Abstract
Background
As proton beam radiation therapy (PBRT) may allow greater normal tissue sparing when compared with intensity-modulated radiation therapy (IMRT), we compared the dosimetry and treatment-related toxicities between patients treated to the ipsilateral head and neck with either PBRT or IMRT.
Methods
Between 01/2011 and 03/2014, 41 consecutive patients underwent ipsilateral irradiation for major salivary gland cancer or cutaneous squamous cell carcinoma. The availability of PBRT, during this period, resulted in an immediate shift in practice from IMRT to PBRT, without any change in target delineation. Acute toxicities were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Results
Twenty-three (56.1%) patients were treated with IMRT and 18 (43.9%) with PBRT. The groups were balanced in terms of baseline, treatment, and target volume characteristics. IMRT plans had a greater median maximum brainstem (29.7 Gy vs. 0.62 Gy (RBE), P < 0.001), maximum spinal cord (36.3 Gy vs. 1.88 Gy (RBE), P < 0.001), mean oral cavity (20.6 Gy vs. 0.94 Gy (RBE), P < 0.001), mean contralateral parotid (1.4 Gy vs. 0.0 Gy (RBE), P < 0.001), and mean contralateral submandibular (4.1 Gy vs. 0.0 Gy (RBE), P < 0.001) dose when compared to PBRT plans. PBRT had significantly lower rates of grade 2 or greater acute dysgeusia (5.6% vs. 65.2%, P < 0.001), mucositis (16.7% vs. 52.2%, P = 0.019), and nausea (11.1% vs. 56.5%, P = 0.003).
Conclusions
The unique properties of PBRT allow greater normal tissue sparing without sacrificing target coverage when irradiating the ipsilateral head and neck. This dosimetric advantage seemingly translates into lower rates of acute treatment-related toxicity.
Keywords: Proton beam, IMRT, Head and neck cancer, Proton beam radiation
Radiation therapy is commonly used in the treatment of head and neck cancer (HNC) in both the definitive and post-operative setting. The anatomical complexity of the head and neck presents a challenging treatment paradigm due to the close proximity of tumors to the surrounding normal tissues [1]. Significant advances in the delivery of photon beam radiation therapy have been made over the last 30 years with the progression from the 2-dimensional era to the 3-dimensional era, and over the last decade with the transition from 3D-conformal to intensity modulated radiation therapy (IMRT). The integration of IMRT in the treatment of head and neck cancer allowed improved tumor dose conformality and normal tissue sparing resulting in reduced late effects such as xerostomia [2,3]. However, a large proportion of patients still experience significant acute effects and require a higher level of supportive care during and after treatment, which has been reported to negatively affect patients’ quality of life [1,4–9]. Importantly, given the increasing incidence of HPV-associated HNC and the long life expectancy of these patients, it is imperative to minimize unnecessary normal tissue exposure to thwart acute and late radiation morbidity.
Given the unique inherent physical properties of proton energy deposition, proton beam radiotherapy (PBRT) has been proposed to lower normal tissue exposure without compromising target coverage, thereby improving the therapeutic ratio. Unlike photons that undergo a continuous reduction in dose with increasing depth, protons deposit the majority of their energy within the Brag peak, resulting in little to no significant dose delivered beyond the target [10]. Multiple publications have shown that proton therapy results in favorable clinical outcomes compared to photon therapy for tumors of the skull base and paranasal sinuses. However, despite the theoretical benefit of PBRT, there are no data on the clinical benefits as compared to IMRT for other types of head and neck cancers.
The purpose of this study was to compare the dosimetry and acute treatment-related toxicities of PBRT versus IMRT in patients who underwent ipsilateral head and neck irradiation for major salivary gland cancers and squamous cell carcinomas of the head. In the absence of randomized data, these data can inform clinicians about differences in acute treatment toxicity between PBRT and IMRT in the treatment of unilateral HNC.
Methods
The institutional review board approved this retrospective study with a waiver of informed consent. Between January 2011 and March 2014, 41 patients underwent unilateral head and neck irradiation for major salivary gland cancer or cutaneous squamous cell carcinoma metastasis to a major salivary gland. The availability of PBRT during this period resulted in an immediate shift in practice from IMRT to PBRT, without any change in target delineation. The same tumor and normal tissue dose constraints were used for treatment planning.
Radiotherapy technique
Patients with resectable disease underwent surgical resection prior to irradiation, while those with unresectable or medically inoperable disease were treated with definitive radiation therapy with or without concurrent chemotherapy. Patients were simulated in the supine position with a thermoplastic mask with 3- or 5-point head, neck, and shoulder immobilization to ensure daily reproducibility of treatments. A simulation CT scan with ≤3mm slice thickness was acquired from the vertex of the scalp through the carina encompassing the entire head and neck. Administration of IV contrast was preferred to help guide the primary tumor volume delineation and neck nodal volumes. Surgical scars were wired if present.
The gross tumor volume (GTV) was defined as the gross extent of tumor visible by imaging studies (CT, PET, and/or MRI) and clinical examination. Primary fusion to the simulation CT was performed when feasible. The clinical target volume (CTV) was defined as the GTV plus a 3–5 mm margin for potential microscopic spread, including high-risk lymph node areas, and dermal involvement. The CTV was expanded by 5 mm to a planning target volume (PTV) to account for intra-fractional patient motion and inter-fractional setup error.
The radiation technique, dose, and fractionation varied according to clinical scenario [11]. Areas of gross disease were treated to 70 Gy (RBE) in 35 fractions, areas of close (≤5 mm from inked margin) or microscopically positive margins were treated to 66 Gy (RBE) in 33 fractions, high-risk areas including the operative or tumor bed were treated to 60 Gy (RBE) in 30 fractions, and low risk nodal regions were treated to 54–45 Gy (RBE). Ipsilateral neck irradiation, nodal levels Ib-IV, was recommended in patients with node-positive disease, T3/T4 primary tumor category, poorly differentiated histology, or high-grade disease. Adenoid cystic or acinic cell carcinomas typically did not require elective nodal irradiation because of the low risk of lymphatic spread [12]. The lingual, hypoglossal, and facial nerves were treated to the skull base in patients with radiographic or pathologic perinueral involvement and for all patients with adenoid cystic histology. Concurrent chemoradiation, preferentially cisplatin based, was recommended in the setting of gross residual or unresectable disease and radiographic/pathologic extranodal extension.
Photon therapy was delivered with 4–6 static IMRT beams. Ipsilateral beams were preferred with care taken not to enter through the oral cavity, but occasionally a contralateral anterior oblique and/or a contralateral posterior oblique beam was used to help improve dose conformality. Depending on the clinical scenario, in patients without low-neck disease the lower neck was either included in the IMRT fields or included in a low anterior neck (LAN) field matched to the IMRT fields at the isocenter just caudal to the arytenoids. The dose was typically 45–50.4 Gy in 25–28 fractions, prescribed to a depth of 3 cm. In patients with gross involvement of the low neck or near the match-line, whole neck IMRT was preferred. Setup accuracy was confirmed with daily kV X-ray orthogonal verification of the isocenter based on bony anatomy. A verification kV cone-beam CT scan was performed weekly in the course of treatment to assess for changes in anatomy and ensure adequate 3D patient setup.
Proton therapy was delivered with uniform scanning beams and planned in CMS XIO (Elekta, Stockholm Sweden). The beam arrangement varied depending on target volume geometry and dose limits to neighboring organs at risk. Three-field plans were typically used (2–4 beams). For some cases, matched fields were used. Physical and biological uncertainties were evaluated and taken into account, and worst- and best-case scenarios with the range uncertainties (2.5% * range + 2 mm) were evaluated. Special care was taken to avoid ranging out into critical structures through selection of beam angles and range feathering if necessary. Dental artifacts were overridden, and as much as possible, attempts were made to avoid beams traversing through dental hardware. Setup accuracy was confirmed with daily X-ray orthogonal verification of the isocenter based on bony anatomy. Verification CT scans were typically performed at least once in the course of treatment to assess for changes in anatomy, most often in the first and/or fourth week of treatment.
Target coverage for both IMRT and PBRT sought to achieve D95 ≥ prescription dose and the PTV D05 ≤108% of the prescription dose. IMRT planning prioritized critical structure constraints, see below, over planning target volumes, which were prioritized over other normal structures. A 3D isodose surface display was reviewed to ensure that hot spots are located within the PTV.
Organs at risk (OAR) including the parotid glands, submandibular glands, cochleas, oral cavity, larynx, esophagus, brachial plexus, brain stem, and spinal cord were contoured. A dose volume histogram was constructed to evaluate target coverage and the doses to the surrounding OAR. The contralateral parotid, contralateral submandibular, and spinal cords were contoured accordingly to visible anatomy on CT images. The brainstem contour included the midbrain, pons, and medulla as identified by CT-based anatomy with an inferior border at the foramen magnum. The oral cavity was defined anteriorly and laterally by the teeth and gums, superiorly by the hard and soft palate, inferiorly by the tongue and floor of mouth and posteriorly by the opening of the pharynx. The larynx was defined as the pharynx contained within the thyroid cartilage and includes the true and false vocal cords. The dose to OAR was limited to <70 Gy (RBE) maximum point dose and <45 Gy (RBE) mean dose to the larynx, <34 Gy (RBE) mean dose to the esophagus, <65 Gy maximum point dose to the brachial plexus, <54 Gy (RBE) maximum point dose to the brainstem, optic nerves, and optic chiasm, <45 Gy (RBE) maximum point dose to the spinal cord, and <40 Gy (RBE) mean dose to the oral cavity excluding any overlapping PTV. Planners were asked to keep the dose to the contralateral submandibular and parotid glands (<6 Gy (RBE) mean dose) as low as feasibly possible.
Toxicity assessment
Patients were assessed weekly by the treating radiation oncologist during the radiation course and in post-treatment follow up visits jointly by head and neck surgery, radiation oncology, and/or medical oncology at approximate intervals of 4, 8, and 12 weeks after completion of treatment, then every 3 months for two years, followed by every 6 months thereafter. A standardized toxicity form was implemented to help improve the accuracy and reproducibility in recording treatment toxicities for dermatitis, nausea, vomiting, mucositis, xerostomia, dysphagia, hoarseness, fatigue, and need for a feeding tube. The incidence of the worst grade toxicity sustained by a patient up to 90 days after the start of radiation therapy was recorded as an acute toxicity event based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v 4.0).
Statistical analysis
Comparisons between cohorts were performed using either the chi-square test or a 2-tailed Student’s t-test. The Kaplan Meier method, with log rank comparison when indicated, was used to calculate locoregional progression-free survival (LPFS), distant metastasis-free survival (DMFS), and overall survival (OS) from end of radiation therapy [13]. A probability value of less than 0.05 was considered statistically significant for all analyses. All analyses were performed in SPSS statistics version 21 (IBM, Armonk, NY, USA).
Results
Patient, tumor, and treatment characteristics
Patient characteristics
The median age was 60.9 years (interquartile range; 50.1– 74.0 years) with an overall median follow up of 8.7 months (interquartile range; 4.1–17.6 months). There was a significant difference in the median follow up between IMRT (16.1 months; interquartile range, 8.7–24.4 months) and PBRT patients (4.7 months; interquartile range 1.6–7.9 months), P < 0.001. Patient, tumor, and treatment characteristics were well balanced, with 23 and 18 patients in the IMRT and PBRT cohorts, respectively, Table 1.
Table 1.
Patient, tumor, and treatment characteristics.
| Characteristic | All patients | IMRT (N = 23) | PBRT (N = 18) | P value |
|---|---|---|---|---|
| Age (median) | 60.9 yrs | 60.9 yrs | 60.4 yrs | 0.483 |
| Tumor size (median) | 2.5 cc | 2.7 cc | 2.2 cc | 0.284 |
| RT dose (median) | 66.0 Gy | 66.0 Gy | 66.0 Gy (RBE) | 0.284 |
| Characteristic | N (%) | N (%) | N (%) | P value |
|
| ||||
| Site of disease | ||||
| Parotid gland | 35 (85.4) | 21 (91.3) | 14 (77.8) | 0.224 |
| Submandibular gland | 6 (14.6) | 2 (9.5) | 4 (22.2) | |
| Unresectable disease | ||||
| Yes | 4 (9.8) | 2 (8.7) | 2 (11.1) | 0.796 |
| No | 37 (90.2) | 21 (91.3) | 16 (88.9) | |
| Perineural involvement* | ||||
| Yes | 19 (51.4) | 12 (57.1) | 7 (43.8) | 0.492 |
| No | 18 (48.6) | 9 (42.9) | 9 (56.2) | |
| Lymphovascular invasion* | ||||
| Yes | 7 (18.9) | 6 (28.6) | 1 (6.2) | 0.086 |
| No | 30 (81.1) | 15 (71.4) | 15 (93.8) | |
| Neck nodal irradiation | ||||
| Yes | 26 (63.4) | 6 (26.1) | 9 (50.0) | 0.115 |
| No | 15 (36.6) | 17 (73.9) | 9 (50.0) | |
| CCRT | ||||
| Yes | 11 (26.9) | 7 (30.4) | 4 (22.2) | 0.556 |
| No | 30 (73.1) | 16 (69.6) | 14 (77.8) | |
Abbreviations: cubic centimeters (cc), gray (Gy), relative biological effectiveness (RBE), years (yrs), concurrent chemoradiation therapy (CCRT)
Analysis restricted to surgery patients.
Tumor characteristics
The parotid gland was the involved site of disease in 35 patients (85.4%) and the submandibular gland in 6 patients (14.6%). Of the 41 patients, 3 patients (7.3%) had unresectable disease and 1 patient (2.4%) was deemed medically inoperable. Of the 37 patients who underwent surgical resection, 20 (54.1%) also underwent an ipsilateral neck dissection. Surgical pathology identified 24 patients (64.9%) with positive surgical margins, 2 patients (5.4%) with very close margins (<0.5 mm), 19 patients (51.4%) with perineural invasion, and 7 patients (18.9%) with lymphovascular invasion. The median tumor size was 2.5 cm (interquartile range; 1.73–3.5 cm), with no significant difference between IMRT (2.7 cm; interquartile range, 2.0–3.5 cm) and PBRT patients (2.2 cm; interquartile range, 1.7–3.0 cm), P = 0.284.
Radiotherapy treatment
The median RT dose to the primary site was 66.0 Gy (RBE) (interquartile range; 66.0–66.0 Gy (RBE)), with no significant difference between IMRT (66.0 Gy; interquartile range, 66.0–66.0 Gy) and PBRT patients (66.0 Gy (RBE); interquartile range, 61.2–66.0 Gy (RBE)), P = 0.284. Twenty-six patients underwent ipsilateral neck irradiation, 17 IMRT and 9 PBRT patients (P = 0.115), to a median dose of 59.4 Gy (RBE) (interquartile range; 54.0–60.0 Gy (RBE)), with no significant difference between IMRT (59.4 Gy; interquartile range, 54.0–60.0 Gy) and PBRT (50.0 Gy (RBE); interquartile range, 50.0–60.0 Gy (RBE)) patients, P = 0.367. Eleven patients (26.9%) were treated with concurrent chemoradiation therapy including 7 IMRT and 4 PBRT patients, P = 0.556.
Dose–volume histogram analysis
Tumor
There was no difference in the parotid PTV between IMRT and PBRT patients (120.5 cc versus 78.3 cc, P = 0.934) or the ipsilateral neck PTV (373.0 cc versus 314.3 cc, P = 0.442), Table 2. PTV V95 > 95% was met for all cases, with no significant difference in the PTV maximum dose to 0.15 cc (72.0 Gy vs. 71.5 Gy (RBE), P = 0.554).
Table 2.
Tumor and organs at risk dose volume characteristics.
| Characteristic | IMRT (N = 23)
|
PBRT (N = 18)
|
P value | ||
|---|---|---|---|---|---|
| Median | Interquartile range | Median | Interquartile range | ||
| Target volume | |||||
| Parotid only | 120.5 cc | 82.3–146.3 cc | 78.3 cc | 63.2–115.9 cc | 0.934 |
| Parotid and ipsilateral neck | 373.0 cc | 273.1–491.7 cc | 314.3 cc | 186.0–383.4 cc | 0.442 |
| PTV max dose to 0.15 cc | 72.0 Gy | 70.1–73.3 Gy | 71.5 Gy (RBE) | 70.0–72.4 Gy (RBE) | 0.554 |
| Organ at risk | |||||
| Brainstem (maximum) | 29.7 Gy | 22.0–46.1 Gy | 0.62 Gy (RBE) | 0.01–6.9 Gy (RBE) | <0.001 |
| Spinal cord (maximum) | 36.3 Gy | 32.2–40.0 Gy | 1.9 Gy (RBE) | 0–6.6 Gy (RBE) | <0.001 |
| Oral cavity (mean) | 20.6 Gy | 11.0–27.2 Gy | 0.94 Gy (RBE) | 0–3.85 Gy (RBE) | <0.001 |
| Parotid gland- contralateral (mean) | 1.4 Gy | 0.84–3.0 Gy | 0.00 Gy (RBE) | 0–0 Gy (RBE) | <0.001 |
| Submandibular gland (contralateral (mean) | 4.1 Gy | 2.5–6.0 Gy | 0.00 Gy (RBE) | 0–0 Gy (RBE) | <0.001 |
| Larynx* (mean) | 21.4 Gy | 11.4–29.6 Gy | 10.3 Gy (RBE) | 8.8–17.3 Gy (RBE) | 0.182 |
Abbreviations: cubic centimeters (cc), gray (Gy), relative biological effectiveness (RBE)
Analysis restricted to the 26 patients who underwent ipsilateral neck irradiation.
Organs at risk (OAR)
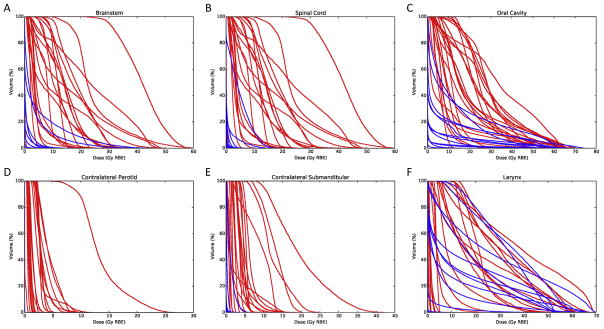
Patients who underwent PBRT had significantly lower brainstem maximum (29.7 Gy versus 0.62 Gy (RBE), P < 0.001), spinal cord maximum (36.3 Gy versus 1.88 Gy (RBE), P < 0.001), oral cavity mean (20.6 Gy versus 0.94 Gy (RBE), P < 0.001), contralateral parotid gland mean (1.4 Gy versus 0.00 Gy (RBE), P < 0.001), and contralateral mean submandibular dose maximum (4.1 Gy versus 0.00 Gy (RBE), P < 0.001), Table 2. Among the 26 patients who underwent ipsilateral neck irradiation there was no difference in the mean larynx dose between cohorts (21.4 Gy versus 10.3 Gy (RBE), P = 0.180), Table 2. Dose–volume histograms of all treated patients are depicted in Fig. 1.
Fig. 1.
Dose–volume histograms of organs at risk for proton (blue) and photon (red) patients: (A) brainstem, (B) spinal cord, (C) oral cavity, (D) contralateral parotid gland, (E) contralateral submandibular gland, (F) larynx. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Acute toxicity
PBRT resulted in significantly lower rates of grade 2 or greater acute mucositis (16.7% versus 52.2%, P = 0.019), nausea (11.1% versus 56.5%, P = 0.003), and dysgeusia (5.6% versus 65.2%, P < 0.001), Table 3, but resulted in a greater rate of grade 2 or greater acute dermatitis when compared to IMRT (100.0% versus 73.9%, P = 0.019).
Table 3.
Acute toxicity.
| Toxicity | IMRT (N = 23) | PBRT (N = 18) | P value |
|---|---|---|---|
| Dermatitis | |||
| Grade 0 | 0 (0.0%) | 0 (0.0%) | 0.032 |
| Grade 1 | 6 (26.1%) | 0 (0.0%) | |
| Grade 2 | 9 (39.1%) | 13 (72.2%) | |
| Grade 3 | 8 (34.8%) | 5 (27.8%) | |
| Grade 4 | 0 (0.0%) | 0 (0.0%) | |
| Mucositis | |||
| Grade 0 | 3 (13.0%) | 12 (66.7%) | 0.005 |
| Grade 1 | 8 (34.8%) | 3 (16.7%) | |
| Grade 2 | 10 (43.5%) | 3 (16.7%) | |
| Grade 3 | 2 (8.7%) | 0 (0.0%) | |
| Grade 4 | 0 (0.0%) | 0 (0.0%) | |
| Nausea | |||
| Grade 0 | 7 (30.4%) | 15 (83.3%) | 0.003 |
| Grade 1 | 3 (13.0%) | 1 (5.6%) | |
| Grade 2 | 13 (56.5%) | 2 (11.1%) | |
| Grade 3 | 0 (0.0%) | 0 (0.0%) | |
| Dysgeusia | |||
| Grade 0 | 4 (17.4%) | 14 (77.8%) | <0.001 |
| Grade 1 | 4 (17.4%) | 3 (16.7%) | |
| Grade 2 | 15 (65.2%) | 1 (5.6%) | |
| Dysphagia | |||
| Grade 0 | 12 (52.2%) | 15 (83.3%) | 0.101 |
| Grade 1 | 9 (39.1%) | 2 (11.1%) | |
| Grade 2 | 2 (8.7%) | 1 (5.6%) | |
| Grade 3 | 0 (0.0%) | 0 (0.0%) | |
| Grade 4 | 0 (0.0%) | 0 (0.0%) | |
| Fatigue | |||
| Grade 0 | 2 (8.7%) | 11 (61.1%) | 0.002 |
| Grade 1 | 19 (82.6%) | 6 (33.3%) | |
| Grade 2 | 2 (8.7%) | 1 (5.6%) | |
| Grade 3 | 0 (0.0%) | 0 (0.0%) | |
There was no difference in the percentage of patients requiring a treatment break (16.7% vs. 21.7%, P = 0.684) between PBRT and IMRT. No patient required a prophylactic or reactive gastrostomy tube or tracheostomy.
Locoregional control
The 1-year actuarial locoregional control rate was 92.8% with no difference between PBRT and IMRT patients (80.0% versus 95.5%; P = 0.473). One patient in the PBRT cohort developed an in-field local recurrence in the parotid bed at 7.7months. Two patients in the IMRT cohort developed local recurrences at 2.1 and 14.7 months.
Distant metastases
Three patients had distant metastases prior to radiation therapy, 1 patient in the PBRT cohort and 2 patients in the IMRT cohort. These were patients who required locoregional treatment despite the presence of metastasis. Of the remaining 38 patients, 5 (1 PBRT and 4 IMRT patients) developed distant metastases at a median of 3.3 months (interquartile range; 2.1–3.5 months) after RT. The 1-year actuarial freedom from distant metastases was 87.8% with no significance between PBRT and IMRT patients (83.3% versus 93.3%; P = 0.662).
Overall survival
The 1-year actuarial overall survival was 89.4% with no difference between PBRT and IMRT patients (83.3% versus 93.3%, P = 0.083).
Discussion
This is the first study, to our knowledge, that demonstrates reduced acute radiation-therapy associated toxicity in adult patients undergoing ipsilateral head and neck irradiation with PBRT as compared to IMRT. PBRT allowed greater sparing of normal tissue without sacrificing target coverage when irradiating the ipsilateral neck. This dosimetric advantage translated into significantly lower rates of acute treatment-related toxicity including dysgeusia, mucositis, and nausea. Longer follow-up is needed to determine whether this translates into fewer late effects and to evaluate long-term tumor control rates.
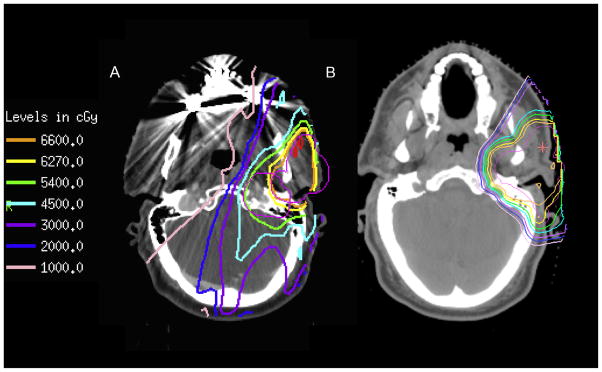
The goal of modern radiation therapy is the optimization of dose delivery to result in adequate tumor control, while sparing adjacent normal tissues. IMRT allowed significant progress in dose conformity compared to previous photon delivery techniques, resulting in improved tumor control and better sparing of normal tissues [14–23]. However, the physical properties of photons result in some inherit limitations that even the most sophisticated IMRT planning and delivery cannot overcome [24]. Conversely, the physical beam properties of protons allow PBRT to lower the exposure to the surrounding normal tissues resulting in decreased acute, and potentially late, radiation-therapy-associated morbidity, Fig. 2.
Fig. 2.
Representative images comparing IMRT (A) and PBRT (B) treatment plans. The steeper dose fall off with PBRT can be appreciated when comparing to the IMRT plan. The planning target volume (PTV) is in pink, with isodose lines as indicated on the legend. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The physical properties of protons can be exploited in one of two ways to improve the therapeutic ratio: (1) to improve tumor control by dose escalation in tumors with suboptimal tumor control (i.e. skull base sarcomas, paraspinal sarcomas, and lung tumors) or (2) to deliver the same dose to the tumor while reducing exposure to surrounding normal tissues in tumors where the local control is already excellent but late effects are significant (pediatric tumors, left sided breast cancer, and lymphomas). For most head and neck cancers, including salivary cancer, tumor control rates with modern surgery and adjuvant therapy is excellent, yet there is a need to minimize treatment related morbidity. The rationale for using proton therapy for ipsilateral head and neck irradiation is thus analogous to other disease sites with excellent tumor control with modern IMRT; the need is to reduce acute and late treatment related morbidty.
Acute radiation-therapy associated toxicity is problematic in patients undergoing head and neck irradiation and can result in malnutrition, treatment breaks, and prolonged recovery and has been correlated with worse late toxicity [3]. Malnutrition and treatment breaks have been associated with higher rates of locoregional failure and death in HNC patients undergoing definitive radiotherapy [25]. As such, efforts to minimize acute toxicities are necessary to improve therapeutic outcomes and improve patient quality of life during radiotherapy. We have shown that PBRT lowers the dose to normal tissues without compromising target coverage, and that this improvement in the therapeutic ratio translates to lower acute toxicity and likely superior patient tolerability and quality of life. It is well documented that patients with higher rates of acute toxicity are more likely to suffer from late toxicity; thus it would be expected that PBRT should reduce late effects but further follow up is needed to confirm this [26–28]
The value of proton therapy in reducing toxicity has been demonstrated in patients with paranasal sinus tumors. A metaanalysis comparing IMRT with PBRT in HNC suggested improved survival for patients treated with PBRT compared to IMRT and less treatment related toxicity [9]. This is likely due to the ability of protons to more adequately cover target volumes while limiting normal tissue exposure. Late toxicities negatively affect patients’ functional outcomes and quality of life, and as such efforts to improve the therapeutic ratio are necessary [3,9]. Normal tissue complication probability (NTCP) models suggest that a lower parotid dose, as can be achieved with PBRT compared to IMRT, will result in a lower complication probability for the parotid gland [10,24,29,30]. In silico data, as reviewed by Van De Water et al., suggested that PBRT would result in lower normal tissue dose, while maintaining similar target coverage [24]. It is important to remember that the relatively higher radiation sensitivity of the parotid gland, as compared to the submandibular gland results from a greater proportion of the radiosensitive serous acinar cells in the parotid as compared to the mixed population of serous and the more radioresistant mucinous acinar cells in the submandibular gland [31]. Serous acinar cells are among the most radiosensitive in the body and no threshold dose has been established, thus it is important to minimize parotid gland exposure to decrease the risk of acute and late xerostomia [31]. As such it is generally accepted that a lower dose to the parotid and submandibular glands will translate into a significantly lower incidence of salivary dysfunction.
Just as techniques for the precise delivery of photon therapy have rapidly improved over the past several decades, methods for the planning and delivering of proton therapy have been rapidly evolving as well [1]. Indeed, proton therapy for head and neck cancers is particularly challenging given the complex anatomy and irregularity of targets in the head and neck [32].
Protons generally have a lower entrance dose than photons and deposit the majority of their energy over a discrete range known as the Brag peak [10]. There is no significant exit dose beyond the target as the proton particles stop in the tissue immediately after the Bragg peak [1]. As tumors are larger than a single point, a spread out Bragg peak (SOBP) is produced, using either variations in proton beam energies or using a tissue compensator when a monoenergetic proton beam is utilized. While the SOBP allows adequate tumor coverage it comes at the expense of an increased entrance or skin dose as the width of the Bragg peak is spread out (i.e. the entrance dose is generally lower for a monoenergetic proton beam, though entrance dose is directly related to the proton beam energy, when compared to photons, but the entrance dose is significantly higher when a SOBP is generated) [32]. As protons travel through body tissues they interact with the nuclei and electrons of atoms, and their heavier mass, as compared to photons, results in a smaller scattering angle and a sharper lateral dose distribution that can be exploited clinically [1]. Most of the newer proton facilities in the US either have incorporated (or are in the process of incorporating) pencil beam scanning (PBS) technology. In PBS, proton spots are guided by magnets to paint the target without the need for apertures and compensators [32]. The proton spots can be delivered in different layers with various energies to allow for dose conformality both distal and proximal to the target, allowing for intensity modulation (i.e. intensity modulated proton therapy (IMPT)) [32]. With passively scattered protons and uniform scanning (as used in our patients), spreading out the Brag peak results in an increased entrance dose. The width of the SOBP cannot be modulated as the width of the target changes, potentially increasing the dose the tissues proximal to the target. Without the ability to vary the modulation in each beam, the skin dose for ipsilateral neck irradiation, in some parts of the target, often approached the prescription dose with essentially no skin sparing. This appears to be the primary disadvantage of PBRT in our study with a greater rate of grade 2 or greater acute dermatitis in the PBRT patients (100.0% versus 73.9%, P = 0.019), but reassuringly no difference in the rate of grade 3 or greater acute dermatitis between IMRT and PBRT (34.8% vs. 27.8%, respectively, P = 0.632). Adoption of IMPT into practice will result in greater skin sparing (i.e. lower entrance dose) and improved dose modulation.
In the current study, PBRT patients had significantly lower normal tissue dose to the oral cavity (median dose 0.94 Gy (RBE) versus 20.6 Gy, P < 0.001) and brainstem (maximum dose 0.62 Gy (RBE) versus 29.7 Gy, P < 0.001), which correlated with significantly lower rates of acute grade 2 or greater mucositis (16.7% versus 52.2%, P = 0.019), dysgeusia (5.6% versus 65.2%, P < 0.001), and nausea (11.1% versus 56.5%, P = 0.003). While PBRT resulted in a lower dose to the contralateral submandibular gland (0.0 Gy (RBE) versus 4.1 Gy, P < 0.001) and parotid gland (0.0 Gy (RBE) versus 1.4 Gy, P < 0.001), it is unlikely that such a small difference will result in a clinically meaningful benefit, as the ultimate estimate of tissue complication is dependent on the shape of the normal tissue complication probabilities (NTCP) curve. While the major salivary glands are predominately responsible for stimuli-associated salivation, the minor salivary glands, which line the oral cavity, are responsible for basal salivation and general oral health and maintenance. As such radiation associated minor salivary gland hypofunction, which correlates with a higher mean oral cavity dose, can also result in significant baseline xerostomia. Given the multifactorial etiology of xerostomia, patient reported quality of life (QOL) outcomes may better capture the patients overall salivary function and physical/physiological impact. Nevertheless, the current study suggests that PBRT results in a lower dose to the adjacent normal tissue, including the oral cavity, contralateral parotid gland, and contralateral submandibular gland, that seemingly translates into lower acute toxicity.
Limitations of this study are subject to biases inherent to retrospective studies and small patient numbers. While our patient cohort was constrained to a defined time period, with an immediate shift in clinical practice from IMRT to PBRT, we were unable to account for any potential differences in target volume delineation that may have intuitively changed over time (i.e. as the treating physician evolved in practice). While our cohorts were well balanced with respect to patient and tumor characteristics our small patient numbers may preclude our ability to detect subtle differences. It is likely that IMPT will result in greater treatment conformality and provide superior normal tissue sparing [33]. Perhaps most importantly, IMPT will allow for modulation of the proximal dose and significant skin sparing. While PBRT resulted in lower acute toxicity, except for dermatitis, longer follow up is necessary to assess the impact on late RT-associated morbidity. These data have prompted us to initiate a prospective randomized study to confirm these findings with an emphasis on patient reported QOL outcomes to verify that the anticipated improvement in acute toxicity translates to improved QOL measures. Longer follow up is also necessary to ensure adequate tumor control, survival, and to assess the impact on late RT-associated morbidity.
Conclusion
Radiotherapy is commonly used in the treatment of HNC, yet the anatomical complexity of this region presents a challenging treatment paradigm because of the close proximity of tumors to critical organs at risk. While significant advances in the delivery of photon beam radiation therapy have been made over the last 20 years with the progression from conventional to 3D-conformal to IMRT, with dramatic improvement in tumor control rates, acute and late radiotherapy associated morbidity continues to negatively affect a substantial proportion of HNC patients’ functional outcomes and quality of life. For this reason it is imperative to find ways to reduce RT-associated morbidity. PBRT results in significantly lower normal tissue exposure and reduced acute toxicity rates (i.e. mucositis, dysgeusia, and nausea) in patients treated to ipsilateral head and neck.
Footnotes
Presented in part as a poster presentation at the 56th Annual for the American Society for Radiation Oncology (ASTRO) on September 14–17th, 2014 in San Francisco, California and as an oral presentation at the inaugural Particle Therapy Cooperative Group – North America in Houston, Texas on October 27–29th, 2014.
Conflict of interest
Oren Cahlon has a minority investment in ProCure.
Funding
None.
References
- 1.Holliday EB, Frank SJ. Proton radiation therapy for head and neck cancer: a review of the clinical experience to date. Int J Radiat Oncol Biol Phys. 2014;89:292–302. doi: 10.1016/j.ijrobp.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Vergeer MR, Doornaert PA, Rietveld DH, Leemans CR, Slotman BJ, Langendijk JA. Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a nonrandomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys. 2009;74:1–8. doi: 10.1016/j.ijrobp.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 3.Romesser PB, Romanyshyn JC, Schupak KD, et al. Percutaneous endoscopic gastrostomy in oropharyngeal cancer patients treated with intensitymodulated radiotherapy with concurrent chemotherapy. Cancer. 2012;118:6072–8. doi: 10.1002/cncr.27633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duke RL, Campbell BH, Indresano AT, et al. Dental status and quality of life in long-term head and neck cancer survivors. Laryngoscope. 2005;115:678–83. doi: 10.1097/01.mlg.0000161354.28073.bc. [DOI] [PubMed] [Google Scholar]
- 5.Hammerlid E, Silander E, Hornestam L, Sullivan M. Health-related quality of life three years after diagnosis of head and neck cancer–a longitudinal study. Head Neck. 2001;23:113–25. doi: 10.1002/1097-0347(200102)23:2<113::aid-hed1006>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 6.Jensen AB, Hansen O, Jorgensen K, Bastholt L. Influence of late side-effects upon daily life after radiotherapy for laryngeal and pharyngeal cancer. Acta Oncol. 1994;33:487–91. doi: 10.3109/02841869409083923. [DOI] [PubMed] [Google Scholar]
- 7.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770–6. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 8.List MA, Bilir SP. Functional outcomes in head and neck cancer. Semin Radiat Oncol. 2004;14:178–89. doi: 10.1053/j.semradonc.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Ramaekers BL, Pijls-Johannesma M, Joore MA, et al. Systematic review and meta-analysis of radiotherapy in various head and neck cancers: comparing photons, carbon-ions and protons. Cancer Treat Rev. 2011;37:185–201. doi: 10.1016/j.ctrv.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 10.van der Laan HP, van de Water TA, van Herpt HE, et al. The potential of intensity-modulated proton radiotherapy to reduce swallowing dysfunction in the treatment of head and neck cancer: a planning comparative study. Acta Oncol. 2013;52:561–9. doi: 10.3109/0284186X.2012.692885. [DOI] [PubMed] [Google Scholar]
- 11.Lee N, Riaz N, Lu J. Target Volume Delineation for Conformal and Intensity- Modulated Radiation Therapy. In: Brady L, Combs S, Lu J, editors. Medical Radiology: Radiation Oncology. New York: Springer; 2015. [Google Scholar]
- 12.Lee NY, Lu JJ. Target Volume Delineation and Field Setup. In: Tham IWK, Lee NY, editors. Majory Salivary Glands. Chapter 7. New York: Springer; 2013. [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:25. [Google Scholar]
- 14.Eisbruch A, Dawson LA, Kim HM, et al. Conformal and intensity modulated irradiation of head and neck cancer: the potential for improved target irradiation, salivary gland function, and quality of life. Acta Otorhinolaryngol Belg. 1999;53:271–5. [PubMed] [Google Scholar]
- 15.Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:695–704. doi: 10.1016/s0360-3016(01)01512-7. [DOI] [PubMed] [Google Scholar]
- 16.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425–39. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 17.Eisbruch A, Ship JA, Dawson LA, et al. Salivary gland sparing and improved target irradiation by conformal and intensity modulated irradiation of head and neck cancer. World J Surg. 2003;27:832–7. doi: 10.1007/s00268-003-7105-6. [DOI] [PubMed] [Google Scholar]
- 18.Hunt MA, Zelefsky MJ, Wolden S, et al. Treatment planning and delivery of intensity-modulated radiation therapy for primary nasopharynx cancer. Int J Radiat Oncol Biol Phys. 2001;49:623–32. doi: 10.1016/s0360-3016(00)01389-4. [DOI] [PubMed] [Google Scholar]
- 19.Lee N, Xia P, Quivey JM, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002;53:12–22. doi: 10.1016/s0360-3016(02)02724-4. [DOI] [PubMed] [Google Scholar]
- 20.Lin A, Kim HM, Terrell JE, Dawson LA, Ship JA, Eisbruch A. Quality of life after parotid-sparing IMRT for head-and-neck cancer: a prospective longitudinal study. Int J Radiat Oncol Biol Phys. 2003;57:61–70. doi: 10.1016/s0360-3016(03)00361-4. [DOI] [PubMed] [Google Scholar]
- 21.Ma BB, Hui EP, Wong SC, et al. Multicenter phase II study of gemcitabine and oxaliplatin in advanced nasopharyngeal carcinoma–correlation with excision repair cross-complementing-1 polymorphisms. Ann Oncol. 2009;20:1854–9. doi: 10.1093/annonc/mdp065. [DOI] [PubMed] [Google Scholar]
- 22.Takamiya R, Missett B, Weinberg V, et al. Simplifying intensity-modulated radiotherapy plans with fewer beam angles for the treatment of oropharyngeal carcinoma. J Appl Clin Med Phys. 2007;8:26–36. doi: 10.1120/jacmp.v8i2.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia P, Lee N, Liu YM, et al. A study of planning dose constraints for treatment of nasopharyngeal carcinoma using a commercial inverse treatment planning system. Int J Radiat Oncol Biol Phys. 2004;59:886–96. doi: 10.1016/j.ijrobp.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 24.van de TA, Lomax AJ, Bijl HP, et al. Potential benefits of scanned intensitymodulated proton therapy versus advanced photon therapy with regard to sparing of the salivary glands in oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2011;79:1216–24. doi: 10.1016/j.ijrobp.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Capuano G, Grosso A, Gentile PC, et al. Influence of weight loss on outcomes in patients with head and neck cancer undergoing concomitant chemoradiotherapy. Head Neck. 2008;30:503–8. doi: 10.1002/hed.20737. [DOI] [PubMed] [Google Scholar]
- 26.Denham JW, Peters LJ, Johansen J, et al. Do acute mucosal reactions lead to consequential late reactions in patients with head and neck cancer? Radiother Oncol. 1999;52:157–64. doi: 10.1016/s0167-8140(99)00107-3. [DOI] [PubMed] [Google Scholar]
- 27.Dorr W, Hendry JH. Consequential late effects in normal tissues. Radiother Oncol. 2001;61:223–31. doi: 10.1016/s0167-8140(01)00429-7. [DOI] [PubMed] [Google Scholar]
- 28.Trotti A. Toxicity in head and neck cancer: a review of trends and issues. Int J Radiat Oncol Biol Phys. 2000;47:1–12. doi: 10.1016/s0360-3016(99)00558-1. [DOI] [PubMed] [Google Scholar]
- 29.Widesott L, Pierelli A, Fiorino C, et al. Intensity-modulated proton therapy versus helical tomotherapy in nasopharynx cancer: planning comparison and NTCP evaluation. Int J Radiat Oncol Biol Phys. 2008;72:589–96. doi: 10.1016/j.ijrobp.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 30.Johansson J, Blomquist E, Montelius A, Isacsson U, Glimelius B. Potential outcomes of modalities and techniques in radiotherapy for patients with hypopharyngeal carcinoma. Radiother Oncol. 2004;72:129–38. doi: 10.1016/j.radonc.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Authors on behalf of I. Stewart FA, Akleyev AV, et al. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs–threshold doses for tissue reactions in a radiation protection context. Ann ICRP. 2012;41:1–322. doi: 10.1016/j.icrp.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Ahn PH, Lukens JN, Teo BK, Kirk M, Lin A. The use of proton therapy in the treatment of head and neck cancers. Cancer J. 2014;20:421–6. doi: 10.1097/PPO.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 33.Lukens JN, Lin A, Hahn SM. Proton therapy for head and neck cancer. Curr Opin Oncol. 2015 doi: 10.1097/CCO.0000000000000181. [DOI] [PubMed] [Google Scholar]