Abstract
Purpose
The purpose of the current study was to assess the penetrance of NRXN1 deletions.
Methods
We compared the prevalence and genomic extent of NRXN1 deletions identified among 19,263 clinically referred cases to that of 15,264 controls. The burden of additional clinically relevant CNVs was used as a proxy to estimate the relative penetrance of NRXN1 deletions.
Results
We identified 41 (0.21%) previously unreported exonic NRXN1 deletions ascertained for developmental delay/intellectual disability, significantly greater than in controls [OR=8.14 (95% CI 2.91–22.72), p< 0.0001)]. Ten (22.7%) of these had a second clinically relevant CNV. Subjects with a deletion near the 3′ end of NRXN1 were significantly more likely to have a second rare CNV than subjects with a 5′ NRXN1 deletion [OR=7.47 (95% CI 2.36–23.61), p=0.0006]. The prevalence of intronic NRXN1 deletions was not statistically different between cases and controls (p=0.618). The majority (63.2%) of intronic NRXN1 deletion cases had a second rare CNV, a two-fold greater prevalence than for exonic NRXN1 deletion cases (p=0.0035).
Conclusions
The results support the importance of exons near the 5′ end of NRXN1 in the expression of neurodevelopmental disorders. Intronic NRXN1 deletions do not appear to substantially increase the risk for clinical phenotypes.
Keywords: NRXN1, copy number variation, genotype-phenotype, variable expression, penetrance
INTRODUCTION
Neurexins are a group of highly polymorphic presynaptic cell adhesion molecules that primarily bind to neuroligins.1 The three neurexin genes (NRXN1, NRXN2 and NRXN3) are highly conserved and undergo extensive alternative splicing2 to produce thousands of isoforms that appear to be both spatially and temporally regulated.3,4 The two main isoforms for each neurexin gene, the longer NRXN-α and the shorter NRXN-β, are transcribed from two independent promoter regions and give rise to proteins with similar C-terminal regions but different N-terminal ectodomains.5 The NRXN-α and NRXN-β isoforms both bind to postsynaptic neuroligins and leucine-rich repeat transmembrane proteins, however with varying degrees of affinity.1,6,7 NRXN-α also binds to dystroglycan and cerebellin.8,9 NRXN1 (OMIM 600565) is the largest neurexin gene (comprising about 1.1 Mb of genetic sequence)2 and the one most implicated as a top candidate gene for neurodevelopmental and neuropsychiatric conditions (Figure 1).10
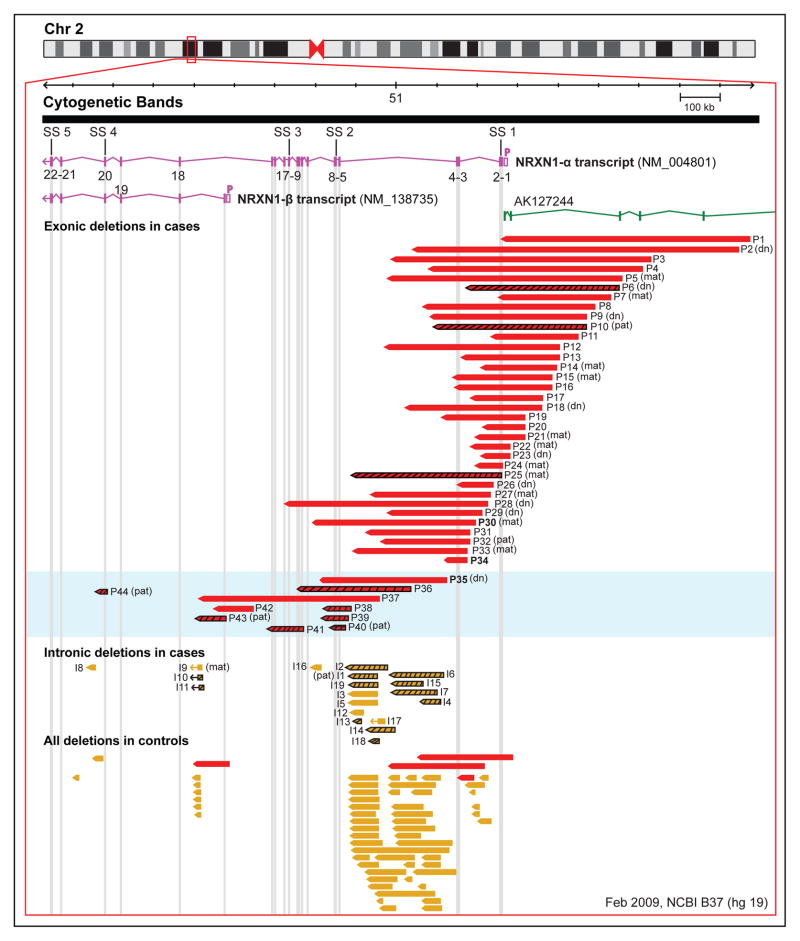
Figure 1. Novel exonic and intronic NRXN1 deletions identified in cases and controls.
The image was modified from the Database of Genomic variants (http://dgv.tcag.ca), NCBI Build 37 (hg 19).37,38 The two primary NRXN1 transcripts (α1 and β1) are shown in pink; other transcripts are not included. The long non-coding RNA (lnRNA) AK127244 is shown in green. Each of the 22 exons is identified by a number according to the NM_004801.4 transcript. The five splice site (SS 1-5) locations are represented above the NRXN1-α transcript. The hollow pink box denoted by a P adjacent to each transcript represents the α and β promoter, respectively. All exonic and intronic deletions (chr2: 50,145,643–51,259,647; hg 19) are represented by solid red and yellow bars, respectively. Deletions with a black grid are subjects that were identified to have a second CNV of potential clinical relevance. Inheritance status of the NRXN1 deletion is represented in brackets following the patient ID number (d.n., de novo; mat, maternal inheritance; pat, paternal inheritance; blank, unknown). P28 and P30 overlap both the 5′ and 3′ ends of NRXN1 and were not included in statistical analyses. P14 and P32 were identified to have maternal uniparental disomy of chromosome 14 and a RAF 1 mutation, respectively. The light blue box designates subjects with deletions overlapping exons ≥5 (3′ deletion). Case numbers were kept consistent throughout the manuscript, tables and supplemental documents. Cases P30, P34 and P35 were obtained from other laboratories and are represented in bold font.
Rare exonic deletions overlapping NRXN1 on chromosome 2p16 were first identified in individuals with autism spectrum disorder (ASD)11,12 and developmental delay/intellectual disability (DD/ID).13 Subsequently, such deletions have been identified in individuals with various neurodevelopmental and neuropsychiatric disorders.14–20 A few exonic NRXN1 deletions have been identified in controls and the majority of transmitting parents are reported to be only mildly affected or clinically unaffected.10,14 Understanding the factors that contribute to this incomplete penetrance is a key goal for clinical genetics.
Exonic NRXN1 deletions are non-recurrent and are found across the entire length of this large gene.2,10,21,22 There is conflicting evidence to suggest that the deletion extent may underlie the NRXN1 phenotypic heterogeneity.10,21 A recent study found that individuals with ASD are significantly more likely to harbor a rare de novo mutation in exons that are under purifying selection (called “critical exons”) than their siblings,23 suggesting that certain sequences within NRXN1 may be more important for clinical expression than others. Also, there is accumulating evidence to suggest that non-coding regions of the genome, including long non-coding RNAs (lncRNAs) and microRNAs (miRNAs), play an important role in the aetiology of neurodevelopmental disorders.24,25 Deletions upstream and within introns of NRXN1 have been identified in disease cases;14,22,26 however the pathogenicity of these deletions remains unclear.
As the use of genome-wide microarray technology in the prenatal setting increases,27 so too does the need for improved understanding of the genetic factors that impact the penetrance of NRXN1 deletions to inform genetic counselling and anticipatory care. We used a large (n=19,263) clinically ascertained cohort from southern Ontario, Canada and 15,264 population-based controls to investigate the penetrance of NRXN1 deletions. CNV data from high resolution genome-wide microarrays allowed systematic evaluation of the burden of secondary CNVs that we used as a proxy to estimate the relative penetrance of exonic and intronic NRXN1 deletions.
MATERIALS AND METHODS
Exonic NRXN1 deletions: clinical cohorts examined
We searched three Province of Ontario accredited clinical cytogenetics laboratory databases for cases with exonic NRXN1 deletions. These included subjects submitted for clinical microarray testing before January 2015 at the Hospital for Sick Children (n=11,727), Trillium Health Partners (n=6,022), and Hamilton Health Sciences (n=1,514), all located in southern Ontario, Canada. All three laboratories are provincially funded to provide clinical constitutional microarray testing for individuals with DD/ID, ASD and/or multiple congenital anomalies (MCA). The NRXN1 deletion cases identified in this study therefore have a strong ascertainment bias for these conditions. Participating physicians completed a detailed clinical checklist (Table S1) for each exonic NRXN1 deletion case based on their own clinical assessment and lifetime chart reviews, where available. For cases without clinical checklist data, ascertainment and demographic data were collected from laboratory requisition forms. We also included three additional cases with an exonic NRXN1 deletion (P30, P34 and P35; Figure 1) detected by other clinical laboratories in order to increase the overall number of cases with genome-wide CNV data. These cases were not included in NRXN1 deletion prevalence calculations for the catchment area.
Molecular methods and validation
All exonic NRXN1 deletions (chr2:50,145,643–51,259,674; hg19) in cases were identified by one of three microarray platforms, the Affymetrix CytoScan® HD Array, the Oxford Gene Technology (OGT) 4×180 CytoSure Oligonucleotide array or the Illumina (formally BlueGnome) 4x180K CytoChip ISCA array. The OGT and Illumina arrays are similar in design with approximately 200–250 oligonucleotide probes (average probe spacing 5.5–7.6 kb) distributed across the NRXN1 gene, allowing for a minimum deletion detection of 10 kb. The CytoScan® HD array has approximately 650 oligonucleotide probes distributed across NRXN1, with similar minimum deletion detection capabilities. All genomic coordinates are given using the Genome Reference Consortium February 2009 build of the human genome (GRCh37/hg 19). Genomic coordinates for the 22 NRXN1 exons (α1 transcript; NM_004801.4) were obtained from the NCBI RefSeq database. Exonic NRXN1 deletions ≥100 kb were confirmed using fluorescence in situ hybridization (FISH). Smaller deletions were confirmed by qPCR or by a second microarray.
Additional clinically relevant CNVs as a proxy for calculating the relative penetrance of NRXN1 deletions
All three clinical laboratories classified a deletion overlapping any one or more of the 22 NRXN1 exons (NM_004801.4) as pathogenic. Therefore, we used the presence of a second clinically relevant CNV as a proxy for estimating the relative penetrance of the NRXN1 deletions. As previously shown,28 CNVs with high penetrance (i.e., not identified in control cohorts) are less likely to harbor a second independent large rare CNV.28 The high-resolution genome-wide CNV data from each NRXN1 deletion case was investigated for the presence of a second CNV that may be clinically relevant. The clinical interpretation of these second CNVs as pathogenic, likely pathogenic, or as a variant of unknown significance (VUS) was provided by one of the three experienced clinical laboratory directors using the American College of Medical Genetics guidelines for CNV interpretation.29 Other clinically relevant variants detected by different laboratory tests were not included in our statistical analyses.
Phenotypic data
The clinical checklist completed for each exonic NRXN1 deletion case is presented in Table S1. Briefly, data were collected on ascertainment features, demographic variables (age, sex), growth parameters (height, weight, head circumference) and growth abnormalities, dysmorphic features, and lifetime developmental, psychiatric, medical, and family history based on clinical assessment and/or lifetime medical chart reviews. If completed, previous clinical genetic testing results were provided. Where known, data on parental phenotypes were collected.
Control cohorts
To examine the prevalence of exonic and intronic NRXN1 deletions in the general population we used 15,264 controls with CNV data available from high-resolution genome-wide arrays (Table S3). These controls were analyzed on several different array platforms with variable probe spacing. Therefore, similar to previous studies,30 we only included exonic and intronic deletions identified by a minimum of two of three CNV calling algorithms (iPattern, PennCNV, ChAS), spanning 5 consecutive array probes and over 10 kb in size. Over 90% of CNVs called using this method validate using other laboratory methods.30
Intronic NRXN1 deletions
Trillium Health Partners was the only clinical laboratory that systematically recorded intronic NRXN1 deletions. These clinical cases (n=6,022) were analyzed using the Illumina 4x180K CytoChip ISCA array which had even probe spacing across introns. We used two strategies to assess the pathogenicity of intronic NRXN1 deletions. First, we compared the prevalence of intronic NRXN1 deletions between clinically referred cases and the 15,254 population-based controls described above. Second, we assessed the prevalence of secondary CNVs among the intronic NRXN1 deletion cases and compared this to the prevalence for exonic NRXN1 deletion cases.
Statistical analyses
Statistical analyses were performed using SAS software (version 9.2; SAS Institute, Cary, NC). For categorical data we used χ2 or Fishers exact test, where appropriate. For continuous data we used Welch’s t-test. All analyses were two tailed, with statistical significance defined as p<0.05. Odds ratios (OR) and 95% confidence intervals were used to assess the prevalence of secondary CNVs between deletions overlapping one or more of exons 1–4 and those overlapping exons ≥5 and the association between the NRXN1 deletions (exonic and intronic) ascertained from the clinical diagnostic population compared to controls.
RESULTS
Prevalence of exonic NRXN1 deletions in cases and controls
As of January 2015, a total of 19,263 individuals were submitted for clinical microarray testing across the three participating cytogenetics laboratories. There were 41 (0.21%) unrelated, previously unpublished, probands identified to have deletions overlapping one or more NRXN1 exons (Figure 1). The prevalence of exonic NRXN1 deletions for the individual laboratories was 0.33%, 0.26%, and 0.14% (Table S4). The prevalence of exonic NRXN1 deletions was significantly greater in cases compared to 15,264 controls [OR=8.14 (95% CI 2.91–22.72), p< 0.0001)] (Table S3).
Exonic NRXN1 deletions
Genomic coordinates for all 44 exonic NRXN1 deletions, including three additional cases with exonic NRXN1 deletions from laboratories outside the catchment area, are represented in Figure 1. All were non-recurrent, with sizes ranging from 29 kb to 806 kb (median=244 kb). The majority of the deletions (n=32; 72.7%) overlapped at least one or more of exons 1–4 (referred to as a 5′ NRXN1 deletion hereafter). Ten (22.7%) deletions overlapped exons ≥5 (hereafter termed 3′ NRXN1 deletions). Two deletions (P28 and P30; Figure 1) that overlapped both the 5′ and 3′ end of NRXN1 were excluded from analyses comparing 5′ and 3′ NRXN1 deletions but not the descriptive statistics. This categorization of exons has been used in previous publications.10,21 The 5′ NRXN1 deletions (median size=242 kb) were significantly larger than the 3′ NRXN1 deletions (median size=83 kb), [t(18.5)=2.55, p=0.019].
The 44 exonic NRXN1 deletions were inherited (11 maternal, 5 paternal) in 16 (64.0%), de novo in nine (36.0%) and unknown in 19 subjects. Of these, 5′ NRXN1 deletions were inherited in thirteen (61.9%) and de novo in 8 (38.1%) subjects; 3′ NRXN1 deletions were inherited in 3 (75.0%) and de novo in 1 (25.0%) subject. Of the 11 transmitting mothers, four were identified as clinically affected: three with mild ID and one with anxiety and depression. Clinical outcomes for the remaining seven transmitting mothers and five transmitting fathers were unknown.
Genome-wide prevalence of additional clinically relevant CNVs
Clinical microarray testing identified 10 (22.7%) exonic NRXN1 deletion cases with one or more additional clinically relevant CNV (n=14), ranging in size from 38 kb to whole chromosomal anomalies (Table 1). The prevalence of males (n=7/28; 25.0%) with a secondary CNV was similar to that of females (n=3/16; 18.8%). Twelve (85.7%) of these CNVs were classified as VUS and two as pathogenic (Table 1). The two pathogenic CNVs were de novo, two of the VUS were paternally inherited and the remaining 10 additional VUS were of unknown inheritance. The prevalence of these secondary CNVs was significantly higher in subjects with a 3′ NRXN1 deletion (n=7/10; 70.0%) compared to subjects with a 5′ NRXN1 deletion (n=3/32; 9.4%) [OR=7.47 (95% CI 2.36–23.61), p=0.0006] (Figure 1). Eleven (78.6%) of the fourteen secondary CNVs overlapped one or more genes known to be involved in central nervous system function (Table 1).
Table I.
Additional clinically relevant CNVs identified in 10 of 44 exonic NRXN1 deletion subjects
| IDa | Main clinical features | NRXN1 deletion | Cytoband | CNV | Start (hg 19) | Size (kb) | # of genes | Inheritance | Protein-coding candidate genesb | Clinical laboratory classification |
|---|---|---|---|---|---|---|---|---|---|---|
| P44 | ID, ASD, ADHD, ODD, anxiety, TS | Exon 20 | X chr (47, XXX) | Gain | - | - | - | de novo | Various, including FMR1, MECP2, SYN1 | Pathogenic |
| P43 | DD | β promoter | Yp11.32-p11.2 | Gain | 10,863 | 4,459 | - | de novo | Various, including | Pathogenic |
| Yq11.21-q12 | Loss | 14,630,081 | 44,700 | - | NLGN4Y | |||||
| P41 | DD | Exons 10–17 | 1q43 | Gain | 236,929,252 | 613 | 3 | Unknown | MTR, RYR2 | VUS |
| 15q13.1-q13.2c | Loss | 28,940,069 | 184 | 9 | APBA2, CHRFAM7A, TJP1 | VUS | ||||
| P40 | DD | Exons 5-8 | 11p11.2-p11.12 | Gain | 48,088,592 | 831 | 8 | Paternal | None | VUS |
| P39 | DD, ASD | Exons 5-8 | 1q23.3 | Gain | 160,927,546 | 428 | 23 | Unknown | USF1, PVRL4, PFDN2, PPOX, B4GALT3, ADAMTS4, NDUFS2, NR1I3, PCP4L1, MPZ, SDHC | VUS |
| P38 | DD/ID, motor and speech delay, PDD, anxiety, failure to thrive | Exons 5-8 | 4q35.2 | Loss | 188,355,766 | 383 | 1 | Unknown | None | VUS |
| P36 | DD, motor and speech delay | Exons 5-10 | 19q13.43 | Loss | 57,656,482 | 794 | 3 | Unknown | None | VUS |
| P13 | DD, behaviour problems | exons 1-2 | 16p13.3 | Gain | 6,679,225 | 38 | 1 | Unknown | RBFOX1 | VUS |
| P10 | DD, speech delay | αP and exons 1-4 | 8p23.3 | Gain | 843,413 | 750 | 4 | Paternal | DLGAP2 | VUS |
| P6 | DD, behavioural problems, hypotonia, bilateral sensorineural loss | αP and exons 1-2 | 1p22.1 | Gain | 92,179,826 | 526 | 6 | Unknown | TGFBR3 | VUS |
| 3q29d | Loss | 192,404,455 | 164 | 2 | Unknown | FGF12 | VUS | |||
| 7q31.2-q31.31 | Gain | 117,382,934 | 1,700 | 3 | Unknown | CTTNBP2 | VUS | |||
| Xp22.23 | Gain | 1,588,945 | 777 | 4 | Unknown | P2RY8, ASMT | VUS |
CNV, copy number variation. #, number; VUS, variant of unknown significance; DD, developmental delay; ASD, autism spectrum disorder; PDD, pervasive developmental disorder; ID, intellectual disability; ADHD, attention deficit hyperactivity disorder; ODD, oppositional defiant disorder; TS, Tourette’s syndrome; MCA, multiple congenital anomalies.
The subject ID’s match those found in Figure 1.
Protein coding genes known to be expressed and/or implicated in nervous system function or cardiac function based on literature search.
This deletion is distal to the Prader-Willi/Angelman syndrome region but proximal to the 15q13.3 deletion syndrome region (OMIM 612001).
Does not overlap the 3q29 microdeletion syndrome region associated with schizophrenia (OMIM 609425).
Clinical characteristics of exonic NRXN1 deletion cases
Completed clinical checklists were returned for 21 (47.7%) exonic NRXN1 deletion cases by referring physicians, with basic ascertainment and demographic data available for the other 23 cases (Table S2). As expected given the criteria for microarray testing, all of the 44 probands (16 female, 28 male) with exonic NRXN1 deletions were ascertained for DD/ID. Thirty-five (79.5%) were children (median 5.2, range 1–6 years) and nine (20.5%) were adults (median 32.0, range 21–59 years).
There were 20 (45.5%) subjects who met diagnostic criteria for another (i.e., additional to DD/ID) neurodevelopmental and/or neuropsychiatric condition, often referred to as ‘dual diagnosis’. Including multiple features per subject, the prevalence of these conditions was: ASD or pervasive developmental disorder (n=14; 31.8%), epilepsy/seizures (n=6; 13.6%), ADHD (n=4; 9.1%), anxiety (n=3; 6.8%), Tourette’s syndrome (n=2; 4.5%) and tardive dyskinesia secondary to antipsychotic treatment in two adults with schizophrenia (n=2; 4.5%). There was no significant difference in the prevalence of dual diagnosis, seizures/epilepsy or macrocephaly between subjects with a 5′ NRXN1 deletion compared to subjects with a 3′ NRXN1 deletion (data not shown).
In contrast to the prominent neurodevelopmental phenotype, congenital anomalies were identified in only four (9.1%) cases: two with tetralogy of Fallot (TOF), one of whom also had a tracheoesophageal fistula and imperforate anus, a third with cryptorchidism, and a fourth with a small atrial septal defect. All four of these subjects had a 5′ NRXN1 deletion and none were identified to have a rare second CNV. However, one TOF case was identified to have a RAF1 sequence mutation causing Noonan syndrome (OMIM 611553).
Eleven (25.0%) cases had additional clinical genetic testing that was found to be normal, including karyotype, fragile X syndrome, 22q11.2 deletion syndrome, Prader-Willi syndrome, muscular dystrophy gene panel and a gene panel for progressive myoclonic epilepsy. Two long contiguous stretches of homozygosity on chromosome 14 (identified on follow-up to be maternal uniparental disomy of chromosome 14) were identified in a single subject (P14; Figure 1).
Intronic NRXN1 deletions
We identified 19 of 6,022 (0.32%) cases submitted for clinical microarray testing and 55 of 15,264 (0.36%) controls to have an intronic NRXN1 deletion (Figure 1). In contrast to the prevalence of exonic NRXN1 deletions identified in this laboratory (0.33% [OR 12.67 (95% CI 4.33–37.08), p<0.0001], there was no significant difference in the prevalence of intronic NRXN1 deletions between cases and controls [OR 0.88 (95% CI 0.52–1.48), p=0.618)]. These intronic NRXN1 deletions ranged in size from 11 kb to 134 kb (median=38 kb), significantly smaller than the 44 exonic deletions (p<0.0001). The majority (n=14; 73.7%) of the intronic deletions were located in the large intron 5 (Figure 1). Inheritance data are limited as most of the intronic deletions were not included in clinical reports; one case had follow-up and was found to be paternal in origin. Several of the intronic deletions were recurrent, including a 70.6 kb deletion in intron 5 and an 11.1 kb deletion in intron 18. Twelve (63.2%) of the 19 intronic NRXN1 deletion cases had a second reportable CNV on clinical microarray (Table 2), a significantly greater prevalence than for the exonic NRXN1 deletion cases [OR=2.59 (95% CI 1.37–4.91); p=0.004].
Table II.
Additional clinically relevant CNVs identified in 12 of 19 intronic NRXN1 deletion subjects
| IDa | Main clinical features | NRXN1 deletion | Cytoband | CNV | Start (hg 19) | Size (kb) | # of genes | Inheritance | Protein-coding candidate genesb | Clinical laboratory classification |
|---|---|---|---|---|---|---|---|---|---|---|
| I8 | DD | Intron 21 | 3q27.1-q27.2 | Loss | 184,027,899 | 1,300 | 4 | Unknown | EIF4G, CLCN2, CHRD, EPHB3, SENP2 | VUS |
| I11c | ASD, heart defect, hip hypoplasia, clubfoot, absent radius | Intron 18 | 1q21.1d | Loss | 144,986,396 | 998 | 20 | Unknown | PDZK1 | Pathogenic |
| I10 | DD | Intron 18 | 4p16.2 | Gain | 3,185,517 | 155 | 3 | de novo | HTT | VUS |
| I6 | DD | Intron 5 | 1p21.2-p21.1 | Gain | 101,742,523 | 554 | 1 | Unknown | OLFM3 | VUS |
| I2c | DD | Intron 5 | 3p13 | Loss | 71,041,637 | 406 | 1 | Unknown | FOXP1 | Likely pathogenic |
| I1 | DD, microcephaly | Intron 5 | 6q22.31-q23.2 | Loss | 125,993,504 | 5,900 | 22 | Maternal | LAMA2 | VUS |
| I13c | MCA | Intron 5 | Mosaic trisomy chr 9 | Gain | - | - | ~800 | Unknown | Various, including SETX | Pathogenic |
| I15 | DD, ASD | Intron 5 | 11p13 | Gain | 33,008,222 | 557 | 6 | Unknown | None | VUS |
| I4 | Absent radius and thumb | Intron 5 | 15q13.1-q13.2 | Gain | 28,859,279 | 1,500 | 4 | Unknown | APBA2 | VUS |
| I14c | DD | Intron 5 | 15q11.2 | Loss | 22,669,082 | 998 | 5 | Unknown | CYFIP1, NIPA2, NIPA1 | Pathogenic |
| I17 | DD | Intron 5 | 16p13.13 | Loss | 10,556,892 | 297 | 4 | Paternal | None | VUS |
| I19 | DD | Intron 5 | 11p15.5 | Gain | 1,222,378 | 318 | 4 | Unknown | BRSK2 | VUS |
CNV, copy number variation; #, number; VUS, variant of unknown significance; DD, developmental delay; ASD, autism spectrum disorder; MCA, multiple congenital anomalies.
The subject ID’s match those found in Figure 1.
Protein coding genes known to be expressed and/or implicated in nervous system function or cardiac function based on literature search.
Subjects with CNVs classified as pathogenic. All other CNVs were classified as a variant of unknown significance.
Overlaps susceptibility locus for Thrombocytopenia-Absent radius syndrome (OMIM 274000).
Of these 19 (11 female, 8 male) unrelated cases with intronic NRXN1 deletions, 17 (89.5%) were children (median=5.0, range 0.5–10 years) and two were adults (Table S2). Sixteen (84.2%) were ascertained for DD/ID and/or ASD and three (6.8%) for MCA (n=1), CHD (n=1) and absent radius/thumb. Of the 16 cases with DD and/or ASD, ten (62.5%) had a second rare CNV that overlapped one or more genes involved in central nervous system function (Table 2).
DISCUSSION
This is the largest study characterizing exonic and intronic NRXN1 deletions to date. Our aim was to use data from a large clinical population-based sample to systematically investigate factors affecting the penetrance of deletions overlapping this large gene. In addition to confirming the predominantly neuropsychiatric phenotypic expression of pathogenic CNVs overlapping NRXN1, novel results support the importance of the genomic extent of these deletions, including the particular region involved and overlapped exons.
Penetrance of exonic NRXN1 deletions
We report on 44 novel exonic NRXN1 deletion cases ascertained for DD/ID (Table S2). We assessed penetrance using multiple factors, both those used previously (prevalence in cases versus controls and the ratio of de novo versus inherited deletions) and those novel to this study (prevalence of secondary CNVs and classification as pathogenic or VUS). We found that the 0.21% prevalence of exonic NRXN1 deletions in this clinical population was over 8-fold greater than in controls and that 34% of these deletions were identified to be de novo, supporting the relatively high penetrance of these deletions for clinically important phenotypes. About one in every four cases with an exonic NRXN1 deletion had one or more other rare CNVs reported on clinical microarray (Table 1). Their distribution amongst the cases indicated a relatively lower penetrance of 3′ NRXN1 deletions.
The effect of genomic position on penetrance of exonic NRXN1 deletions
Similar to previous studies,10,21,22,31 the majority of the exonic NRXN1 deletions identified in clinical cases overlapped the promoter and the first few exons of the NRXN1-α transcript. For subjects with deletions overlapping the 3′ end of NRXN1 there was an over 7-fold increased likelihood of having a second clinically relevant CNV compared to subjects with a 5′ NRXN1 deletion (Figure 1). Further, none of the additional rare CNVs identified in the 5′ NRXN1 deletion subjects were classified as pathogenic, demonstrating the relative importance of 5′ NRXN1 deletions over 3′ NRXN1 deletions for the expression of clinical phenotypes.
A potential explanation for the higher penetrance of 5′ NRXN1 deletions may be that these deletions directly overlap or indirectly influence the lncRNA AK127244 that is adjacent to the promoter of NRXN1-α (Figure 1). There is accumulating evidence to suggest that AK127244 may play a role in the etiology of neuropsychiatric disorders.26,32,33 This includes the identification of two deletions that overlap this lncRNA (and not NRXN1) in a child with borderline IQ and early onset schizophrenia.32 A recent report also described five ASD cases with deletions overlapping AK127244.26 The biological function of AK127244 has yet to be elucidated. However, a significant proportion of lncRNAs are expressed in the brain and have important roles in neurodevelopmental processes.34
There was little evidence that additional phenotypic features were indicators of higher penetrance in individuals with exonic NRXN1 deletions in this study. This could be due in part to a high phenotypic floor effect present in clinically recruited subjects. It is interesting to note that the two adults with comorbid ID and schizophrenia in this study had deletions overlapping the 5′ end of NRXN1. Similarly, in the largest study of NRXN1 deletions in schizophrenia to date there were 10 exonic NRXN1 deletions identified, the majority of which overlapped the 5′ end of NRXN1.14 Also, two cases from this study had a deletion overlapping AK127244 but not NRXN1,14 providing support for the possible role of this lncRNA in the etiology of schizophrenia.
In contrast to our findings supporting the reduced penetrance of 3′ NRXN1 deletions, the limited number of these deletions reported in the literature had previously led to the hypothesis that they are associated with severe phenotypes, including prenatal lethality.31 To further investigate if deletions overlapping the 3′ end of NRXN1 are associated with prenatal lethality we examined ten studies of miscarriages and/or stillbirths (see refs 6 and 17–26 in Rosenfeld et al.)35 for rare CNVs overlapping NRXN1. Among >900 products of conception there was only one paternally inherited 95 kb deletion overlapping NRXN1-α (exons 2–4) identified.35 We also identified one deletion overlapping the NRXN1-β promoter among 15,254 controls. These data provide further support for the increased penetrance of 5′ relative to 3′ NRXN1 deletions.
Interpretation of intronic NRXN1 deletions
We identified 19 novel cases with an intronic NRXN1 deletion, 16 with DD/ID and/or ASD and three with a congenital anomaly. Our group used two strategies to assess the penetrance of intronic NRXN1 deletions. First, we compared the prevalence of intronic NRXN1 deletions between cases and population-based controls and detected no significant difference. Indeed, 18 of the 19 intronic NRXN1 deletions identified in cases had breakpoints similar to those seen in controls (Figure 1). Second, we determined that intronic NRXN1 deletion cases were two-fold more likely to harbour a second clinically relevant CNV compared to exonic NRXN1 deletion cases. Therefore, intronic NRXN1 deletions appear unlikely to substantially increase the risk for a neurodevelopmental disorder and/or MCA.
However, seven (36.8%) of the intronic NRXN1 deletion cases, each with severe phenotypes had no additional rare CNV reported on clinical microarray. One of these seven NRXN1 deletions overlapped intron 9 (Case I16; Figure 1), which had no corresponding deletion identified in controls. Using the VISTA enhancer browser (https://enhancer.lbl.gov)36 we identified a known enhancer element (hs1348) located 37 kb upstream of the intron 9 deletion, which if perturbed could potentially alter the transcriptional levels of NRXN1 and thus increase the penetrance of this deletion.
Advantages and limitations
There are several advantages to this study. We used data from three clinical laboratories to compile the largest cohort of clinically referred individuals with exonic and intronic NRXN1 deletions assembled to date. Our systematic approach to the detection and interpretation of additional rare CNVs allowed us to use the burden of these secondary CNVs as a proxy for determining the relative penetrance of NRXN1 deletions. We employed robust methods for CNV detection, to evaluate the prevalence of intronic NRXN1 deletions in cases and controls which had previously been ignored due to the use of different CNV calling algorithms and reporting practices across clinical laboratories.
A limitation of this study is that the prevalence of individual clinical features would necessarily be influenced by the ascertainment bias inherent in clinically referred cases. This tends to overestimate DD/ID and underestimate other features. The prevalence of NRXN1 deletions was about an order of magnitude different between each clinical laboratory, reaching statistical significance between the Hospital for Sick Children (0.14%) and Trillium Health Partners (0.33%) [OR 2.29 (95% CI 1.19–4.39), p=0.012)] (Table S3). This may be due to differences in indications for referral, with the Hospital for Sick Children and Hamilton Health Sciences servicing children with the most severe and intractable neurological disorders from this catchment area. Given that some NRXN1 deletions are identified in control subjects,14,31 it is possible that the prevalence of these deletions is highest at Trillium Health Partners since it is a community-based hospital that services individuals with comparatively milder clinical phenotypes. The resolution of the microarrays used to define the NRXN1 deletion breakpoints and detect genome-wide structural variants limited us to those >10 kb that could influence phenotypic expression.
Only one control cohort (OPGP; Table S3) was systematically screened for neurodevelopmental and/or neuropsychiatric conditions. Control subjects with NRXN1 exonic or intronic deletions may thus have had mild and/or subclinical symptoms. This could have reduced the effect size of the case-control results. We did not have access to the individual SNP data for the NRXN1 deletion cases or the controls and as a result were unable to genetically confirm that each individual was unrelated to any other. However, given that none of the exonic NRXN1 deletions identified in cases or controls had similar breakpoints it is unlikely that these individuals were related. Further, only two of the eight control cohorts (Table S3) were ascertained from the same catchment area as our cases and they included adults only. This makes it unlikely that the intronic NRXN1 deletion cases (89% children) were the same individuals as those among the controls.
Future directions
The major challenge moving forward will be to determine how genetic and non-genetic factors converge to explain the variable expression and incomplete penetrance of exonic and intronic NRXN1 deletions. Examination of the genes overlapped by additional rare CNVs as well as applying next generation sequencing to detect variants within the coding and non-coding regions of the genome in subjects with a NRXN1 deletion may serve as a key step towards identifying novel pathways to disease expression. Interestingly, none of the second rare CNVs identified in this study have been previously reported in combination with an exonic NRXN1 deletion,10,21,22,31 suggesting that a large NRXN1 deletion consortium may be required to compile enough cases to identify novel biological patterns among the additional variants.
Conclusions
The expression of exonic NRXN1 deletions appears to be primarily neuropsychiatric, with DD/ID often comorbid with another neuropsychiatric condition. The results of this study suggest that deletions near the 5′ end of NRXN1 have higher penetrance, potentially related to perturbation of the lncRNA AK127244 located adjacent to the NRXN1-α promoter. Subjects with a 3′ NRXN1 deletion had a 7-fold increased likelihood of having a second rare CNV detected by clinical microarray, supporting reduced penetrance for these deletions. There is insufficient evidence as yet to suggest that all intronic NRXN1 deletions are benign.
Supplementary Material
Acknowledgments
The authors thank the patients and their families for their participation and Dr. Gregory Costain for his critical review of the manuscript. Study support was provided by the Canadian Institutes of Health Research (MOP 111238 and MOP 89066 to A.S.B.). A.S.B. holds the Canadian Research Chair in Schizophrenia Genetics and Genomic Disorders and the Dalglish Chair in 22q11.2 Deletion Syndrome. C.L. is supported by a Frederick Banting and Charles Best CIHR Doctoral Award. S.W.S. holds the GlaxoSmithKline-CIHR Endowed Chair in Genomic Sciences at the Hospital for Sick Children and University of Toronto. Acknowledgements for control data sets are in Table S5.
Footnotes
Disclosure
The authors have no conflicts of interest to disclose.
Supplementary information is available at the Genetics in Medicine website.
References
- 1.Siddiqui TJ, Pancaroglu R, Kang Y, Rooyakkers A, Craig AM. LRRTMs and neuroligins bind neurexins with a differential code to cooperate in glutamate synapse development. J Neurosci. 2010;30(22):7495–7506. doi: 10.1523/JNEUROSCI.0470-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabuchi K, Sudhof TC. Structure and evolution of neurexin genes: insight into the mechanism of alternative splicing. Genomics. 2002;79(6):849–859. doi: 10.1006/geno.2002.6780. [DOI] [PubMed] [Google Scholar]
- 3.Puschel AW, Betz H. Neurexins are differentially expressed in the embryonic nervous system of mice. J Neurosci. 1995;15(4):2849–2856. doi: 10.1523/JNEUROSCI.15-04-02849.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng Z, Sharpe CR, Simons JP, Gorecki DC. The expression and alternative splicing of alpha-neurexins during Xenopus development. Int J Dev Biol. 2006;50(1):39–46. doi: 10.1387/ijdb.052068zz. [DOI] [PubMed] [Google Scholar]
- 5.Ushkaryov YA, Hata Y, Ichtchenko K, et al. Conserved domain structure of beta-neurexins. Unusual cleaved signal sequences in receptor-like neuronal cell-surface proteins. J Biol Chem. 1994;269(16):11987–11992. [PubMed] [Google Scholar]
- 6.Koehnke J, Katsamba PS, Ahlsen G, et al. Splice form dependence of beta-neurexin/neuroligin binding interactions. Neuron. 2010;67(1):61–74. doi: 10.1016/j.neuron.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48(2):229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Uemura T, Lee SJ, Yasumura M, et al. Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell. 2010;141(6):1068–1079. doi: 10.1016/j.cell.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 9.Sugita S, Saito F, Tang J, Satz J, Campbell K, Sudhof TC. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154(2):435–445. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bena F, Bruno DL, Eriksson M, et al. Molecular and clinical characterization of 25 individuals with exonic deletions of NRXN1 and comprehensive review of the literature. Am J Med Genet B Neuropsychiatr Genet. 2013;162B(4):388–403. doi: 10.1002/ajmg.b.32148. [DOI] [PubMed] [Google Scholar]
- 11.Szatmari P, Paterson AD, Zwaigenbaum L, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39(3):319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall CR, Noor A, Vincent JB, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82(2):477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zahir FR, Baross A, Delaney AD, et al. A patient with vertebral, cognitive and behavioural abnormalities and a de novo deletion of NRXN1alpha. J Med Genet. 2008;45(4):239–243. doi: 10.1136/jmg.2007.054437. [DOI] [PubMed] [Google Scholar]
- 14.Rujescu D, Ingason A, Cichon S, et al. Disruption of the neurexin 1 gene is associated with schizophrenia. Hum Mol Genet. 2009;18(5):988–996. doi: 10.1093/hmg/ddn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nag A, Bochukova EG, Kremeyer B, et al. CNV analysis in Tourette syndrome implicates large genomic rearrangements in COL8A1 and NRXN1. PloS One. 2013;8(3):e59061. doi: 10.1371/journal.pone.0059061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moller RS, Weber YG, Klitten LL, et al. Exon-disrupting deletions of NRXN1 in idiopathic generalized epilepsy. Epilepsia. 2013;54(2):256–264. doi: 10.1111/epi.12078. [DOI] [PubMed] [Google Scholar]
- 17.Noor A, Lionel AC, Cohen-Woods S, et al. Copy number variant study of bipolar disorder in Canadian and UK populations implicates synaptic genes. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(4):303–313. doi: 10.1002/ajmg.b.32232. [DOI] [PubMed] [Google Scholar]
- 18.McGrath LM, Yu D, Marshall C, et al. Copy number variation in obsessive-compulsive disorder and tourette syndrome: a cross-disorder study. J Am Acad Child Adolesc Psychiatry. 2014;53(8):910–919. doi: 10.1016/j.jaac.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swaminathan S, Shen L, Kim S, et al. Analysis of copy number variation in Alzheimer’s disease: the NIALOAD/NCRAD Family Study. Current Alzheimer Res. 2012;9(7):801–814. doi: 10.2174/156720512802455331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Cheng R, Ye X, et al. Increased rate of sporadic and recurrent rare genic copy number variants in Parkinson’s Disease among Ashkenazi Jews. Mol Genet Genomic Med. 2013;1(3):142–154. doi: 10.1002/mgg3.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaaf CP, Boone PM, Sampath S, et al. Phenotypic spectrum and genotype-phenotype correlations of NRXN1 exon deletions. Eur J Hum Genet. 2012 Dec;20(12):1240–1247. doi: 10.1038/ejhg.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curran S, Ahn JW, Grayton H, Collier DA, Ogilvie CM. NRXN1 deletions identified by array comparative genome hybridisation in a clinical case series - further understanding of the relevance of NRXN1 to neurodevelopmental disorders. J Mol Psychiatry. 2013;1(1):4. doi: 10.1186/2049-9256-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uddin M, Tammimies K, Pellecchia G, et al. Brain-expressed exons under purifying selection are enriched for de novo mutations in autism spectrum disorder. Nat Genet. 2014;46(7):742–747. doi: 10.1038/ng.2980. [DOI] [PubMed] [Google Scholar]
- 24.Noor A, Whibley A, Marshall CR, et al. Disruption at the PTCHD1 Locus on Xp22. 11 in Autism spectrum disorder and intellectual disability. Sci Transl Med. 2010;2(49):49ra68. doi: 10.1126/scitranslmed.3001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brzustowicz LM, Bassett AS. miRNA-mediated risk for schizophrenia in 22q11. 2 deletion syndrome. Front Genet. 2012;3:291. doi: 10.3389/fgene.2012.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker S, Scherer SW. Identification of candidate intergenic risk loci in autism spectrum disorder. BMC Genomics. 2013;14:499. doi: 10.1186/1471-2164-14-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayden EC. Prenatal-screening companies expand scope of DNA tests. Nature. 2014;507(7490):19. doi: 10.1038/507019a. [DOI] [PubMed] [Google Scholar]
- 28.Girirajan S, Rosenfeld JA, Coe BP, et al. Phenotypic heterogeneity of genomic disorders and rare copy-number variants. N Engl J Med. 2012;367(14):1321–1331. doi: 10.1056/NEJMoa1200395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kearney HM, Thorland EC, Brown KK, Quintero-Rivera F, South ST Working Group of the American College of Medical Genetics Laboratory Quality Assurance Committee. American College of Medical Genetics standards and guidelines for interpretation and reporting of postnatal constitutional copy number variants. Genet Med. 2011;13(7):680–685. doi: 10.1097/GIM.0b013e3182217a3a. [DOI] [PubMed] [Google Scholar]
- 30.Lionel AC, Crosbie J, Barbosa N, et al. Rare copy number variation discovery and cross-disorder comparisons identify risk genes for ADHD. Sci Transl Med. 2011;3(95):95ra75. doi: 10.1126/scitranslmed.3002464. [DOI] [PubMed] [Google Scholar]
- 31.Dabell MP, Rosenfeld JA, Bader P, et al. Investigation of NRXN1 deletions: clinical and molecular characterization. Am J Med Genet A. 2013;161A(4):717–731. doi: 10.1002/ajmg.a.35780. [DOI] [PubMed] [Google Scholar]
- 32.Duong LT, Hoeffding LK, Petersen KB, et al. Two rare deletions upstream of the NRXN1 gene (2p16. 3) affecting the non-coding mRNA AK127244 segregate with diverse psychopathological phenotypes in a family. Eur J Med Genet. 2015;58(12):650–653. doi: 10.1016/j.ejmg.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Pedrosa E, Kaushik S, Lachman HM. ChIP-chip analysis of neurexins and other candidate genes for addiction and neuropsychiatric disorders. J Neurogenet. 2010;24(1):5–17. doi: 10.3109/01677060903305658. [DOI] [PubMed] [Google Scholar]
- 34.Briggs JA, Wolvetang EJ, Mattick JS, Rinn JL, Barry G. Mechanisms of Long Non-coding RNAs in Mammalian Nervous System Development, Plasticity, Disease, and Evolution. Neuron. 2015;88(5):861–877. doi: 10.1016/j.neuron.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 35.Rosenfeld JA, Tucker ME, Escobar LF, et al. Diagnostic utility of microarray testing of pregnancy losses. Ultrasound Obstet Gynecol. 2015;46(4):478–486. doi: 10.1002/uog.14866. [DOI] [PubMed] [Google Scholar]
- 36.Visel A, Minovitsky S, Dubchak I, Pennacchio LA. VISTA Enhancer Browser--a database of tissue-specific human enhancers. Nucleic Acids Res. 2007;35(Database issue):D88–92. doi: 10.1093/nar/gkl822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macdonald JR, Ziman R, Yuen RK, Feuk L, Scherer SW. The Database of Genomic Variants: a curated collection of structural variation in the human genome. Nucleic Acids Res. 2014;42(1):D986–992. doi: 10.1093/nar/gkt958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zarrei M, MacDonald JR, Merico D, Scherer SW. A copy number variation map of the human genome. Nat RevGenet. 2015;16(3):172–183. doi: 10.1038/nrg3871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.