Abstract
Background
The use of NVBG for mandibular defects seems to be gradually giving way for more advanced reconstructive techniques but it has the advantages of shorter operating time and lesser amount of blood loss. The aim of this study was to review the available data on the success and failure rates of use of NVBG and factors associated with failure.
Method
Studies published from 1978 to 2014 regarding the use of NVBG were searched. Most important information was graft success and failure outcomes. Information on graft success with particular focus on large defects was extracted and the quality of papers was rated. Defects > 6cm were considered long defects.
Results
Twenty publications were included in this review. The quality of the studies was low and there was high heterogeneity. All articles reported high graft success rates ranging from 67 to 100%. Success in defects > 6cm was considerable, the range was 72–100%. Three main factors were associated with failure; these were defect length, fixation method and infection. Infection contributed the highest complication rate which was 48.2%.
Conclusion
NVBG seems to be appropriate for mandible reconstruction when there is vascular recipient bed and sufficient soft tissue cover and mucosal lining are achievable.
Keywords: defects, mandible, non-vascularized, grafts, outcome
Mandibular continuity defect is defined as loss of a portion of the bone resulting in a gap of ∼2cm or more in the lower jaw. The etiology is mainly acquired and rarely congenital.1 2 3 4 Causes include cysts, benign and malignant tumors, trauma, and chronic osteomyelitis.5 6 7 8 Resection of benign invasive odontogenic tumors and avulsion due to trauma frequently cause the defects in sub-Saharan Africa and Asia, while malignancies, osteoradionecrosis and bisphosphonate related osteonecrosis are mostly responsible for the defects in Europe, America and parts of Asia.9 10 11 12 13 14 Prevalence rates of the defects are not available in literature probably because of diverse and multiple etiologies. Reconstruction of mandibular continuity defects is a great challenge to surgeons because of the form and biomechanical functions of the bone.15 16 17 18 19 20 21 22 23 Currently, the state of the art technique to reconstruct this type of defects is vascularized bone grafting (VBG) because it is able to provide immediate blood supply to the bone graft and a soft tissue paddle for external cover and intraoral lining. This results in faster wound healing and better resistance to infection and radiation effects.1 2 5 6 However this is a complicated technique that requires high skills, technology, infrastructure and materials. In addition, it has the disadvantages of longer operating time, increased blood loss and lower cost.5 6 10 13 An alternative for reconstruction of mandibular defects is the use of non-vascularized bone grafts (NVBG), which involves harvesting only bone grafts from sites like the ilium, rib, fibula, calvarium or parts of the mandible itself.24 25 26 This technique has the advantages of shorter operating time, lesser amount of blood loss and more affordable to patients. This is particularly important in centers which lack sufficient expertise or the infrastructure and economic resources to perform microvascular anastomosis, required for the VBG.
Failure to reconstruct mandibular defects causes collapse of the portion of the face leading to aesthetic, functional and psychosocial challenges for the patients.15 16 17 18 These challenges have socioeconomic impact on the patients and to improve their quality of life, reconstruction of the defects takes utmost priority in the patients' management.
Defects up to 6cm long (such as those extending from the first premolar to the third molar), are regarded as short defects, while defects longer than 6cm, are considered long defects. Several articles described the use of NVBG for reconstruction of the mandibular defects ranging from 3 to 14cm3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 and achieved success rates of 38% to 100%.1 2 3 Most articles reported their outcomes for a period of 6–12 months, evaluation of long-term outcomes (> 1year) is useful to assess the survival of NVBG for the treatment of mandibular bone defects. The aim of this study was to review the available data on success/failure rates and complications associated with the use of NVBG for the treatment of mandibular bone defects and, possibly identify the causes of failure.
Materials and Methods
This systematic review was performed from April 2015 to June 2015 in accordance with the PRISMA statement.27 Available literatures published from 1978 to 2014 regarding the use of NVBG for the treatment of mandibular defects was searched in PubMed.
A combination of search strings was used as follows: (Mandible [mesh] AND reconstruction) OR (mandible AND continuity defect), autogenous bone graft OR “bone transplantation”[mesh] OR bone graft NOT Flap OR “vascularized” OR allograft, with predefined filter which were 'human' and 'age'. The inclusion criteria were: reported outcomes for a minimum of 5 patients who had been treated with NVBG; follow-up of 12 months and with reported information on graft success and failure outcomes. Graft success was defined as absence or control of infection after 12 month follow-up, complete extraoral and intraoral wound closure, less than 60% of resorption, as well as high radio density of bone grafts (> 50%). Information regarding complication, functional and aesthetic outcomes was also extracted when available. Articles were excluded if they were case reports, if all cases were done on infants/young children below 8 years or if the techniques were distraction osteogenesis, tissue engineering of any sort, cancellous bone and alveolar ridge reconstruction.
Review articles and references of included articles were screened to further identify potentially eligible articles. Articles were selected by author (BA). Information regarding; author, year of publication, journal, study type/design, sample size, causes of defect, approach for resection, site and extent of the defect, primary or secondary reconstructive procedure, site of graft, type of fixation, antibiotic coverage, and use of radiotherapy was extracted. The classification by Jewer et al28 was used to describe the extent of the defects. This was classified as (L) for defects extending from parasymphysis to ramus on one side, (C) for defects in the anterior portion, (CL or LC) for right or left lateral defects that extend to the symphysis, (LCL) for defects extending to both bodies/angles/ramus, and (H) for defects affecting the whole of one side of the mandible. In publications that documented the defect lengths, and the proportions of patients with long defects (>6cm) were 50% and above, it was possible to calculate success rates (worst case) for defects > 6cm. Success rate in patients (worst case) with defect > 6cm is equal to number of success (worst case) with defect > 6cm divided by number of patients with defect > 6cm multiplied by 100.
In this review, success was defined as absence or control of infection, complete wound closure, bony continuity and mild (30%) or moderate (60%) resorption after 12 month follow-up. Failure was defined as inability to control infection, discharging sinus/fistula, nonunion and gross resorption after 1 year follow-up were documented. Complications at both donor and recipient sites were extracted. Whenever possible, factors associated with failure were identified. Aesthetic outcomes in terms of appearance of the scar, symmetry of the lower jaw and contour of the chin were extracted when available as well as functional outcome such as mouth opening, satisfactory occlusion, jaw movements for mastication with prosthesis or remaining dentition.
Quality Assessment
Two reviewers(ER, BA) independently assessed the quality of each study. Most current quality assessments are designed for randomized clinical trials (RCTs) but none of the publications identified in this literature search was an RCT. Recently, a quality assessment list applicable to observational studies was published by Huisstede et al29 and we slightly modified this score. If item is documented, it was scored 1, if not documented or unclear, it was scored 0. The maximum score was 18. There were 5 domains containing the following individual items:
Study Population
1 Inclusion and exclusion criteria clearly described
2 Sufficient descriptions of baseline characteristics which include demographics, sites and length of defects
3 Number of cases ≥ 50
4 Analysis available for non-vascularized bone grafts only.
Interventions
5 Description of surgical technique which include the surgical approach (extraoral/intraoral/combined) and the stage of reconstruction (Primary/secondary)
6a) Information on source/site of bone graft used
6b) Information on fixation used: number or proportion of patients for each fixation method (Reconstruction plate, miniplate and transosseous wires)
Study Design
7 Prospective study designs
8a) Explicit statement given on drop-out/loss to follow up rates
8b) Explicit information on whether there were no drop-outs or number is <2% per year (applicable if 8a is positive)
8c) Explicit information given about completers versus loss to follow up/dropouts
9 Mean Follow up time clearly stated as 1 year or more
Outcome Measurements
10a) Information on success/failure/survival provided
10b) Information or explanation on causes of failure provided
11 Description of adverse events like complications provided
12 Interval between measurements identical for all patients
13 Criteria for success/failure clearly defined
14 Analysis of prognostic factors
Statistical Analyses
The characteristics of the included publications were summarized and presented in tables. The statistical heterogeneity was determined by random effect model analysis (I2 statistics). The inter-rater agreement was also determined.
Results
The initial Pubmed search resulted in 262 potentially eligible articles. After screening of titles and abstracts, 21 potentially eligible full text articles were selected. The articles were further assessed and 17 articles were deemed appropriate for inclusion. The reference lists of these publications were scanned for further potentially eligible citations which led to identification of 3 additional eligible articles. After the whole screening process, 20 publications were included in this review (Fig. 1). The results of quality assessment are presented in (Table 1). The study characteristics are reported in Tables 2, 3 and 4. Only 2 studies were prospective. One was a cohort study and 2 were case control designs while the remaining were case series. The inter rater agreement with regards to the quality assessment between reviewers was 100% and heterogeneity, I2 value of 77.4%, p = 0.000 (Fig. 2).
Fig. 1.
Flowchart of literature review.
Table 1. Quality assessment of 20 Studies.
| Population | Intervention | Study type/Design | Outcome Measurement | Analysis | Score | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6a | 6b | 7 | 8a | 8b | 8c | 9 | 10a | 10b | 11 | 12 | 13 | 14 | Total | |
| Adekeye, 1978 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 7 |
| Kudo,1978 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 5 |
| Persson,1978 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 6 |
| Egyedi,1986 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| Hong,1989 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 7 |
| Taher,1990 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 6 |
| El-Sheikh,1992 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 7 |
| Kudo,1992 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 6 |
| Ardary,1993 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 10 |
| Pogrel,1997 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 7 |
| Foster,1999 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 8 |
| August,2000 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| Obiechina,2003 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 8 |
| Chiapasco,2008 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 8 |
| Van Germet,2009 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14 |
| Maurer,2010 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 6 |
| Gadre,2011 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 14 |
| Sajid,2011 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 8 |
| Guerrier,2012 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 11 |
| Okoje,2012 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 7 |
| Range | 5–14 | ||||||||||||||||||
Table 2. Study characteristics and patients demographics in reported studies.
| Author/year | Study type/design | Demographics | Cause of defect | Reconstruction stage | Site of graft | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size | Male | Female | Mean age, range | Malignant | Benign | Trauma | Infection | Congenital | Immediate | Secondary | Ilium | Rib | Others | ||
| Adekeye, 1978 | Retrospective case series | 37 | NR | NR | NR,NR | 0 | 7 | 30 | 0 | 0 | 7 | 30 | 33 | 4 | 0 |
| Kudo,1978 | Retrospective case series | 38 | NR | NR | NR, 8–68 |
8 | 28 | 2 | 0 | 0 | 25 | 13 | 30 | 3 | 5 |
| Persson,1978 | Retrospective, case series | 21 | 19 | 2 | 51.1, 8–67 |
2 | 14 | 2 | 2 | 1 | 17 | 4 | 16 | 5 | 0 |
| Egyedi,1986 | Retrospective case series | 38 | NR | NR | NR,NR | 12 | 26 | 0 | 0 | 0 | 17 | 21 | 36 | 2 | 0 |
| Hong,1989 | Retrospective case series | 55 | NR | NR | NR,NR | 8 | 37 | 7 | 3 | 0 | NR | NR | 0 | 0 | 55 |
| Taher,1990 | Retrospective case series | 93 | 93 | 0 | NR, 16–55 |
0 | 0 | 93 | 0 | 0 | 0 | 93 | 93 | 0 | 0 |
| El-Sheikh,1992 | Retrospective case series | 38 | 26 | 12 | 23.0, 11–45 | 36 | 2 | 0 | 0 | 0 | 34 | 4 | 0 | 38 | 0 |
| Kudo,1992 | Retrospective, case series | 8 | NR | NR | NR,NR | 8 | 0 | 0 | 0 | 0 | 1 | 7 | 8 | 0 | 0 |
| Ardary,1993 | Prospective, case series | 8 | 5 | 3 | 35.4, 18–63 | 1 | 3 | 2 | 2 | 0 | 6 | 2 | 8 | 0 | 0 |
| Pogrel,1997 | Cohort | 25 | NR | NR | 39.2, 14–89 | 5 | 16 | 1 | 3 | 0 | 2 | 23 | 22 | 3 | 0 |
| Foster,1999 | Retrospective case control | 22 | NR | NR | 42.0, NR | 0 | 22 | 0 | 0 | 0 | 14 | 8 | 22 | 0 | 0 |
| August,2000 | Retrospective case series | 68 | 46 | 22 | 41.8, 9–69 |
32 | 36 | 0 | 0 | 0 | 0 | 68 | 68 | 0 | 0 |
| Obiechina,2003 | Retrospective case series | 18 | 12 | 6 | 25.5, 11–42 | 0 | 18 | 0 | 0 | 0 | 18 | 0 | 18 | 0 | 0 |
| Chiapasco,2008 | Retrospective case series | 16 | NR | NR | 35.7, 17–54 | 0 | 16 | 0 | 0 | 0 | 11 | 5 | 14 | 0 | 2 |
| Van Germet,2009 | Retrospective, case series | 74 | 41 | 33 | 54.3, 12–73 |
26 | 29 | 11 | 8 | 0 | 34 | 40 | 74 | 0 | 0 |
| Maurer,2010 | Retrospective, case series | 30 | NR | NR | NR,NR | NR | NR | NR | NR | NR | 30 | 0 | 26 | 0 | 4 |
| Gadre,2011 | Retrospective case series | 87 | 59 | 28 | NR, 13–85 | 33 | 54 | 0 | 0 | 0 | 87 | 0 | 68 | 3 | 16 |
| Sajid,2011 | Prospective, case series | 26 | 16 | 10 | 38.0, 15–65 | 3 | 15 | 5 | 3 | 0 | 17 | 9 | 15 | 8 | 3 |
| Guerrier,2012 | Retrospective case control | 35 | 30 | 5 | 33.0,, 15–57 | 0 | 0 | 35 | 0 | 0 | 0 | 35 | 35 | 0 | 0 |
| Okoje,2012 | Retrospective case series | 47 | 26 | 21 | 24.6, 12–45 | 3 | 42 | 2 | 0 | 0 | 47 | 0 | 47 | 0 | 0 |
* NR-NOT REPORTED.
Table 3. Study characteristics and outcomes in reported studies.
| Author/year | Sample size | Defect length >6cm | Surgical approach at recipient site | Method of fixation | Follow-up duration | Success Outcome | Complication | Functional outcome | Aesthetics outcome | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % of pts. with defects > 6cm > 6cm ddddddefects >6cm <6cm | Extraoral | Intraoral | Combined | Reconstruction plate | Miniplates | Wires | 12 months | 1.1–5.0years | 5.1–10years | Success | Failure | Drop-outs | Success rate | Infections | Graft resorption | Non-union | Dehiscence | |||||
| Adekeye, 1978 | 37 | NR | − | 30 | 0 | 7 | 0 | 0 | 37 | 30 | 0 | 0 | 35 | 2 | 0 | 95(35/37) | 2 | 0 | 0 | 2 | NR | NR |
| Kudo,1978 | 38 | NR | − | 28 | 0 | 10 | 0 | 0 | 38 | 0 | 38 | 0 | 30 | 8 | 0 | 79(30/38) | 6 | 8 | 6 | 6 | NR | NR |
| Persson,1978 | 21 | NR | − | 20 | 1 | 0 | 0 | 4 | 17 | 6 | 10 | 5 | 19 | 2 | 0 | 86(19/21) | 2 | 2 | 0 | 2 | NR | NR |
| Egyedi,1986 | 38 | NR | − | 38 | 0 | 0 | 0 | 0 | 0 | 38 | 0 | 0 | 31 | 4 | 3 | 82(31/38) | 10 | 3 | 0 | 0 | NR | NR |
| Hong,1989 | 55 | 33 | 60 | NR | NR | NR | 18 | NR | 30 | 2 | 49 | 0 | 49 | 2 | 4 | 89(49/55) | 1 | 0 | 0 | 0 | 48 | 48 |
| Taher,1990 | 93 | NR | − | 93 | 0 | 0 | 0 | 93 | 0 | 80 | 13 | 0 | 91 | 2 | 0 | 98(91/93) | 2 | 0 | 0 | 2 | 93 | 93 |
| El-Sheikh,1992 | 38 | 20 | 53 | 5 | 0 | 33 | 0 | 0 | 38 | 0 | 38 | 0 | 38 | 0 | 0 | 100(38/38) | 7 | 7 | 0 | 5 | 23 | 23 |
| Kudo,1992 | 8 | NR | − | 7 | 0 | 1 | 3 | 0 | 5 | 8 | 0 | 0 | 6 | 2 | 0 | 75(6/8) | 2 | 0 | 0 | 2 | NR | NR |
| Ardary,1993 | 8 | 6 | 75 | 8 | 0 | 0 | 8 | 0 | 0 | 2 | 4 | 2 | 8 | 0 | 0 | 100(8/8) | 1 | 1 | 0 | 0 | 8 | 8 |
| Pogrel,1997 | 25 | 2 | 8 | 25 | 0 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 18 | 7 | 0 | 72(18/25) | 11 | 0 | 0 | 0 | NR | NR |
| Foster,1999 | 22 | NR | − | NR | NR | NR | 22 | 0 | 0 | 0 | 22 | 0 | 15 | 7 | 0 | 68(15/22) | 0 | 7 | 0 | 0 | 18 | NR |
| August,2000 | 68 | NR | − | NR | NR | NR | 0 | 0 | 68 | 0 | 66 | 0 | 46 | 22 | 0 | 68(46/68) | 13 | 4 | 3 | 0 | NR | NR |
| Obiechina,2003 | 18 | 12 | 67 | 18 | 0 | 0 | 0 | 0 | 18 | 6 | 12 | 0 | 17 | 1 | 0 | 94(17/18) | 1 | 0 | 1 | 1 | 12 | 17 |
| Chiapasco,2008 | 16 | NR | − | 2 | 14 | 0 | 0 | 16 | 0 | 16 | 0 | 0 | 16 | 0 | 0 | 100(16/16) | 1 | 1 | 0 | 0 | 12 | 12 |
| Van Germet,2009 | 74 | NR | − | 42 | 0 | 32 | 54 | 20 | 0 | 74 | 0 | 0 | 56 | 18 | 0 | 76(56/74) | 22 | 18 | 0 | 37 | 63 | NR |
| Maurer,2010 | 30 | NR | − | NR | NR | NR | 9 | 21 | 0 | 0 | 0 | 30 | 20 | 10 | 0 | 67(20/30) | NR | NR | NR | NR | NR | NR |
| Gadre,2011 | 87 | 60 | 69 | 0 | 43 | 44 | 53 | 34 | 0 | 0 | 87 | 0 | 77 | 10 | 0 | 89(77/87) | 7 | 3 | 0 | 5 | 87 | 87 |
| Sajid,2011 | 26 | NR | − | 11 | 15 | 0 | 0 | 13 | 13 | 26 | 0 | 0 | 23 | 3 | 0 | 88(23/26) | 2 | 3 | 0 | 0 | NR | NR |
| Guerrier,2012 | 35 | 25 | 71 | 35 | 0 | 0 | 33 | 2 | 0 | 0 | 35 | 0 | 28 | 5 | 2 | 80(28/35) | 33 | 7 | 0 | 2 | 23 | NR |
| Okoje,2012 | 47 | 14 | 30 | 0 | 0 | 47 | 9 | 0 | 38 | 47 | 0 | 0 | 41 | 6 | 0 | 87(41/47) | 13 | 0 | 0 | 8 | NR | 42 |
*NR- NOT REPORTED.
Table 4. Number and percentages of patients with prognostic factors and outcomes.
| Factors | No of Articles that reported | Total no. of patients | Percentage |
|---|---|---|---|
| Defect length | |||
| Length of defect <6cm | 8 | 141 | 45.0 |
| Length of defect >6cm | 8 | 172 | 55.0 |
| Cause of defect | |||
| Malignant | 19 | 177 | 23.4 |
| Benign | 19 | 365 | 48.7 |
| Trauma | 19 | 190 | 25.1 |
| Infection | 19 | 21 | 2.7 |
| Congenital | 19 | 1 | 0.1 |
| Site of Graft | |||
| Ilium | 20 | 633 | 80.7 |
| Rib | 20 | 66 | 8.4 |
| Others | 20 | 85 | 10.9 |
| Stage of Reconstruction | |||
| Immediate | 19 | 367 | 50.3 |
| Secondary | 19 | 362 | 49.7 |
| Surgical approach | |||
| Extraoral | 19 | 362 | 59.4 |
| Intraoral | 19 | 73 | 12.0 |
| Combined | 19 | 174 | 28.6 |
| Method of Fixation | |||
| Reconstruction plate | 11 | 234 | 31.7 |
| Miniplate | 11 | 203 | 27.5 |
| Wire | 11 | 302 | 40.8 |
| Outcomes | |||
| Success | 20 | 664 | 84.7 |
| Failure | 20 | 111 | 14.2 |
| Drop outs | 20 | 9 | 1.1 |
| Functional | 10 | 389 | 49.6 |
| Aesthetics | 8 | 330 | 42.1 |
| Complications | |||
| Infection | 19 | 136 | 48.2 |
| Dehiscence | 19 | 72 | 25.5 |
| Resorption | 19 | 64 | 22.7 |
| Non Union | 19 | 10 | 3.6 |
Fig. 2.
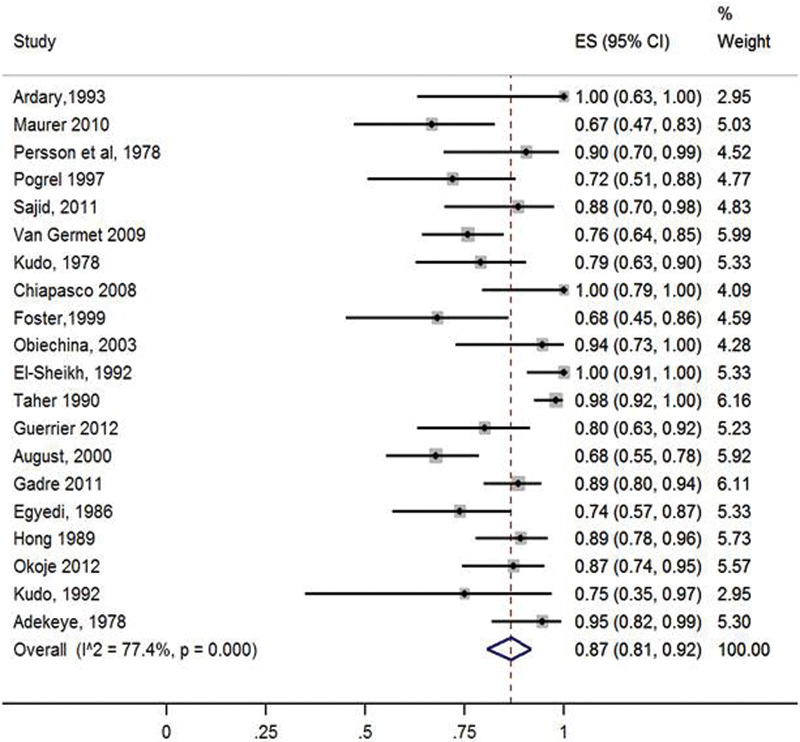
Forest plot of success in patients with NVBG (random effects model).
Five articles scored 50% or more of the maximum attainable score. The variability in quality was high and ranged between 5 and 14 with a mean score was 7.9. Six (30%) of the articles clearly described inclusion and exclusion criteria. A total of 784 patients treated with NVBG were included in this study. Gender characteristics were reported in 11 articles, and with the majority of cases being males (72.4%). Age was reported in 12 articles as means, of which the highest was 54.3 and lowest was 23.0 years. In 14 articles age was reported as range and the highest range was 14–89 years. Eight articles reported the lengths of defects, 141 (45%) patients had defects on the body of the mandible with length less than 6cm while 172(55.0%) patients had defects more than 6cm (extending beyond the body). Cause of defect was clearly reported in 19 publications, of which the majority were benign tumors 365 (48.8%), followed by trauma 190 (25.2%).
All the publications presented information on the graft used. There was no clear relationship in the choice for site of graft with the cause of the defect. The Ilium was the most commonly used cortico-cancellous bone grafts, used in 633(80.7%) patients. Rib was used in 66(8.4%) patients while fibula, calvarium and ramus of mandible were used in 85(10.8%) patients. Immediate reconstruction was done in 367 (50.3%) cases and secondary in 362(49.7%) cases out of a total 729 cases in 19 publications. It was not reported in 1 publication with 55 cases. Extraoral approach was used in 362 (59.4%) patients. Method of fixation was stated in all the articles but the exact numbers on the use of reconstruction plates or miniplates was not given in 9 of them. It was generally reported that reconstruction plates offered more rigid fixation than mini-plates and transosseous wires. Reconstruction plates were used in 234(31.7%) patients and miniplates in 203(27.5%) patients. Success and failure rates were not clearly specified in relation to surgical approach and method of fixation. (Table 2)
Success was reported in 664(84.7%) patients out of 784 patients with a range of 67–100%. Failure was reported in 111(14.2%) patients with a range of 0–23%, total drop-outs were 9(1.2%) patients. Only 8 articles documented number of patients with defect lengths. In 6 of these, 50% or more patients had defect length >6cm, success rates (worst case) in defects > 6cm had a range of 72–100%, in 2 studies, with proportions of patients less than 50%, success rate (worst case) could not be calculated because number of failures (worst case) with defect > 6cm could not be determined (Table 5). One or more complications were reported in total of 282 (36.0%) patients out of the 784 patients, information about complications was not available in 30(3.8%) patients. Infection was the main reported complication reported in 136(48.2%) patients out of the 282 patients with complications, followed by dehiscence, 72(25.5%) and mild(30%) to moderate(60%) resorption, 64(22.7%)(Tables 3). Complication rates were slightly higher in the population with malignancies where infections contributed 37(60%) while resorption and radiation effects were 16(40%), out of 177 patients with malignancies. Causes of graft failure were documented in 16 publications, 11 of them performed analysis of the prognostic factors. All together from these 16 studies, 10 causes of failure were extracted and these were avascular recipient bed, intraoperative blood loss, radiation, recurrence of malignancy, anterior defects, intraoral surgical approach, intraoral/extraoral communication, length of defect > 6cm, fixation method with transosseous wires and infection. Infection contributed 39(35%) of the causes of failure followed by fixation method, 32(28.8%) and length of defect was 24(21.6%).
Table 5. Success rates in long defects.
| Author, Year | Sample size | No. of success | No. of failure | No. of dropout | Success rate | No.(%) of pts. with defect >6cm | No. of failure >6cm(worst case) | No. of success >6 (worst case) | Success rate in pt. > 6cm (worst case) |
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | |
| Hong,1989 | 55 | 49 | 2 | 4 | 89(49/55) | 33(60.0) | 6 | 27 | 81.8 |
| El-Sheikh,1992 | 38 | 38 | 0 | 0 | 100(38/38) | 20(52.6) | 0 | 20 | 100.0 |
| Ardary,1993 | 8 | 8 | 0 | 0 | 100(8/8) | 6(75.0) | 0 | 6 | 100.0 |
| Obiechina,2003 | 18 | 17 | 1 | 0 | 94(17/18) | 12(66.7) | 1 | 11 | 91.7 |
| Gadre,2011 | 87 | 77 | 10 | 0 | 89(77/87) | 60(69.0) | 10 | 50 | 83.3 |
| Guerrier,2012 | 35 | 28 | 5 | 2 | 80(28/35) | 25(71.4) | 7 | 18 | 72.0 |
# of failure with defect > 6cm (worst case) = # of failure + # of drop-out. G = C + D.
# of success with defect > 6cm (worst case) = # with defect > 6cm – # of failure with defect > 6cm (worst case). H = (F – G).
Success rate with defect > 6cm (worst case) = # of success with defect > 6cm (worst case) ÷ # with defect > 6cm × 100. I = H/F × 100.
Only 2 articles were designed to provide information about graft survival beyond 1 year. In one article,3 none of the grafts survived after 5 years when sternocleidomastoid muscle flap was used as covering whereas when it was not used, survival was 72.5%. In the study by Hong et al, there was union of all grafts after the 3rd year.13 Functional outcome was reported in 10 of the publications. The parameters were closely similar and included mouth opening, jaw movements and mastication. Two studies reported the use of implants for prosthetic rehabilitation while the others used conventional tissue borne prosthesis; outcome was stated as satisfactory outcome in 389(49.6%) patients. Aesthetic outcome based on the appearance of scar and jaw symmetry was documented in 8 publications with satisfactory outcome stated in 330(42.1%) patients (Table 4).
Discussion
The success rate with the use of NVBG in this review was 84.7%. In a Cohort study, Pogrel et al5 found a 95% success rate in 39 vascularized bone grafts compared with a 72% success rate in 29 non-vascularized bone grafts. Failure of the nonvascularized bone grafts was closely correlated to the length of the defect. It was documented that the failure rate for grafts of 6 cm or shorter was 17%, increasing to 75% for grafts over 12 cm in length. He reported an increased rate of failure with continuity defects greater than 9 cm and recommended the use of vascularized grafts in such cases to improve outcome. Foster et al.6 also compared VBG and NVBG, reporting an overall success of 88% for VBG and, 68% for NVBG of which there was 75% success rate for bone defects < 6cm, 44% for ≥ 6cm, 46% for 6–10cm and 40% for 10–14cm. The number of cases in each subgroup was not specified. They also corroborated few studies that stated that outcome in strictly lateral (body) defects were better than defects extending to the symphysis, angle and ramus region.24 25 26
Many prognostic factors of success/failure outcome have been discussed in the various articles including age of patients and cause of defects. The defects can occur in a wide age range as documented in the findings, but from this review, increasing age of patients has not clearly been associated with failure outcome. However, success outcome seems to be better in benign tumors and trauma compared with malignant tumors. August et al.3 stated that failure mostly occurs in one year and defined graft failure as graft removal or enormous loss of bone volume postoperatively that makes regrafting necessary. They classified variables into patient factors, preoperative, intraoperative and post-operative and follow-up variables analyzed ∼30 variables that could potentially affect the outcome of reconstruction of mandibular continuity defects. For 4 of these, they found an association with the outcome in a univariate regression analysis, namely amount of intraoperative blood loss, presence of postoperative recipient site complications, length of graft and duration of antibiotic use. In a multivariate regression analysis, intraoperative blood loss and presence of postoperative recipient site complications (dehiscence, infections) were the most important factors.
High blood loss may reflect higher operative time, increased difficulty, and maybe more manipulation with contamination of the recipient bed and graft.3 A longer operation may also indicate a greater interval between graft harvesting and its placement in the recipient bed. In the course of time, there will be desiccation of nonvascularized bone graft and release of enzymes toxic to pluripotential cells thereby affecting graft viability and survival. Eygedi et al4 stated that for autogenous grafts to have “self-defense”, antibiotic coverage must be for at least 10 days and this was practiced in many centers.
Ilium is usually the preferred graft donor site for NVBG because of its bulky cancellous content. Non-vascularized grafts from ribs, fibula, ramus of the mandible and calvarium were also used in this present review, with very good success rates and satisfactory functional and aesthetic outcome. Hayden et al,14 in their review, documented that fibula was the preferred choice for VBG because of large amount of bone available for multiple osteotomies and double barrel design for implants as well as large and highly vascular skin paddle.
In this review, despite the fact that many of the studies did not clearly specify the site and length of the defects, from 6 studies it was found that the success rates were between 72–100% for defects more than 6cm. This appears considerable and it is reasonable to state that when conditions such as vascularity and inherent regenerative capacity are similar in all parts of the recipient bed, this can improve the take of the whole length.22 With extraoral approach the risk of contamination from the oral cavity is lower; however, when combined approaches are used, a “watertight” seal intraoral closure should be achieved.8 9 10 11 12 In this review, many more cases were done via an extraoral than an intraoral approach and this may have contributed to the high success rate.4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
Obiechina et al8 and Okoje et al9 did a descriptive comparison between fixations done with wires and plates and they observed better success rates in those with plates. Nonetheless, in comparison to the findings of this present review, there seems to be minimal superiority between reconstructive plates (>2.0 mm) or miniplates (<2.0 mm). Proponents of miniplates documented lower profile, easy application, and less disruption of the vascular supply to the bone.10 Advocates of reconstructive plates stated that condyle positioning is better ensured and that this more rigid fixation improves bone union with decreased risk of plate fracture but the disadvantage of disuse atrophy due to stress shielding. This present review identified a higher number for reconstruction plates with good success.10 Robey et al10 studied 117 patients; 86 had miniplates and 31 had reconstructive plates. They found no significant difference in the complication rate between the two groups, but had a decreased incidence of osteonecrosis in the miniplate group.
In this review, complication rates were higher in the population with malignancies because of the radiation such patients receive, this supports the general belief stated in the study by August et al,3 However in the study of Guerrier et al,11 all the cases were due to trauma and there was infection in almost all the cases. This contradicted the statement that complications are less in trauma cases by August et al,3 but in another study by Taher et al12 with even a larger population of all trauma cases, there was a low infection rate. These differences may be explained based on varying compliance to infection control practice in hospital set-ups or the nature of trauma in each case. Notwithstanding, it should be stressed that infections do not necessarily result in failure when they are well controlled.4
From the existing literature and this review also, only 2 articles were identified that documented prediction factors that determined the graft survival rates after 1 year.3 13 The first used proportional hazard models and identified the diagnosis of malignant disease, the use of sternocleidomastoid muscle (SCM) flaps for soft tissue augmentation, longer duration of suction drainage, and the presence of postoperative recipient site complications as significant positive predictors of graft survival time.3 There was necrosis of the SCM flaps which led to the loss of all the grafts in the study by August et al.3 Persistent infection from necrotic soft tissue accounted for failure in the study.3 Hong et al.13 documented union at 1, 2, 3 and >3 years and found out that survival rate and functional/aesthetic outcomes improved by the year. Long-term success rates of ∼90% have been documented almost universally with vascularized bone transfer.14 However, there are difficulties in performing vascularized bone grafts in developing countries due to its high cost as well as limited trained manpower and hospital facilities. Therefore, with considerable success rate from individual studies in this review, NVBG is still an acceptable method of reconstruction in both the developing and the developed world.
An attempt to perform a meta-analysis by means of a random effect model analyses resulted in a considerable statistical heterogeneity (I2 value of 77.4%). Possible reasons are of the differences in the population in terms of patient baseline demographics and, interventions. Metaregression analyses could not be performed because insufficient data on the potential prognostic factors were available. Some studies used infection, severe resorption and bony union15 16 17 18 19 20 21 22 as their criteria for success while some used infection, gross resorption and bony continuity/stability,8 9 16 as their criteria, to ensure uniformity, it would be useful to clearly define and quantify the criteria for success/failure. We therefore, recommend the following; At 1 year follow-up, on clinical evaluation, infection must have been fully controlled and extraoral/intraoral wounds completely closed. On radiologic evaluation, there must be complete graft union with residual bone and volume of graft resorption less than 60% and high radiodensity (> 50%). When substantial amount of bone graft is lost, it has implication on the aesthetic appearance and there is need to specify amount of resorption that will need regrafting. Since bone resorbs at a rate of ∼1.5mm the first year and 0.2mm for subsequent years,7 for survival outcome at 5 years, in addition to first 3 criteria, graft resorption must be less than 65%, and for 10 years, it should be less than 70% in addition to high radiodensity (> 60%) as assessed by OPG or CT scan value. Of recent, 3DCT scan has been very useful in surgical planning and resection, and for the latest techniques of reconstruction.
An obvious limitation of this review is the deficiencies in the quality of studies that exist on this very important aspect of oral and maxillofacial surgery. However, there appears to be an overall high success rate, this effect cannot be fully statistically substantiated considering the high degree of heterogeneity but the clinical significance cannot be nullified or overlooked. In conclusion, from this review, success/failure outcome was influenced by interaction of multiple factors, and there was a very high success rate in all articles supporting the relevance and appropriateness of the use of this traditional technique of reconstruction especially when sufficient tissue cover and vascular recipient bed is present. NVBG can be harvested and used as bicortical, corticocancellous grafts (blocks) or particulate bone marrow in meshes/trays (cancellous chips). Particulate bone marrow graft offers better resistance to infection because of higher vascularity and ready availability of pluripotential cells; blocks also result in bulkier reconstructions.7 16
From the findings in this study, NVBG was mostly indicated for defects caused by benign tumors, trauma, osteomyelitis and congenital disease, when vascularity and soft tissue cover/mucosal lining of the recipient bed were sufficient. Relative indications included small to moderate defects due to malignant tumors that do not require radiotherapy or when secondary reconstruction was achievable at least 6 months after radiotherapy. This period allow restoration of blood supply to the recipient bed as well as detection of recurrence.1 2 7 Absolute contraindications to the use of NVBG are immediate post-irradiation, osteoradionecrosis, immune-compromised patients and end stage organ failure.3 13 From the descriptions or analysis in the studies reviewed, 10 factors linked to failure were extracted and of these, length of defect > 6cm, non-rigid fixation method with transosseus wires and infection, were prominent. Despite the fact that success in long defects was appreciable in some of the articles, NVBG cannot be used when there is gross soft tissue loss. Future studies may need to focus on the impact of this factor, on failure outcome, with high quality, long-term follow-up, large sample sized, randomized clinical trial study designs.
Acknowledgments
The author is grateful to Elke Rometsch, Alexander Joeris, Anahí Hurtado-Chong, Irene Santi, Julia Gujan and the rest of the staff at CID for their support during the AOCID clinical research fellowship.
Footnotes
Conflict of Interest The author hereby declares that there is no conflict of interest with regard to this review. Funding The author was supported by the AO Foundation via an AOCMF fellowship at AOCID.
References
- 1.Adamo A K, Szal R L. Timing, results, and complications of mandibular reconstructive surgery: report of 32 cases. J Oral Surg. 1979;37(10):755–763. [PubMed] [Google Scholar]
- 2.Tidstrom K D Keller E E Reconstruction of mandibular discontinuity with autogenous iliac bone graft: report of 34 consecutive patients J Oral Maxillofac Surg 1990484336–346., discussion 347 [DOI] [PubMed] [Google Scholar]
- 3.August M Tompach P Chang Y Kaban L Factors influencing the long-term outcome of mandibular reconstruction J Oral Maxillofac Surg 2000587731–737., discussion 738 [DOI] [PubMed] [Google Scholar]
- 4.Egyedi P. Wound infection after mandibular reconstruction with autogenous graft. Ann Acad Med Singapore. 1986;15(3):340–345. [PubMed] [Google Scholar]
- 5.Pogrel M A, Podlesh S, Anthony J P, Alexander J. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J Oral Maxillofac Surg. 1997;55(11):1200–1206. doi: 10.1016/s0278-2391(97)90165-8. [DOI] [PubMed] [Google Scholar]
- 6.Foster R D, Anthony J P, Sharma A, Pogrel M A. Vascularized bone flaps versus nonvascularized bone grafts for mandibular reconstruction: an outcome analysis of primary bony union and endosseous implant success. Head Neck. 1999;21(1):66–71. doi: 10.1002/(sici)1097-0347(199901)21:1<66::aid-hed9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Chiapasco M, Colletti G, Romeo E, Zaniboni M, Brusati R. Long-term results of mandibular reconstruction with autogenous bone grafts and oral implants after tumor resection. Clin Oral Implants Res. 2008;19(10):1074–1080. doi: 10.1111/j.1600-0501.2008.01542.x. [DOI] [PubMed] [Google Scholar]
- 8.Obiechina A E, Ogunlade S O, Fasola A O, Arotiba J T. Mandibular segmental reconstruction with iliac crest. West Afr J Med. 2003;22(1):46–49. doi: 10.4314/wajm.v22i1.27979. [DOI] [PubMed] [Google Scholar]
- 9.Okoje V N, Obimakinde O S, Arotiba J T, Fasola A O, Ogunlade S O, Obiechina A E. Mandibular defect reconstruction with nonvascularized iliac crest bone graft. Niger J Clin Pract. 2012;15(2):224–227. doi: 10.4103/1119-3077.97334. [DOI] [PubMed] [Google Scholar]
- 10.Robey A B, Spann M L, McAuliff T M, Meza J L, Hollins R R, Johnson P J. Comparison of miniplates and reconstruction plates in fibular flap reconstruction of the mandible. Plast Reconstr Surg. 2008;122(6):1733–1738. doi: 10.1097/PRS.0b013e31818a9ac5. [DOI] [PubMed] [Google Scholar]
- 11.Guerrier G, Alaqeeli A, Al Jawadi A, Foote N, Baron E, Albustanji A. Reconstruction of residual mandibular defects by iliac crest bone graft in war-wounded Iraqi civilians, 2006-2011. Br J Oral Maxillofac Surg. 2015;53(6):e27–e31. doi: 10.1016/j.bjoms.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Taher A AY. Reconstruction of gunshot wounds of the mandible. 128 cases treated by autogenous iliac crest bone grafts. J Craniomaxillofac Surg. 1990;18(7):310–314. doi: 10.1016/s1010-5182(05)80538-4. [DOI] [PubMed] [Google Scholar]
- 13.Hong M, Chen Z H, Sun H. et al. Clinical application of autogenous mandibular bone grafts. Analysis of 166 cases. Chin Med J (Engl) 1989;102(10):791–794. [PubMed] [Google Scholar]
- 14.Hayden R E, Mullin D P, Patel A K. Reconstruction of the segmental mandibular defect: current state of the art. Curr Opin Otolaryngol Head Neck Surg. 2012;20(4):231–236. doi: 10.1097/MOO.0b013e328355d0f3. [DOI] [PubMed] [Google Scholar]
- 15.Ardary W C Reconstruction of mandibular discontinuity defects using autogenous grafting and a mandibular reconstruction plate: a prospective evaluation of nine consecutive cases J Oral Maxillofac Surg 1993512125–130., discussion 131–132 [DOI] [PubMed] [Google Scholar]
- 16.Sajid M AH, Warraich R A, Abid H, Ehsan-ul-Haq M, Shah K L, Khan Z. Reconstruction of mandibular defects with autogenous bone grafts: a review of 30 cases. J Ayub Med Coll Abbottabad. 2011;23(3):82–85. [PubMed] [Google Scholar]
- 17.Kudo K, Fujioka Y. Review of bone grafting for reconstruction of discontinuity defects of the mandible. J Oral Surg. 1978;36(10):791–793. [PubMed] [Google Scholar]
- 18.Maurer P, Eckert A W, Kriwalsky M S, Schubert J. Scope and limitations of methods of mandibular reconstruction: a long-term follow-up. Br J Oral Maxillofac Surg. 2010;48(2):100–104. doi: 10.1016/j.bjoms.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Persson G, Astrand P, Lundgren A, Stenström S. Mandibular reconstruction with bone grafts. Int J Oral Surg. 1978;7(6):512–522. doi: 10.1016/s0300-9785(78)80067-2. [DOI] [PubMed] [Google Scholar]
- 20.van Gemert J TM, van Es R J, Van Cann E M, Koole R. Nonvascularized bone grafts for segmental reconstruction of the mandible—a reappraisal. J Oral Maxillofac Surg. 2009;67(7):1446–1452. doi: 10.1016/j.joms.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 21.el-Sheikh M M, Zeitoun I M, Medra A M. The split rib bundle graft in mandibular reconstruction. J Craniomaxillofac Surg. 1992;20(8):326–332. doi: 10.1016/s1010-5182(05)80359-2. [DOI] [PubMed] [Google Scholar]
- 22.Adekeye E O. Reconstruction of mandibular defects by autogenous bone grafts: a review of 37 cases. J Oral Surg. 1978;36(2):125–128. [PubMed] [Google Scholar]
- 23.Kudo K, Shoji M, Yokota M, Fujioka Y. Evaluation of mandibular reconstruction techniques following resection of malignant tumors in the oral region. J Oral Maxillofac Surg. 1992;50(1):14–21. doi: 10.1016/0278-2391(92)90186-4. [DOI] [PubMed] [Google Scholar]
- 24.Gadre P K, Ramanojam S, Patankar A, Gadre K S. Nonvascularized bone grafting for mandibular reconstruction: myth or reality? J Craniofac Surg. 2011;22(5):1727–1735. doi: 10.1097/SCS.0b013e31822e633b. [DOI] [PubMed] [Google Scholar]
- 25.Ndukwe K C, Aregbesola S B, Ikem I C. et al. Reconstruction of mandibular defects using nonvascularized autogenous bone graft in nigerians. Niger J Surg. 2014;20(2):87–91. doi: 10.4103/1117-6806.137309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adenike O A, Olukunle A T, Olusegun I A, Ifeolu A V, Tunde A J. Perioperative findings and complications of non-vascularised iliac crest graft harvest: The experience of a Nigerian tertiary hospital. Niger Med J. 2014;55(3):224–229. doi: 10.4103/0300-1652.132046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D Liberati A Tetzlaff J Altman D G; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement PLoS Med 200967e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jewer D D Boyd J B Manktelow R T et al. Orofacial and mandibular reconstruction with the iliac crest free flap: a review of 60 cases and a new method of classification Plast Reconstr Surg 1989843391–403., discussion 404–405 [PubMed] [Google Scholar]
- 29.Huisstede B, Miedema H S, van Opstal T, de Ronde M T, Verhaar J A, Koes B W. Interventions for treating the radial tunnel syndrome: a systematic review of observational studies. J Hand Surg Am. 2008;33(1):72–78. doi: 10.1016/j.jhsa.2007.10.001. [DOI] [PubMed] [Google Scholar]


