cm petri dish to which embryos of the appropriate stage were added. Embryos were washed of drug at the indicated stage through three changes of filtered seawater and allowed to develop in a new petri dish of filtered seawater and antibiotics.
Xenopus Methods
Animals
Females were induced for ovulation by a subcutaneous injection of human chorionic gonadotropin hormone as described (Sive et al., 2007). Eggs and sperm were collected for in vitro fertilization as described (Sive et al., 2000).
Morpholino injections
X. laevis CAV3.2 morpholinos (MOs) were designed according to the sequence of Genbank entry GQ120631 and alignment with scaffold 22599 in X. laevis genome version 6 (xenbase.org). Two controls were used: the standard control morpholino (Gene Tools, CTL MO1) and a mismatch MO containing a 5bp mismatch from CAV3.2 splice disrupting morpholino 1 (CTL-MO2). Sequences for both CAV3.2 MOs and CAV3.2 mismatch MOs are in Table S1.
Fertilized eggs were microinjected MOs at final doses of 40ng, 20ng and 10ng in a 10nl drop. After dose determination, subsequent embryos used for coronal section imaging or RNA isolation, were injected with a combination of CAV3.2 MO1 and MO2 at 40ng final, or the CTL-MO2 at the same concentration. The same combination and concentrations of MOs were used in the GCamP3 co-injection experiments. The embryos were imaged for developmental defects from neurula to tailbud stages, then fixed in 4% Paraformaldehyde + MEM for immunohistochemistry or lysed in Trizol for RT-PCR analysis(Blackiston et al., 2010). RNAs for GCaMP3 were made from a gene construct using the T3 mMessage in vitro Transcription kit (Ambion), 1ng of RNA was co-injected with the MOs as indicated.
Immunohistochemistry and imaging
X. laevis embryos were fixed, embedded and sectioned as described (Blackiston et al., 2010). Primary antibody sera to NCAM (4d-s) and E-cadherin (5D3) were obtained from the Developmental Studies Hybridoma Bank at University of Iowa and used at final concentrations of 5μg/ml. Fluorophore conjugated secondary antibodies (Invitrogen) were used at 1:500. Sections were mounted in glycerol.
For GCamP3 imaging, GFP expressing embryos from each experimental condition were placed in 1% low melting pointing agarose and 1/3X MMR on a coverslip-bottom dish. Embryos were oriented with neural plates facing down and then imaged on an inverted Zeiss 780 LSM or an Olympus FV1000. Imaging was done with a 20X objective and images captured every second for 8–10 minutes. The analysis of GCamP3 images was done with ImageJ and described in supplemental procedures.
RT-PCR
Primers used for RT-PCR on X. laevis single tadpole cDNA are listed in Table S2. Single embryos were lysed in TRIZOL for RNA isolation and 400ng of total RNA was used to make cDNA (Invitrogen Superscript III).
Supplementary Material
Highlights.
T-type calcium channels are required for neural tube closure in Ciona and Xenopus
T-type calcium channels repress EphrinA expression in neural tube closure
Acknowledgments
We thank Erin Newman-Smith, Eli Spina and Matt Kourakis for their critical reading of this manuscript. We also thank Jasmine Anderson for her efforts in cloning the C. intestinalis guide RNA constructs and Alberto Stolfi for sharing CRISPR and dnEPH3 plasmids. This work was supported by award HD038701 from the National Institutes of Health to W.C.S.
Footnotes
Author contributions. SA and WCS conceived and designed experiments. SA, HM and SMK performed experiments. SA and WCS wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ABDUL-AZIZ NM, TURMAINE M, GREENE ND, COPP AJ. EphrinA-EphA receptor interactions in mouse spinal neurulation: implications for neural fold fusion. The International journal of developmental biology. 2009;53:559–68. doi: 10.1387/ijdb.082777na. [DOI] [PubMed] [Google Scholar]
- ABDUL-WAJID S, VEEMAN MT, CHIBA S, TURNER TL, SMITH WC. Exploiting the extraordinary genetic polymorphism of ciona for developmental genetics with whole genome sequencing. Genetics. 2014;197:49–59. doi: 10.1534/genetics.114.161778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARVANITIS D, DAVY A. Eph/ephrin signaling: networks. Genes Dev. 2008;22:416–29. doi: 10.1101/gad.1630408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTHIER C, MONTEIL A, LORY P, STRUBE C. Alpha(1H) mRNA in single skeletal muscle fibres accounts for T-type calcium current transient expression during fetal development in mice. J Physiol. 2002;539:681–91. doi: 10.1113/jphysiol.2001.013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKISTON D, VANDENBERG LN, LEVIN M. High-throughput Xenopus laevis immunohistochemistry using agarose sections. Cold Spring Harb Protoc. 2010 doi: 10.1101/pdb.prot5532. pdb prot5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRANDLI AW, KIRSCHNER MW. Molecular cloning of tyrosine kinases in the early Xenopus embryo: identification of Eck-related genes expressed in cranial neural crest cells of the second (hyoid) arch. Dev Dyn. 1995;203:119–40. doi: 10.1002/aja.1002030202. [DOI] [PubMed] [Google Scholar]
- BRONNER-FRASER M, WOLF JJ, MURRAY BA. Effects of antibodies against N-cadherin and N-CAM on the cranial neural crest and neural tube. Developmental biology. 1992;153:291–301. doi: 10.1016/0012-1606(92)90114-v. [DOI] [PubMed] [Google Scholar]
- CHEN CC, LAMPING KG, NUNO DW, BARRESI R, PROUTY SJ, LAVOIE JL, CRIBBS LL, ENGLAND SK, SIGMUND CD, WEISS RM, WILLIAMSON RA, HILL JA, CAMPBELL KP. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science. 2003;302:1416–8. doi: 10.1126/science.1089268. [DOI] [PubMed] [Google Scholar]
- DESCHET K, SMITH WC. Frimousse — a spontaneous ascidian mutant with anterior ectodermal fate transformation. Current Biology. 2004;14:R408–R410. doi: 10.1016/j.cub.2004.05.029. [DOI] [PubMed] [Google Scholar]
- FERRON L, RUCHON Y, RENAUD JF, CAPUANO V. T-type Ca(2)+ signalling regulates aldosterone-induced CREB activation and cell death through PP2A activation in neonatal cardiomyocytes. Cardiovasc Res. 2011;90:105–12. doi: 10.1093/cvr/cvq379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCOIS A, SCHUETTER N, LAFFRAY S, SANGUESA J, PIZZOCCARO A, DUBEL S, MANTILLERI A, NARGEOT J, NOEL J, WOOD JN, MOQRICH A, PONGS O, BOURINET E. The Low-Threshold Calcium Channel Cav3.2 Determines Low-Threshold Mechanoreceptor Function. Cell Rep. 2015 doi: 10.1016/j.celrep.2014.12.042. [DOI] [PubMed] [Google Scholar]
- FRISCHKNECHT F, RANDALL AD. Voltage- and ligand-gated ion channels in floor plate neuroepithelia of the rat. Neuroscience. 1998;85:1135–49. doi: 10.1016/s0306-4522(97)00641-6. [DOI] [PubMed] [Google Scholar]
- GHOSH S, MARQUARDT T, THALER JP, CARTER N, ANDREWS SE, PFAFF SL, HUNTER T. Instructive role of aPKCzeta subcellular localization in the assembly of adherens junctions in neural progenitors. Proc Natl Acad Sci USA. 2008;105:335–40. doi: 10.1073/pnas.0705713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTMANN K, DIETZEL ID, LUX HD, HUCK S, ROHRER H. Development of inward currents in chick sensory and autonomic neuronal precursor cells in culture. J Neurosci. 1988;8:3722–32. doi: 10.1523/JNEUROSCI.08-10-03722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENE ND, COPP AJ. Neural tube defects. Annual review of neuroscience. 2014;37:221–42. doi: 10.1146/annurev-neuro-062012-170354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HACKLEY C, MULHOLLAND E, KIM GJ, NEWMAN-SMITH E, SMITH WC. A transiently expressed connexin is essential for anterior neural plate development in Ciona intestinalis. Development. 2013;140:147–55. doi: 10.1242/dev.084681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAIGO SL, HILDEBRAND JD, HARLAND RM, WALLINGFORD JB. Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr Biol. 2003;13:2125–37. doi: 10.1016/j.cub.2003.11.054. [DOI] [PubMed] [Google Scholar]
- HARRINGTON MJ, HONG E, BREWSTER R. Comparative analysis of neurulation: first impressions do not count. Mol Reprod Dev. 2009;76:954–65. doi: 10.1002/mrd.21085. [DOI] [PubMed] [Google Scholar]
- HAUPAIX N, ABITUA PB, SIROUR C, YASUO H, LEVINE M, HUDSON C. Ephrin-mediated restriction of ERK1/2 activity delimits the number of pigment cells in the Ciona CNS. Developmental biology. 2014;394:170–80. doi: 10.1016/j.ydbio.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUPAIX N, STOLFI A, SIROUR C, PICCO V, LEVINE M, CHRISTIAEN L, YASUO H. p120RasGAP mediates ephrin/Eph-dependent attenuation of FGF/ERK signals during cell fate specification in ascidian embryos. Development. 2013;140:4347–52. doi: 10.1242/dev.098756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMBERG J, CLARKE DL, FRISEN J. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature. 2000;408:203–6. doi: 10.1038/35041577. [DOI] [PubMed] [Google Scholar]
- HOTTA K, MITSUHARA K, TAKAHASHI H, INABA K, OKA K, GOJOBORI T, IKEO K. A web-based interactive developmental table for the ascidian Ciona intestinalis, including 3D real-image embryo reconstructions: I. From fertilized egg to hatching larva. Developmental dynamics : an official publication of the American Association of Anatomists. 2007;236:1790–805. doi: 10.1002/dvdy.21188. [DOI] [PubMed] [Google Scholar]
- IMAI KS, STOLFI A, LEVINE M, SATOU Y. Gene regulatory networks underlying the compartmentalization of the Ciona central nervous system. Development. 2009;136:285–93. doi: 10.1242/dev.026419. [DOI] [PubMed] [Google Scholar]
- JI YJ, HWANG YS, MOOD K, CHO HJ, LEE HS, WINTERBOTTOM E, COUSIN H, DAAR IO. EphrinB2 affects apical constriction in Xenopus embryos and is regulated by ADAM10 and flotillin-1. Nat Commun. 2014;5:3516. doi: 10.1038/ncomms4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG D, TRESSER JW, HORIE T, TSUDA M, SMITH WC. Pigmentation in the sensory organs of the ascidian larva is essential for normal behavior. J Exp Biol. 2005;208:433–8. doi: 10.1242/jeb.01420. [DOI] [PubMed] [Google Scholar]
- LECLERC C, NEANT I, MOREAU M. The calcium: an early signal that initiates the formation of the nervous system during embryogenesis. Front Mol Neurosci. 2012;5:3. doi: 10.3389/fnmol.2012.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS BB, WESTER MR, MILLER LE, NAGARKAR MD, JOHNSON MB, SAHA MS. Cloning and characterization of voltage-gated calcium channel alpha1 subunits in Xenopus laevis during development. Developmental dynamics : an official publication of the American Association of Anatomists. 2009;238:2891–902. doi: 10.1002/dvdy.22102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN SS, TZENG BH, LEE KR, SMITH RJ, CAMPBELL KP, CHEN CC. Cav3.2 T-type calcium channel is required for the NFAT-dependent Sox9 expression in tracheal cartilage. Proc Natl Acad Sci USA. 2014;111:E1990–8. doi: 10.1073/pnas.1323112111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOUHIVUORI LM, LOUHIVUORI V, WIGREN HK, HAKALA E, JANSSON LC, NORDSTROM T, CASTREN ML, AKERMAN KE. Role of low voltage activated calcium channels in neuritogenesis and active migration of embryonic neural progenitor cells. Stem Cells Dev. 2013;22:1206–19. doi: 10.1089/scd.2012.0234. [DOI] [PubMed] [Google Scholar]
- MANNI L, AGNOLETTO A, ZANIOLO G, BURIGHEL P. Stomodeal and neurohypophysial placodes in Ciona intestinalis: insights into the origin of the pituitary gland. J Exp Zool B Mol Dev Evol. 2005;304:324–39. doi: 10.1002/jez.b.21039. [DOI] [PubMed] [Google Scholar]
- MARTIN RL, LEE JH, CRIBBS LL, PEREZ-REYES E, HANCK DA. Mibefradil block of cloned T-type calcium channels. J Pharmacol Exp Ther. 2000;295:302–8. [PubMed] [Google Scholar]
- MURDOCH JN, DOUDNEY K, PATERNOTTE C, COPP AJ, STANIER P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet. 2001;10:2593–601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- NELSON MT, TODOROVIC SM, PEREZ-REYES E. The role of T-type calcium channels in epilepsy and pain. Curr Pharm Des. 2006;12:2189–97. doi: 10.2174/138161206777585184. [DOI] [PubMed] [Google Scholar]
- NISHIDA H. Cell lineage analysis in ascidian embryos by intracellular injection of a tracer enzyme. III. Up to the tissue restricted stage. Developmental biology. 1987;121:526–41. doi: 10.1016/0012-1606(87)90188-6. [DOI] [PubMed] [Google Scholar]
- OKAMURA Y, NISHINO A, MURATA Y, NAKAJO K, IWASAKI H, OHTSUKA Y, TANAKA-KUNISHIMA M, TAKAHASHI N, HARA Y, YOSHIDA T, NISHIDA M, OKADO H, WATARI H, MEINERTZHAGEN IA, SATOH N, TAKAHASHI K, SATOU Y, OKADA Y, MORI Y. Comprehensive analysis of the ascidian genome reveals novel insights into the molecular evolution of ion channel genes. Physiological genomics. 2005;22:269–82. doi: 10.1152/physiolgenomics.00229.2004. [DOI] [PubMed] [Google Scholar]
- OLINSKI RP, DAHLBERG C, THORNDYKE M, HALLBOOK F. Three insulin-relaxin-like genes in Ciona intestinalis. Peptides. 2006;27:2535–46. doi: 10.1016/j.peptides.2006.06.008. [DOI] [PubMed] [Google Scholar]
- PARK EC, CHO GS, KIM GH, CHOI SC, HAN JK. The involvement of Eph-Ephrin signaling in tissue separation and convergence during Xenopus gastrulation movements. Developmental biology. 2011;350:441–50. doi: 10.1016/j.ydbio.2010.12.012. [DOI] [PubMed] [Google Scholar]
- PEREZ-REYES E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–61. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- PEREZ-REYES E, LORY P. Molecular biology of T-type calcium channels. CNS Neurol Disord Drug Targets. 2006;5:605–9. doi: 10.2174/187152706779025508. [DOI] [PubMed] [Google Scholar]
- PICCO V, HUDSON C, YASUO H. Ephrin-Eph signalling drives the asymmetric division of notochord/neural precursors in Ciona embryos. Development. 2007;134:1491–7. doi: 10.1242/dev.003939. [DOI] [PubMed] [Google Scholar]
- PYRGAKI C, LIU A, NISWANDER L. Grainyhead-like 2 regulates neural tube closure and adhesion molecule expression during neural fold fusion. Developmental biology. 2011;353:38–49. doi: 10.1016/j.ydbio.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAIBLE F, BRAND M. Divide et Impera--the midbrain-hindbrain boundary and its organizer. Trends Neurosci. 2004;27:727–34. doi: 10.1016/j.tins.2004.10.003. [DOI] [PubMed] [Google Scholar]
- RAN FA, HSU PD, WRIGHT J, AGARWALA V, SCOTT DA, ZHANG F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBERG SS, SPITZER NC. Calcium signaling in neuronal development. Cold Spring Harb Perspect Biol. 2011;3:a004259. doi: 10.1101/cshperspect.a004259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROURE A, ROTHBACHER U, ROBIN F, KALMAR E, FERONE G, LAMY C, MISSERO C, MUELLER F, LEMAIRE P. A multicassette Gateway vector set for high throughput and comparative analyses in ciona and vertebrate embryos. PLoS One. 2007;2:e916. doi: 10.1371/journal.pone.0000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIVE HL, GRAINGER RM, HARLAND RM. Early development of Xenopus laevis : a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- SIVE HL, GRAINGER RM, HARLAND RM. Inducing Ovulation in Xenopus laevis. CSH Protoc. 2007 doi: 10.1101/pdb.prot4734. pdb prot4734. [DOI] [PubMed] [Google Scholar]
- SMITH A, ROBINSON V, PATEL K, WILKINSON DG. The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Curr Biol. 1997;7:561–70. doi: 10.1016/s0960-9822(06)00255-7. [DOI] [PubMed] [Google Scholar]
- SOLECKI DJ, GOVEK EE, TOMODA T, HATTEN ME. Neuronal polarity in CNS development. Genes Dev. 2006;20:2639–47. doi: 10.1101/gad.1462506. [DOI] [PubMed] [Google Scholar]
- STOLFI A, GANDHI S, SALEK F, CHRISTIAEN L. Tissue-specific genome editing in Ciona embryos by CRISPR/Cas9. Development. 2014;141:4115–20. doi: 10.1242/dev.114488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOLFI A, WAGNER E, TALIAFERRO JM, CHOU S, LEVINE M. Neural tube patterning by Ephrin, FGF and Notch signaling relays. Development. 2011;138:5429–39. doi: 10.1242/dev.072108. [DOI] [PubMed] [Google Scholar]
- TAKAMURA K, OKA N, AKAGI A, OKAMOTO K, OKADA T, FUKUOKA T, HOGAKI A, NAITO D, OOBAYASHI Y, SATOH N. EST analysis of genes that are expressed in the neural complex of Ciona intestinalis adults. Zoolog Sci. 2001;18:1231–6. doi: 10.2108/zsj.18.1231. [DOI] [PubMed] [Google Scholar]
- TIAN L, HIRES SA, MAO T, HUBER D, CHIAPPE ME, CHALASANI SH, PETREANU L, AKERBOOM J, MCKINNEY SA, SCHREITER ER, BARGMANN CI, JAYARAMAN V, SVOBODA K, LOOGER LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–81. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRESSER J, CHIBA S, VEEMAN M, EL-NACHEF D, NEWMAN-SMITH E, HORIE T, TSUDA M, SMITH WC. doublesex/mab3 related-1 (dmrt1) is essential for development of anterior neural plate derivatives in Ciona. Development. 2010;137:2197–203. doi: 10.1242/dev.045302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUDA M, KUSAKABE T, IWAMOTO H, HORIE T, NAKASHIMA Y, NAKAGAWA M, OKUNOU K. Origin of the vertebrate visual cycle: II. Visual cycle proteins are localized in whole brain including photoreceptor cells of a primitive chordate. Vision research. 2003;43:3045–53. doi: 10.1016/j.visres.2003.09.012. [DOI] [PubMed] [Google Scholar]
- VEEMAN MT, CHIBA S, SMITH WC. Ciona genetics. Methods in molecular biology. 2011;770:401–22. doi: 10.1007/978-1-61779-210-6_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VEEMAN MT, NAKATANI Y, HENDRICKSON C, ERICSON V, LIN C, SMITH WC. Chongmague reveals an essential role for laminin-mediated boundary formation in chordate convergence and extension movements. Development. 2008;135:33–41. doi: 10.1242/dev.010892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VEEMAN MT, NEWMAN-SMITH E, EL-NACHEF D, SMITH WC. The ascidian mouth opening is derived from the anterior neuropore: reassessing the mouth/neural tube relationship in chordate evolution. Developmental biology. 2010;344:138–49. doi: 10.1016/j.ydbio.2010.04.028. [DOI] [PubMed] [Google Scholar]
- WALLINGFORD JB. Neural tube closure and neural tube defects: studies in animal models reveal known knowns and known unknowns. Am J Med Genet C Semin Med Genet. 2005;135C:59–68. doi: 10.1002/ajmg.c.30054. [DOI] [PubMed] [Google Scholar]
- WALLINGFORD JB, HARLAND RM. Xenopus Dishevelled signaling regulates both neural and mesodermal convergent extension: parallel forces elongating the body axis. Development. 2001;128:2581–92. doi: 10.1242/dev.128.13.2581. [DOI] [PubMed] [Google Scholar]
- WALLINGFORD JB, NISWANDER LA, SHAW GM, FINNELL RH. The continuing challenge of understanding, preventing, and treating neural tube defects. Science. 2013;339:1222002. doi: 10.1126/science.1222002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YBOT-GONZALEZ P, COPP AJ. Bending of the neural plate during mouse spinal neurulation is independent of actin microfilaments. Dev Dyn. 1999;215:273–83. doi: 10.1002/(SICI)1097-0177(199907)215:3<273::AID-AJA9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.