Abstract
In this paper, we introduce arylphosphinic acid aminoquinoline amides as competent substrates for cobalt-catalyzed sp2 C–H bond functionalization. Specifically, the feasibility of their coupling with alkynes, alkenes, and allyl pivalate has been demonstrated. Reactions are catalyzed by simple Co(NO3)2 hydrate in ethanol or mixed dioxane/tBuOH solvent in the presence of Mn(OAc)3·2H2O additive, sodium pivalate, or acetate base and use oxygen from the air as an oxidant. Directing group removal affords ortho-functionalized P,P-diarylphosphinic acids.
Keywords: cobalt, C–H functionalization, phosphinic amides, alkenes, alkynes
Graphical abstract
Transition-metal-catalyzed coupling of sp2 C–H bonds with alkenes and alkynes has been extensively investigated.1 In most cases, these transformations are promoted by second-row transition-metal complexes.2 Relatively few papers describe the use of abundant first-row transition metals in these reactions.3 Among base metals, cobalt catalysis shows perhaps the highest versatility in coupling of sp2 C–H bonds with alkenes and alkynes and in other C–H bond functionalization reactions.4–8 An early example showing low-valent cobalt catalysis was reported by Brookhart.5 Yoshikai has disclosed a series of relevant low-valent cobalt-catalyzed reactions.6 Cyclopentadienylcobalt(III) complexes were introduced by Kanai as effective catalysts in the coupling of sp2 C–H bonds with alkynes.7 We recently reported aminoquinoline- and picolinamide-directed coupling of alkynes, alkenes, and carbon monoxide with sp2 C–H bonds catalyzed by simple cobalt salts.8a–c Dimerization of aminoquinoline benzamides was also described.8d Excellent functional group tolerance was observed, the reactions employed oxygen as a terminal oxidant, and silver additives were not required. In these transformations, the aminoquinoline auxiliary is attached to the aryl group via a carbonyl linkage, which allows the removal of the directing group after the functionalization step and increases the synthetic versatility of the methodology.9 After the directing group was cleaved, a benzoic acid derivative was obtained.
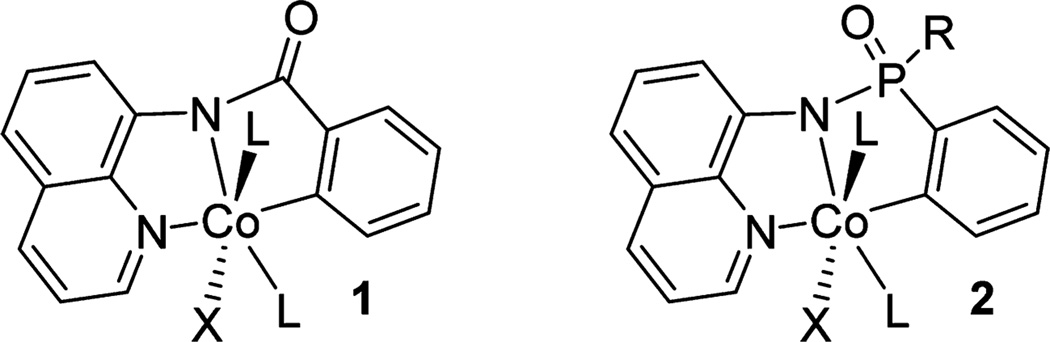
A successful bidentate, monoanionic auxiliary requires a coordinating group and an acidic NH group that can bind to transition metals in a bidentate fashion to form a five-membered-ring chelate (Figure 1).9c This requirement is met in aminoquinoline amides (1). It is conceivable that the amide functionality in these complexes could be replaced with a phosphinic amide group (2). A related 2-pyridylsulfonyl group is efficient in directing the functionalization of amino acid sp3 C–H bonds.10 Since phosphorus-containing compounds are important as pesticides, as organocatalysts, and in materials chemistry,11 we decided to investigate C–H bond functionalization of phosphinamides possessing an aminoquinoline directing group. Relatively few examples of phosphorus-containing directing groups in catalytic sp2 C–H bond functionalization have been disclosed, and nearly all of these employ second-row transition metals.12 A rare example of cobalt-catalyzed intramolecular C–H amination with phosphoryl azide has been reported.13
Figure 1.
Aminoquinoline directing group.
We show here that aminoquinoline-containing phosphinic amides are competent substrates in C–H bond functionalization. Cobalt-catalyzed, aminoquinoline-directed couplings of sp2 C–H bonds with alkynes and ethylene as well as vinyl and allyl pivalates have been reported.
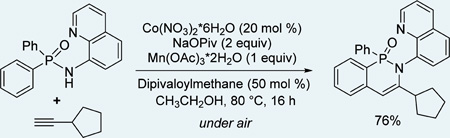
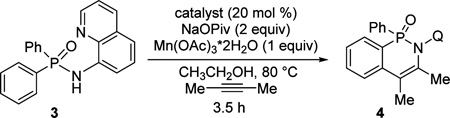
Gratifyingly, minor modification of the conditions that were successful for our previous cobalt-catalyzed reactions8 worked for phosphinic amide functionalization. Reaction optimization with respect to the ligand and control experiments are presented in Table 1. The use of Co(acac)2 catalyst, Mn(OAc)3·2H2O cocatalyst, and Na pivalate (NaOPiv) base in ethanol at 80 °C gave a respectable 69% yield of 4 (entry 1). If Co(NO3)2·6H2O was used instead of Co(acac)2, a 30% yield of product was obtained (entry 2). Addition of dipivaloylmethane ligand to Co(NO3)2·6H2O increased the yield to 99% (entry 3). If Co catalyst was omitted, 4 was not formed (entry 4). Omission of Mn(OAc)3·2H2O reduced the yield to 49% (entry 5). If the reaction was run in degassed solvent under argon, less than 5% of 4 was formed (entry 6). Diketones other than dipivaloylmethane afforded lower yields (entries 7 and 8). Addition of a catalytic amount of Mn(OAc)3 also lowered the conversion (entry 9). Similarly, use of Co(acac)2 with added acetylacetone afforded a lower yield (entry 10).
Table 1.
Optimization of Reaction Conditions and Control Experimentsa
 | |||
|---|---|---|---|
| entry | catalyst | cooxidant | yield of 4, % |
| 1 | Co(acac)2 | Mn(OAc)3·2H2O | 69 |
| 2 | Co(NO3)2·6H2O | Mn(OAc)3·2H2O | 30 |
| 3b | Co(NO3)2·6H2O | Mn(OAc)3·2H2O | >99 |
| 4b | none | Mn(OAc)3·2H2O | 0 |
| 5b | Co(NO3)2·6H2O | none | 49 |
| 6b,c | Co(NO3)2·6H2O | Mn(OAc)3·2H2O | <5 |
| 7d | Co(NO3)2·6H2O | Mn(OAc)3·2H2O | 74 |
| 8e | Co(NO3)2·6H2O | Mn(OAc)3·2H2O | 53 |
| 9b,f | Co(NO3)2·6H2O | Mn(OAc)3·2H2O | 82 |
| 10g | Co(acac)2 | Mn(OAc)3·2H2O | 76 |
Conditions unless specified otherwise: amide (0.1 mmol), alkyne (0.12 mmol), catalyst (0.02 mmol), Na pivalate (0.2 mmol), CH3CH2OH (1 mL), air. Yields were determined by NMR of reaction mixtures, with triphenylmethane internal standard.
Dipivaloylmethane (50 mol %) added.
Degassed ethanol, under an Ar atmosphere.
PhCOCH2COtBu (50 mol %) added.
PhCOCH2COPh (50 mol %) added.
Mn(OAc)3·2H2O (50 mol %).
Acetylacetone (10 mol %) added. Q = 8-quinolinyl.
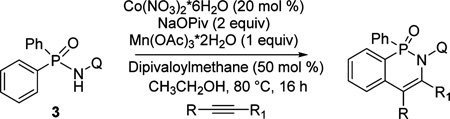
The reaction scope with respect to alkyne coupling partner is presented in Table 2. Nearly all products exist as atropisomer mixtures that likely equilibrate by rotation about the N-quinoline bond. Coalescence of their 1H NMR signals is observed above 85 °C. The coupling reaction is successful for simple, dialkyl-substituted alkynes such as 2-butyne and 3-hexyne, affording products in good yields (entries 1 and 2). Scaling the reaction up from 0.5 to 3 mmol affords the product in nearly the same yield (entry 1). Diphenylacetylene gives the coupling product in 78% yield (entry 3). An ester functionality is tolerated (entry 4). Arylacetylenes are competent coupling partners (entries 5–8). Steric hindrance in the case of mesitylacetylene lowers the yield of the product to 24% (entry 8). The thiophene functionality is compatible with the reaction conditions (entry 9). Aliphatic terminal alkynes afford products in good yields (entries 10–12). 1-Phenyl-1-propyne gives the coupling product in 72% yield (entry 13). Terminal alkynes and 1-phenyl-1-propyne form regioisomeric mixtures. However, selectivities are reasonably good, ranging from about 9:1 for aryl alkynes (entries 5–7) to >20:1 for 1-hexyne and cyclopentylacetylene (entries 10 and 12).
Table 2.
Reaction Scope with Respect to Alkynesa
 | |||
|---|---|---|---|
| entry | alkyne | product | yield, % |
| 1 | 2-butyne | 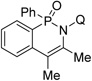 |
78 79b |
| 2 | 3-hexyne | 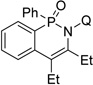 |
87 |
| 3 | diphenylacetylene | 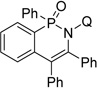 |
78 |
| 4 | butyne-1,4-dioldipivalate | 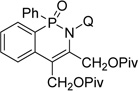 |
61 |
| 5c | phenylacetylene | 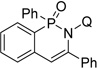 |
81 |
| 6c | 4-F-phenylacetylene | 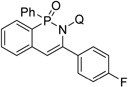 |
80 |
| 7c | 3-Cl-phenylacetylene | 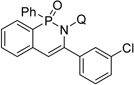 |
81 |
| 8d | mesitylacetylene | 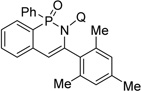 |
24 |
| 9e | 3-ethynylthiophene | 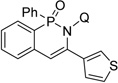 |
80 |
| 10f | 1-hexyne | 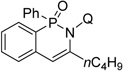 |
74 |
| 11g | prop-2-ynylpivalate | 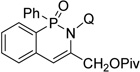 |
65 |
| 12f | cyclopentylacetylene | 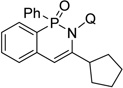 |
76 |
| 13h | 1-phenyl-1-propyne | 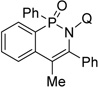 |
72 |
Conditions: phosphinic amide (0.5 mmol), alkyne (0.6 mmol), Co(NO3)2·6H2O (0.1 mmol), Na pivalate (1 mmol), Mn(OAc)3·2H2O (0.5 mmol), dipivaloylmethane (0.25 mmol), ethanol (5 mL), air. Yields are isolated yields. Please see the Supporting Information for details.
Scale: 3 mmol, 24 h.
Isolated as a 9:1 regioisomer mixture.
Isolated as a 14:1 regioisomer mixture, 1 mmol scale.
Isolated as a 10:1 regioisomer mixture.
Regioisomer ratio: >20:1.
Isolated as a 14:1 regioisomer mixture.
Isolated as a 20:1 regioisomer mixture.
Piv = pivalate.
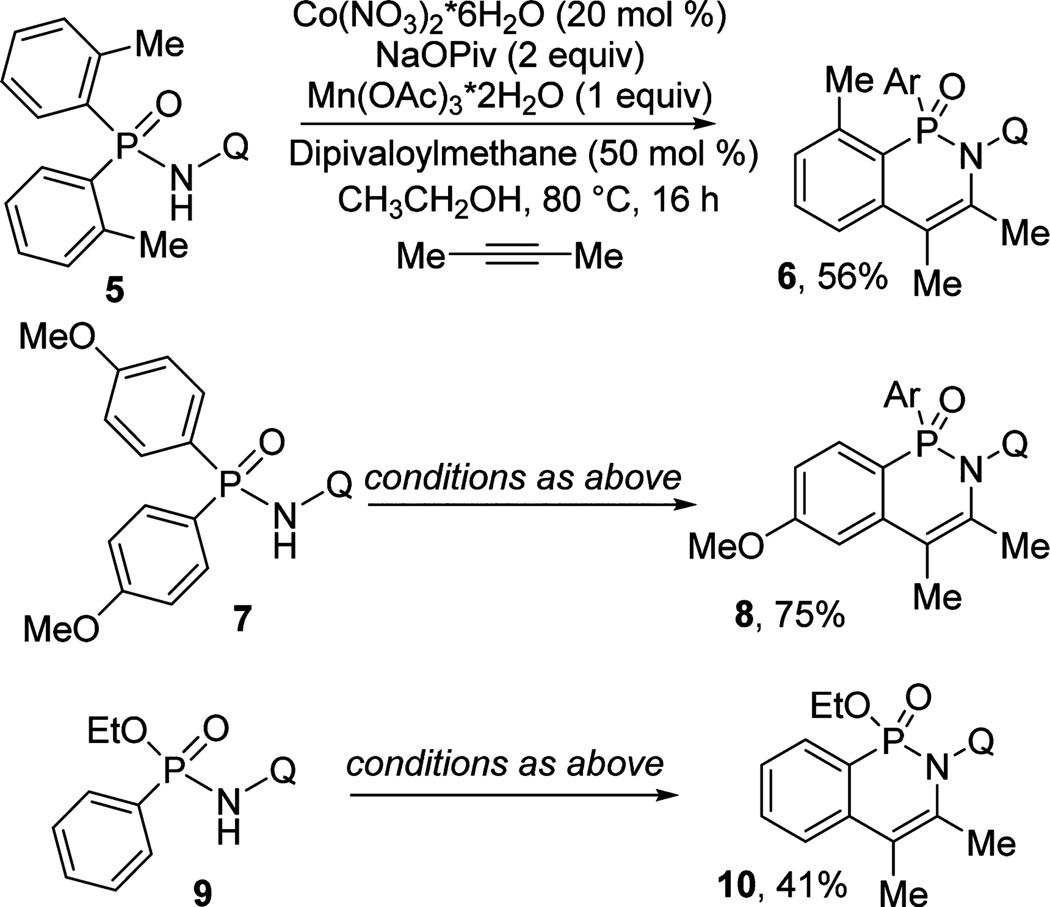
Substituted diarylphosphinamides are also reactive (Scheme 1). Thus, 2-methyl- and 4-methoxyphenyl derivatives 5 and 7 were reacted with 2-butyne under standard conditions to afford products 6 and 8 in 56 and 75% yields, respectively. Ethoxy-substituted 9 gave 10 in 41% yield.
Scheme 1.
Substituted Phosphinamides
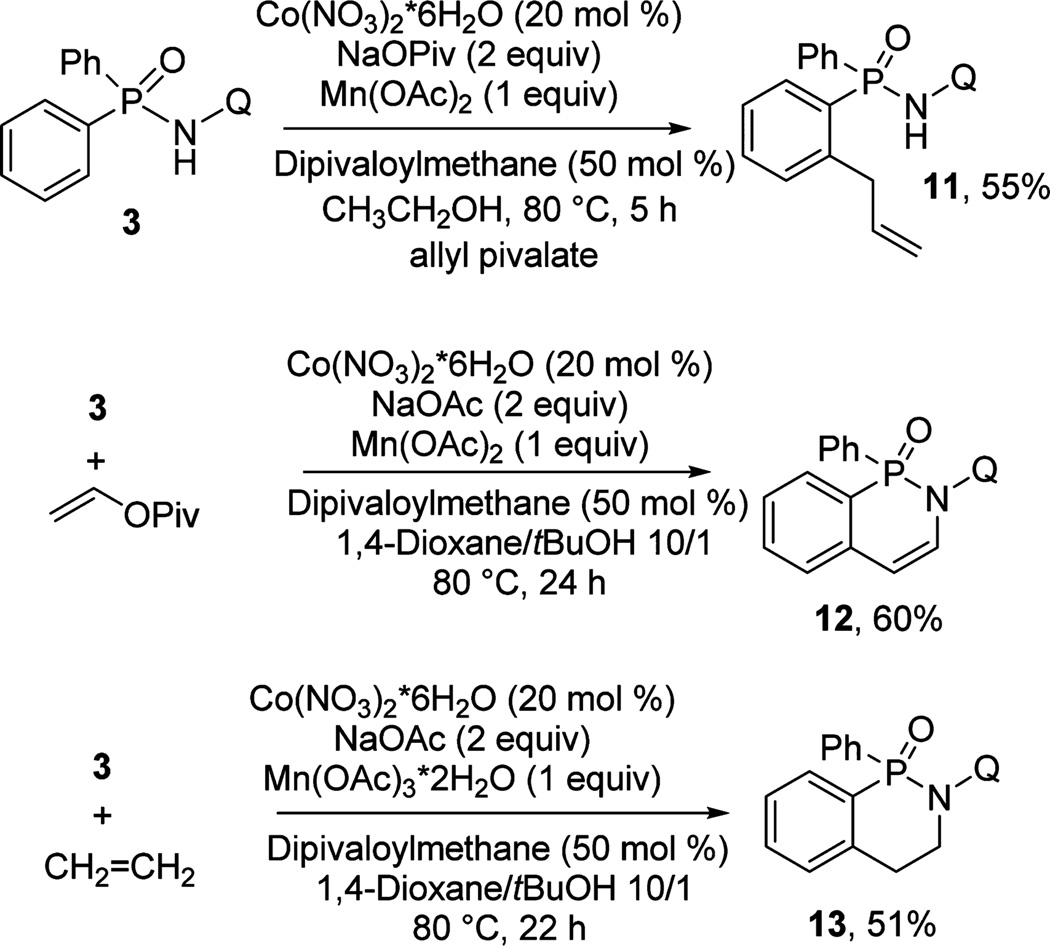
Reactions with alkenes were also investigated (Scheme 2). Thus, substrate 3 was allylated with allyl pivalate, affording noncyclic 11 in 55% yield. Vinyl pivalate reacted with 3, giving the product of pivalate elimination 12. These transformations do not require oxidant; however, omission of Mn(OAc)2 gave products in lower yields. Finally, ethylene was coupled with 3, affording 13 in 51% yield. Reactions with monosubstituted alkenes were not pursued due to the formation of complicated diastereomer mixtures.
Scheme 2.
Reaction with Alkenes
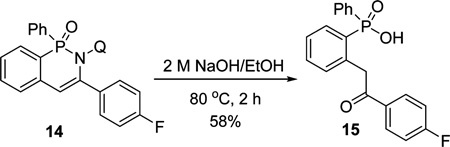
The directing group can be cleaved under basic conditions, giving ortho-functionalized phosphinic acids (eq 1). The p-fluorophenyl derivative 14 was treated with NaOH in ethanol to afford 15 in 58% yield.
 |
(1) |
In conclusion, a method for cobalt-catalyzed, aminoquinoline-directed functionalization of phosphinic acid amide sp2 C–H bonds has been developed. Specifically, the feasibility of their coupling with alkynes, alkenes, and allyl pivalate has been demonstrated. Reactions proceed in ethanol or mixed dioxane/tBuOH solvent in the presence of Mn(OAc)3 additive, sodium pivalate, or acetate base and use oxygen from air as an oxidant. Good to excellent regioselectivities are observed for unsymmetrical and terminal alkynes. Directing group removal gives ortho-functionalized arylphosphinic acids.
Supplementary Material
Acknowledgments
We thank the Welch Foundation (Chair E-0044), NIGMS (Grant No. R01GM077635), and the University of Houston for supporting this research.
Footnotes
ASSOCIATED CONTENT
Supporting Information
- Detailed experimental procedures and characterization data for new compounds (PDF)
The authors declare no competing financial interest.
REFERENCES
- 1.Reviews: Ritleng V, Sirlin C, Pfeffer M. Chem. Rev. 2002;102:1731–1769. doi: 10.1021/cr0104330. Colby DA, Bergman RG, Ellman JA. Chem. Rev. 2010;110:624–655. doi: 10.1021/cr900005n. Messaoudi S, Brion J-D, Alami M. Eur. J. Org. Chem. 2010:6495–6516. Ye B, Cramer N. Acc. Chem. Res. 2015;48:1308–1318. doi: 10.1021/acs.accounts.5b00092. Engle KM, Mei T-S, Wasa M, Yu J-Q. Acc. Chem. Res. 2012;45:788–802. doi: 10.1021/ar200185g.
- 2.Selected examples: Kakiuchi F, Yamamoto Y, Chatani N, Murai S. Chem. Lett. 1995:681–682. Dong C-G, Yeung P, Hu Q-S. Org. Lett. 2007;9:363–366. doi: 10.1021/ol062885a. Tunge JA, Foresee LN. Organometallics. 2005;24:6440–6444. Jia C, Piao D, Oyamada J, Lu W, Kitamura T, Fujiwara Y. Science. 2000;287:1992–1995. doi: 10.1126/science.287.5460.1992. Chernyak N, Tilly D, Li Z, Gevorgyan V. Chem. Commun. 2010;46:150–152. doi: 10.1039/b919991h. Ackermann L, Lygin AV, Hofmann N. Angew. Chem., Int. Ed. 2011;50:6379–6382. doi: 10.1002/anie.201101943. Yi CS, Yun SY. J. Am. Chem. Soc. 2005;127:17000–17006. doi: 10.1021/ja055608s. Guimond N, Gouliaras C, Fagnou K. J. Am. Chem. Soc. 2010;132:6908–6909. doi: 10.1021/ja102571b. Ueura K, Satoh T, Miura M. J. Org. Chem. 2007;72:5362–5367. doi: 10.1021/jo070735n. Zhang YJ, Skucas E, Krische MJ. Org. Lett. 2009;11:4248–4250. doi: 10.1021/ol901759t. Colby DA, Bergman RG, Ellman JA. J. Am. Chem. Soc. 2008;130:3645–3651. doi: 10.1021/ja7104784. Hong P, Cho B-R, Yamazaki H. Chem. Lett. 1979:339–342. Wang H, Grohmann C, Nimphius C, Glorius F. J. Am. Chem. Soc. 2012;134:19592–19595. doi: 10.1021/ja310153v. Lu Y, Wang D-H, Engle KM, Yu J-Q. J. Am. Chem. Soc. 2010;132:5916–5921. doi: 10.1021/ja101909t. Miura M, Tsuda T, Satoh T, Pivsa-Art S, Nomura M. J. Org. Chem. 1998;63:5211–5215. Tani M, Sakaguchi S, Ishii Y. J. Org. Chem. 2004;69:1221–1226. doi: 10.1021/jo035568f.
- 3.(a) Nakao Y, Kashihara N, Kanyiva KS, Hiyama T. J. Am. Chem. Soc. 2008;130:16170–16171. doi: 10.1021/ja807258m. [DOI] [PubMed] [Google Scholar]; (b) Shiota H, Ano Y, Aihara Y, Fukumoto Y, Chatani N. J. Am. Chem. Soc. 2011;133:14952–14955. doi: 10.1021/ja206850s. [DOI] [PubMed] [Google Scholar]; (c) Liu W, Zell D, John M, Ackermann L. Angew. Chem., Int. Ed. 2015;54:4092–4096. doi: 10.1002/anie.201411808. [DOI] [PubMed] [Google Scholar]; (d) Zhou B, Chen H, Wang C. J. Am. Chem. Soc. 2013;135:1264–1267. doi: 10.1021/ja311689k. [DOI] [PubMed] [Google Scholar]
- 4.(a) Gandeepan P, Cheng C-H. Acc. Chem. Res. 2015;48:1194–1206. doi: 10.1021/ar500463r. [DOI] [PubMed] [Google Scholar]; (b) Gao K, Yoshikai N. Acc. Chem. Res. 2014;47:1208–1219. doi: 10.1021/ar400270x. [DOI] [PubMed] [Google Scholar]; (c) Hyster TK. Catal. Lett. 2015;145:458–467. [Google Scholar]; (d) Wang A, Sun H, Li X. Organometallics. 2008;27:5434–5437. [Google Scholar]; (e) Hummel JR, Ellman JA. J. Am. Chem. Soc. 2015;137:490–498. doi: 10.1021/ja5116452. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Ackermann L. J. Org. Chem. 2014;79:8948–8954. doi: 10.1021/jo501361k. [DOI] [PubMed] [Google Scholar]; (g) Li J, Ackermann L. Angew. Chem., Int. Ed. 2015;54:8551–8554. doi: 10.1002/anie.201501926. [DOI] [PubMed] [Google Scholar]; (h) Zhao D, Kim JH, Stegemann L, Strassert CA, Glorius F. Angew. Chem., Int. Ed. 2015;54:4508–4511. doi: 10.1002/anie.201411994. [DOI] [PubMed] [Google Scholar]; (i) Zhang L-B, Hao X-Q, Zhang S-K, Liu Z-J, Zheng X-X, Gong J-F, Niu J-L, Song M-P. Angew. Chem., Int. Ed. 2015;54:272–275. doi: 10.1002/anie.201409751. [DOI] [PubMed] [Google Scholar]; (j) Fallon BJ, Derat E, Amatore M, Aubert C, Chemla F, Ferreira F, Perez-Luna A, Petit M. J. Am. Chem. Soc. 2015;137:2448–2451. doi: 10.1021/ja512728f. [DOI] [PubMed] [Google Scholar]; (k) Schaefer BA, Margulieux GW, Small BL, Chirik PJ. Organometallics. 2015;34:1307–1320. [Google Scholar]; (l) Pawar AB, Chang S. Org. Lett. 2015;17:660–663. doi: 10.1021/ol503680d. [DOI] [PubMed] [Google Scholar]
- 5.Lenges CP, White PS, Brookhart M. J. Am. Chem. Soc. 1998;120:6965–6979. [Google Scholar]
- 6.(a) Lee PS, Fujita T, Yoshikai N. J. Am. Chem. Soc. 2011;133:17283–17295. doi: 10.1021/ja2047073. [DOI] [PubMed] [Google Scholar]; (b) Ding Z, Yoshikai N. Angew. Chem., Int. Ed. 2012;51:4698–4701. doi: 10.1002/anie.201200019. [DOI] [PubMed] [Google Scholar]; (c) Gao K, Lee P-S, Fujita T, Yoshikai N. J. Am. Chem. Soc. 2010;132:12249–12251. doi: 10.1021/ja106814p. [DOI] [PubMed] [Google Scholar]; (d) Yamakawa T, Yoshikai N. Org. Lett. 2013;15:196–199. doi: 10.1021/ol303259m. [DOI] [PubMed] [Google Scholar]
- 7.(a) Yoshino T, Ikemoto H, Matsunaga S, Kanai M. Chem. - Eur. J. 2013;19:9142–9146. doi: 10.1002/chem.201301505. [DOI] [PubMed] [Google Scholar]; (b) Ikemoto H, Yoshino T, Sakata K, Matsunaga S, Kanai M. J. Am. Chem. Soc. 2014;136:5424–5431. doi: 10.1021/ja5008432. [DOI] [PubMed] [Google Scholar]
- 8.(a) Grigorjeva L, Daugulis O. Angew. Chem., Int. Ed. 2014;53:10209–10212. doi: 10.1002/anie.201404579. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Grigorjeva L, Daugulis O. Org. Lett. 2014;16:4684–4687. doi: 10.1021/ol502005g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Grigorjeva L, Daugulis O. Org. Lett. 2014;16:4688–4690. doi: 10.1021/ol502007t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Grigorjeva L, Daugulis O. Org. Lett. 2015;17:1204–1207. doi: 10.1021/acs.orglett.5b00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Zaitsev VG, Shabashov D, Daugulis O. J. Am. Chem. Soc. 2005;127:13154–13155. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]; (b) Shabashov D, Daugulis O. J. Am. Chem. Soc. 2010;132:3965–3972. doi: 10.1021/ja910900p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Daugulis O, Roane J, Tran LD. Acc. Chem. Res. 2015;48:1053–1064. doi: 10.1021/ar5004626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poveda A, Alonso I, Fernández-Ibáñez MA. Chem. Sci. 2014;5:3873–3882. [Google Scholar]
- 11.(a) Marinetti A, Voituriez A. Synlett. 2010:174–194. [Google Scholar]; (b) Baumgartner T. Acc. Chem. Res. 2014;47:1613–1622. doi: 10.1021/ar500084b. [DOI] [PubMed] [Google Scholar]; (c) Eto M. Biosci., Biotechnol., Biochem. 1997;61:1–11. [Google Scholar]
- 12.(a) Du Z-J, Guan J, Wu G-J, Xu P, Gao L-X, Han F-S. J. Am. Chem. Soc. 2015;137:632–635. doi: 10.1021/ja512029x. [DOI] [PubMed] [Google Scholar]; (b) Park S, Seo B, Shin S, Son J-Y, Lee PH. Chem. Commun. 2013;49:8671–8673. doi: 10.1039/c3cc44995e. [DOI] [PubMed] [Google Scholar]; (c) Ma W, Ackermann L. Synthesis. 2014;46:2297–2304. [Google Scholar]; (d) Unoh Y, Hashimoto Y, Takeda D, Hirano K, Satoh T, Miura M. Org. Lett. 2013;15:3258–3261. doi: 10.1021/ol4012794. [DOI] [PubMed] [Google Scholar]; (e) Gwon D, Lee D, Kim J, Park S, Chang S. Chem. - Eur. J. 2014;20:12421–12425. doi: 10.1002/chem.201404151. [DOI] [PubMed] [Google Scholar]; (f) Lin Z-Q, Wang W-Z, Yan S-B, Duan W-L. Angew. Chem., Int. Ed. 2015;54:6265–6269. doi: 10.1002/anie.201500201. [DOI] [PubMed] [Google Scholar]; (g) Park Y, Jeon I, Shin S, Min J, Lee PH. J. Org. Chem. 2013;78:10209–10220. doi: 10.1021/jo401608v. [DOI] [PubMed] [Google Scholar]; (h) Zhao D, Nimphius C, Lindale M, Glorius F. Org. Lett. 2013;15:4504–4507. doi: 10.1021/ol402053n. [DOI] [PubMed] [Google Scholar]; (i) Itoh M, Hashimoto Y, Hirano K, Satoh T, Miura M. J. Org. Chem. 2013;78:8098–8104. doi: 10.1021/jo401393b. [DOI] [PubMed] [Google Scholar]
- 13.Lu H, Tao J, Jones JE, Wojtas L, Zhang XP. Org. Lett. 2010;12:1248–1251. doi: 10.1021/ol100110z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.