Abstract
Brown adipose tissue (BAT) has evolved as a unique thermogenic organ that allows placental mammals to withstand cold environmental temperatures through the dissipation of metabolic energy in the form of heat. Although traditionally believed to be lost shortly after birth, metabolically active BAT depots have recently been identified in a large percentage of human adults. Besides classical brown cells, a distinct type of thermogenic adipocytes named beige or brite (brown in white) cells are recruited in white adipose tissue depots under specific stimuli. Given the well-known energy-dissipating properties of thermogenic adipose tissue and its function of metabolic sink for glucose and lipids, this tissue has attracted considerable research interest as a possible target for treating obesity and metabolic disease. The complex network of interorgan connections that regulate BAT and brite tissue mass and function is a major hurdle for the development of therapeutic strategies against metabolic disorders. This review provides an overview of the current knowledge on the regulation of BAT and brite adipose tissue function. The possibility of targeting these tissues to treat obesity and other metabolic disorders is also discussed.
General keywords: adiposity, diabetes mellitus, energy metabolism, weight loss, beige cells, mitochondrial respiration, positron emission tomography (PET), inflammation, sympathicomimetics
Specific keywords: obesity, insulin resistance, cold exposure, uncoupling protein, brite adipose tissue, thermogenesis, adipokines, thyroid hormone receptor
Image keywords: bone morphogenetic protein (BMP), fibroblast growth factor (FGF), uncoupling protein (UCP), natriuretic peptide, white adipose tissue (WAT), brown adipose tissue (BAT), thyroid hormones, thermoregulation
Adipose tissue: much more than a fat reservoir
Adipose tissue is a complex organ with significant effects on the physiology and pathophysiology of an organism. Contrary to the original view of a lipid droplet-containing, inert variant of connective tissue, adipose tissue is now recognized as an integral component in nutrient homeostasis, energy metabolism and modulation of inflammatory pathways.1 Adipose tissue also serves protective and cushioning functions as well as thermal insulation and thermogenesis. In addition, the distribution of adipose tissue depots has an important role in defining secondary sexual characteristics in several species, including humans.
Traditionally, two forms of adipose tissue are distinguished based on their peculiar morphological, physiological and biochemical characteristics: white adipose tissue (WAT) and brown adipose tissue (BAT). WAT is the largest energy store in humans. Morphologically, white adipocytes are characterized by the presence of a single large lipid droplet that comprises the majority of cell volume, with the cytoplasm and nucleus found at the cell periphery. They only possess few mitochondria. Besides its role as an energy-storing tissue, WAT and resident macrophages also act as an endocrine organ by releasing an array of signaling molecules, collectively termed adipokines.2 Adiponectin, leptin, interleukin (IL) 6, tumor necrosis factor α (TNF-α), resistin, and omentin 1 are among the best known adipokines. Alterations in WAT plasticity and expandability in the context of overnutrition are key factors in the pathogenesis of obesity-associated metabolic dysregulations.3 Indeed, the imbalance between pro- and antinflammatory adipokines is involved in the development of insulin resistance and diabetes through a state of chronic low-grade inflammation.3
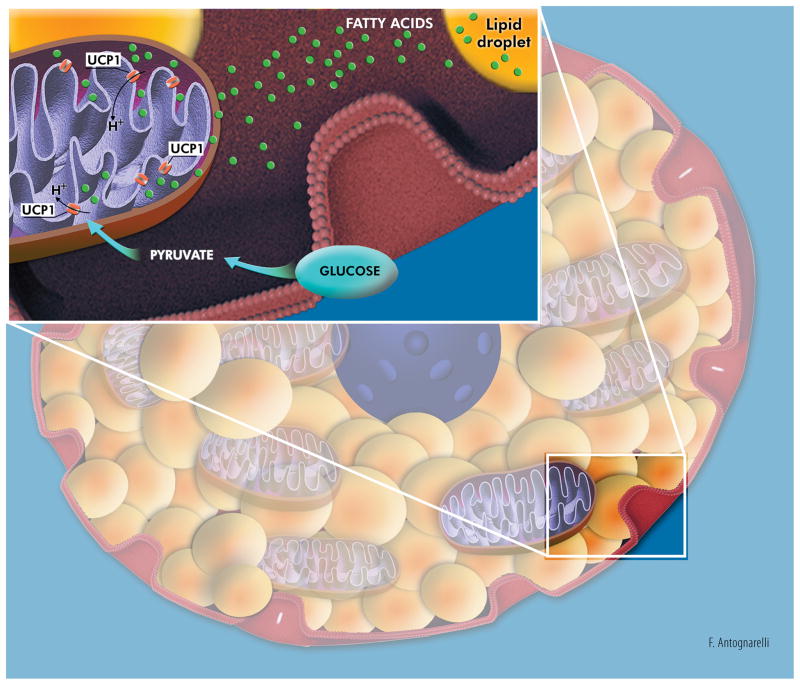
BAT differs markedly from WAT. Brown adipocytes contain multiple small lipid droplets that confer cells a multilocular appearance, and are enriched in mitochondria. BAT is densely innervated by the sympathetic nervous system (SNS) and is highly vascularized (up to five capillaries per cell).1 The main function of BAT is nonshivering thermogenesis (NST), which serves to maintain body temperature in small mammals and hibernating species, and allows human newborns to cope with the thermal shock of delivery.1 Indeed, brown adipocytes actively oxidate fatty acids and glucose to sustain heat generation through uncoupling mitochondrial substrate oxidation from ATP production (Figure 1).1 A role for BAT beyond thermogenesis has also been postulated. Studies in experimental rodents have shown that brown adipocytes may release a number of signaling molecules with autocrine and paracrine actions [e.g., triiodothyronine, prostaglandins, angiotensinogen, IL1α, IL6, insulin-like growth factor I, fibroblast growth factor (FGF) 2 and 21, etc.].4 However, the actual existence of BAT-derived endocrine factors and their relevance to human health are yet to be established.
Figure 1. Simplified representation of the metabolic activity of a brown adipocyte.
Energy produced via oxidation of glucose and fatty acids is used to generate heat through dissipation of the mitochondrial proton-motive force by uncoupling protein 1 (UCP1) located in the mitochondrial inner membrane. Artwork by Francesco Antognarelli.
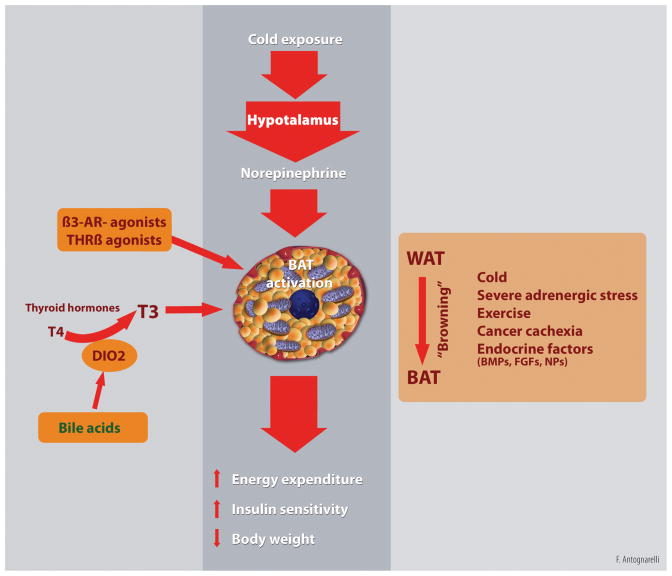
A distinct type of brown adipocyte has recently been identified, termed the beige or brite (“brown in white”) adipocyte.5 Beige cells share characteristics with both white (i.e., low basal expression of uncoupling protein 1, UCP1) and brown adipocytes (i.e., presence of multilocular fat droplets and high mitochondrial density), but show a gene expression pattern distinct from either WAT or BAT.5 Upon stress stimuli (e.g., chronic cold exposure, exercise and severe adrenergic stress), pharmacological treatments (e.g., long-term treatment with PPARγ agonists) and pathological conditions (e.g., cancer cachexia), brite adipocytes appear at WAT anatomical sites through differentiation of precursor cells distinct from BAT and closer to the white cell lineage.6 This phenomenon, known as “browning” of WAT, is followed by the triggering of a thermogenic program similar to BAT (Figure 2).6 Beige cells can also be induced by a vast array of endocrine factors and drugs via both central (SNS activation) and peripheral stimuli.1
Figure 2. Regulation of brown adipose tissue (BAT) thermogenesis.
Cold exposure activates the hypothalamic thermoregulatory center that, in turn, stimulates brown adipose tissue (BAT) thermogenesis through sympathetic efferent signals. Other stimuli, including physical exercise, severe adrenergic stress, disease conditions (e.g., cancer cachexia), endocrine factors [e.g., thyroid hormones (T3 and T4), β3-adrenergic agonists, bone morphogenetic proteins (BMPs), fibroblast growth factors (FGFs), and natriuretic peptides (NPs)] can activate BAT and induce the conversion of white adipocytes into thermogenic beige/brite adipose tissue (“browning” process). BAT activation can also occur as a result of bile acid-mediated stimulation of local type 2 iodothyronine deiodinase (DIO2) that converts T4 into T3. Sustained activation of BAT thermogenesis impacts whole-body metabolism by increasing energy expenditure and improving insulin sensitivity. These adaptations may be exploited to obtain therapeutic gain in obesity and type II diabetes. Artwork by Francesco Antognarelli.
This review summarizes the current understanding of signaling pathways that orchestrate BAT- and brite adipose tissue-mediated thermogenesis. The possibility of targeting NST to treat metabolic disorders is also discussed.
BAT and brite adipose tissue thermogenesis: burning from within
About 140 million years ago, the emergence of BAT-mediated NST in placental mammals offered them a remarkable evolutionary advantage by conferring the ability of maintaining body temperature in the cold. Simplifying, cutaneous thermal receptors activated by the exposure to cold environmental temperatures signal to thermoregulatory areas of the hypothalamus. This evokes efferent SNS signaling and subsequent stimulation of β3-adrenergic receptors in BAT, followed by intracellular lipolysis, activation of mitochondrial electron transport chain and uncoupling of respiration (Figure 2).1 UCP1, a mitochondrial carrier protein inserted into the inner mitochondrial membrane, is the major actor in BAT-mediated NST.7 Indeed, UCP1 promotes a state of metabolic inefficiency by inducing maximum mitochondrial respiration through dissipation of the proton-motive force in the form of heat (Figure 1).7 Cold adaptation is achieved through different mechanisms in wild-type and UCP-ablated mice. Indeed, UCP1-null mice may still develop cold tolerance after adaptation, but, contrary to their NST-competent littermates, they only rely on shivering for heat production.7
Although BAT depots have long been assumed to regress shortly after birth, recent studies using 18F-fluordeoxyglucose positron emission tomography/computed tomography (18FDG-PET/CT) have revealed that human adults possess BAT located in cervical-supraclavicular (the most common location), perirenal/adrenal, and paravertebral regions and around the major arteries.8 Indeed, BAT depots are highly prevalent in adult humans.1 The abundance of BAT is greater in women than in men, whilst it is inversely related to outdoor temperature, age, beta-blocker use, body mass index, percent body fat, and plasma glucose levels.1,8
Interestingly, recent studies employing 3D magnetic resonance imaging in combination with histological and biochemical analyses have shown that while BAT in the cervical region consists of classic brown adipocytes, BAT depots found in other anatomical sites show a molecular signature consistent with beige cells.9 What is more, gene expression profiling of BAT samples obtained from the supraclavicular region revealed that molecular markers of both brown and beige adipocytes coexist in human adults.10 This suggests that human BAT might indeed consist of both brown and recruitable beige adipocytes.1
As previously mentioned, BAT prevalence and activity are lower in overweight and obese individuals relative to lean subjects. On the other hand, weight loss via bariatric surgery increases BAT activity in morbid obese patients.11 Weight loss also leads to the appearance of functional BAT depots in obese persons previously void of such tissue.12 Similar to obesity, BAT activity is inversely associated with diabetes and fasting glucose levels.13 Interestingly, prolonged (5–8 hours) cold-induced BAT activation has shown to improve resting energy expenditure, whole-body glucose disposal, plasma glucose oxidation, and insulin sensitivity in middle-aged overweight men with 18FDG-PET/CT-detectable BAT.14 Collectively, these observations raise the possibility that BAT dysfunction might contribute to the development of obesity and insulin resistance.
As a whole, evidence accumulated so far strongly suggests a pivotal role for BAT in energy balance and metabolic homeostasis. This tissue may therefore represent a promising target for interventions against obesity and metabolic disorders.
Integrating central and peripheral signals: the BAT choreography
The regulation of BAT mass and function is achieved via a Daedalus of signals and feedbacks involving multiple organs and tissues. Almost all organs may produce, under either physiological or pathological stimuli, specific signals that positively or negatively modulate BAT activity.
According to the classical view, sympathetic neuro-adipose connections induce the BAT thermogenic program and browning of WAT in response to cold exposure.1 A cold environment may also activate a thermogenic circuit consisting of eosinophils, cytokines, and alternatively activated (type 2/M2) macrophages.15 Once activated by eosinophil-derived interleukin 4 and 13, M2 macrophages are recruited to subcutaneous WAT and release catecholamines to activate WAT browning process and drive thermogenesis.15
Several neuropeptides and hormones, including leptin, thyroid hormones, estradiol, brain-derived neurotrophic factor (BDNF), irisin, FGF21, bone morphogenetic protein (BMP) 7 and 8B, glucagon-like peptide 1, nesfatin 1, and cannabinoids, modulate BAT function by acting both centrally (on different hypothalamic nuclei) and peripherally to adapt thermogenesis and energy homeostasis to various stimuli (Figure 2).16
Exercise has been shown to confer some of its beneficial effects through the induction of WAT browning. Exercise-derived myokines and metabolites [e.g., irisin, IL6, β-aminoisobutyric acid (BAIBA), lactate, meteorin-like peptide, FGF21] stimulate beige adipocyte development and increase energy expenditure.17
Conversely, under obesity conditions, WAT browning is impaired by multiple factors, including increased activation of signaling pathways that inhibit beige adipocyte development (e.g., transforming growth factor β and TNF-α) and concomitant reduced SNS activity.18
A number of other signaling molecules are involved in the formation and activation of brown adipocytes. The identification of their peripheral and central targets would provide valuable tools to fine-tune whole-body energy expenditure and improve metabolic control.
Targeting BAT to fight metabolic disease
Overweight and obesity result from prolonged imbalance between energy intake and energy expenditure. Fat mass reduction in obese persons can be achieved via either decreasing food consumption or increasing energy expenditure to obtain sustained negative energy balance. Unfortunately, this task is not easy to achieve, because of the sedentary way of living and unrestricted availability of calorie-dense, inexpensive food that characterize modern societies. Indeed, with the exception of bariatric surgery, most anti-obesity interventions tackling energy intake result in moderate, often temporary improvements. Pharmacological agents that have so far been proposed to increase energy expenditure are either inefficacious or toxic. Finally, physical activity, the most physiological way of burning energy, is not easy to sustain for the long term.
In recent years, the possibility has emerged of achieving weight loss by harnessing the thermogenic properties of BAT.19 Chronic cold exposure is obviously not a strategy worth pursuing. An alternative might be the administration of sympaticomimetic agents (Figure 2). For instance, the highly specific β3-adrenoceptor agonist CL316,243 promoted thermogenesis, induced BAT hypertrophy and the appearance of brown adipocytes adipocytes in WAT, and reduced obesity in rats on a high-fat diet.20 CL 316,243 administration increased insulin action and fat oxidation in lean men, but the effects were markedly diminished after 8 weeks of treatment.21 The compound did not affect resting energy expenditure, body weight or body composition at any time over the course of treatment. In contrast, acute administration of the β3-adrenoceptor agonist L-796568 increased lipolysis and energy expenditure in overweight men.22 Similar to CL 316,243, L-796568 effects were lost when the compound was administered chronically, likely as a result of β3-adrenoceptor down-regulation.23
Stimulation of thyroid hormone receptor β (THRβ) might represent an alternative strategy to increase BAT-mediated thermogenesis (Figure 2). For instance, the selective THRβ agonist GC-24 improved metabolic control in rats fed a hypercaloric diet.24 Furthermore, treatment with GC-1, another selective THRβ agonist, increased energy expenditure and prevented fat mass accumulation in rats.25 Notably, administration of chenodeoxycholic acid activated type 2 iodothyronine deiodinase (DIO2) in BAT of health women, resulting in increased whole-body energy expenditure and mitochondrial uncoupling in brown adipocytes.26
A very appealing strategy may be the transformation of the more abundant WAT into beige fat. In this regard, a number of compounds may be explored, including BMP4 and BMP7, FGF19 and FGF21, natriuretic peptides, β-aminoisobutyric acid (BAIBA), capsinoids, and inhibitors of phosphodiesterase 5 (e.g., sildenafil) (Figure 2).27 Further studies are needed to establish their effectiveness and safety profile, in order to avoid the side effects encountered with other BAT activators (e.g., cardiovascular toxicity).
Conclusion
The escalating prevalence of obesity conveys a number of detrimental consequences, including an increased risk of type 2 diabetes, dyslipidemia, cardiovascular disease, and several cancers. With the exception of bariatric surgery, none of the available interventions is able to achieve substantial weight loss over the long term. The recent discovery of active BAT and recruitable beige cells in most human adults, together with the role played by these tissues in energy balance and insulin sensitivity, has sparked considerable interest into the possibility of targeting BAT to treat obesity and its negative correlates. While a number of factors and pathways have been discovered that enhance BAT and beige fat recruitment and function in rodents, the mechanisms of activation of these tissues in humans are only partly characterized. This is also reflected by the conflicting results obtained by interventions targeting BAT in rodents and humans.
An increased understanding of the function and regulation of BAT is therefore instrumental for devising pharmacological and nutritional interventions that harness the thermogenic properties of this tissue to treat metabolic disorders.
Acknowledgments
The present work was partly funded by grants from the Innovative Medicines Initiative – Joint Undertaking (IMI–JU 115621; E.M., R.C., E.d.A.) and Fondazione Roma. The work was also partly supported by the “Centro Studi Achille e Linda Lorenzon” (E.M., R.C.), a grant from the Italian Ministry of Education, Universities and Research (MIUR – linea D3.2 2013; E.M.), and the University of Florida’s Institute on Aging and Claude D. Pepper Older Americans Independence Center (NIA 1P30AG028740; C.L.). The authors thank Mr. Francesco Antognarelli for his invaluable assistance with illustrations.
Footnotes
Competing interests: no potential conflicts of interest relevant to this article were reported.
References
- 1.Betz MJ, Enerbäck S. Human brown adipose tissue: what we have learned so far. Diabetes. 2015;64(7):2352–2360. doi: 10.2337/db15-0146. [DOI] [PubMed] [Google Scholar]
- 2.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Sethi JK, Vidal-Puig AJ. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48(6):1253–1262. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villarroya J, Cereijo R, Villarroya F. An endocrine role for brown adipose tissue? Am J Physiol Endocrinol Metab. 2013;305(5):E567–72. doi: 10.1152/ajpendo.00250.2013. [DOI] [PubMed] [Google Scholar]
- 5.Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sidossis LS, Porter C, Saraf MK, et al. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab. 2015;22(2):219–227. doi: 10.1016/j.cmet.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta. 2001;1504(1):82–106. doi: 10.1016/S0005-2728(00)00247-4. [DOI] [PubMed] [Google Scholar]
- 8.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharp LZ, Shinoda K, Ohno H, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7(11):e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jespersen NZ, Larsen TJ, Peijs L, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17(5):798–805. doi: 10.1016/j.cmet.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Vijgen GH1, Bouvy ND, Teule GJ, et al. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2012;97(7):E1229–1233. doi: 10.1210/jc.2012-1289. [DOI] [PubMed] [Google Scholar]
- 12.Vijgen GH1, Bouvy ND, Teule GJ, et al. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2012;97(7):E1229–1233. doi: 10.1210/jc.2012-1289. [DOI] [PubMed] [Google Scholar]
- 13.Ouellet V, Routhier-Labadie A, Bellemare W, et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab. 2011;96(1):192–199. doi: 10.1210/jc.2010-0989. [DOI] [PubMed] [Google Scholar]
- 14.Chondronikola M, Volpi E, Børsheim E, et al. Brown adipose tissue improves whole body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63(12):4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu Y, Nguyen KD, Odegaard JI, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157(6):1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contreras C1, Gonzalez F, Fernø J, et al. The brain and brown fat. Ann Med. 2015;47(2):150–168. doi: 10.3109/07853890.2014.919727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Delgado G, Martinez-Tellez B, Olza J, Aguilera CM, Gil Á, Ruiz JR. Role of exercise in the activation of brown adipose tissue. Ann Nutr Metab. 2015;67(1):21–32. doi: 10.1159/000437173. [DOI] [PubMed] [Google Scholar]
- 18.Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 2015;125(2):478–486. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calvani R, Leeuwenburgh C, Marzetti E. Brown adipose tissue and the cold war against obesity. Diabetes. 2014;63(12):3998–4000. doi: 10.2337/db14-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Himms-Hagen J, Cui J, Danforth E, Jr, et al. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol. 1994;266(4 Pt 2):R1371–1382. doi: 10.1152/ajpregu.1994.266.4.R1371. [DOI] [PubMed] [Google Scholar]
- 21.Weyer C, Tataranni PA, Snitker S, Danforth E, Jr, Ravussin E. Increase in insulin action and fat oxidation after treatment with CL 316,243, a highly selective beta3-adrenoceptor agonist in humans. Diabetes. 1998;47(10):1555–1561. doi: 10.2337/diabetes.47.10.1555. [DOI] [PubMed] [Google Scholar]
- 22.van Baak MA, Hul GB, Toubro S, Astrup A, Gottesdiener KM, DeSmet M, Saris WH. Acute effect of L-796568, a novel beta 3-adrenergic receptor agonist, on energy expenditure in obese men. Clin Pharmacol Ther. 2002;71(4):272–279. doi: 10.1067/mcp.2002.122527. [DOI] [PubMed] [Google Scholar]
- 23.Larsen TM, Toubro S, van Baak MA, Gottesdiener KM, Larson P, Saris WH, Astrup A. Effect of a 28-d treatment with L-796568, a novel beta(3)-adrenergic receptor agonist, on energy expenditure and body composition in obese men. Am J Clin Nutr. 2002;76(4):780–788. doi: 10.1093/ajcn/76.4.780. [DOI] [PubMed] [Google Scholar]
- 24.Amorim BS, Ueta CB, Freitas BC, et al. A TRbeta-selective agonist confers resistance to diet-induced obesity. J Endocrinol. 2009;203(2):291–299. doi: 10.1677/JOE-08-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villicev CM, Freitas FR, Aoki MS, et al. Thyroid hormone receptor beta-specific agonist GC-1 increases energy expenditure and prevents fat-mass accumulation in rats. J Endocrinol. 2007;193(1):21–29. doi: 10.1677/joe.1.07066. [DOI] [PubMed] [Google Scholar]
- 26.Broeders EP, Nascimento EB, Havekes B, et al. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab. 2015;22(3):418–426. doi: 10.1016/j.cmet.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Pfeifer A, Hoffmann LS. Brown, beige, and white: the new color code of fat and its pharmacological implications. Annu Rev Pharmacol Toxicol. 2015;55:207–227. doi: 10.1146/annurev-pharmtox-010814-124346. [DOI] [PubMed] [Google Scholar]