Abstract
The use of virtual surgical planning and computer-aided design/computer-aided manufacturing has been reported to enhance the planning for the reconstruction of mandibular continuity defects. This case report illustrates the use of this technology in the fabrication of a custom-made titanium prosthesis to restore a segmental mandibular defect. The design specifications and sequence of the custom-made titanium prosthesis are discussed. Although successful in this case, there are limitations in its application and case selection is of vital importance.
Keywords: titanium prosthesis, mandibular reconstruction
Reconstructive options for mandibular continuity defects have included the use of microvascular free flaps, free bone grafts, and alloplastic implants including titanium reconstruction plates and titanium trays.1 2 3 4 5 6 With the establishment of these reconstructive options, advances in three-dimensional (3D) imaging software, virtual surgical planning (VSP), and computer-aided design/computer-aided manufacturing (CAD/CAM) have further enhanced the planning and application of these options in restoring mandibular defects. Advantages of this technology have included visualization of tumor margins, definition of surgical margins, fabrication of surgical templates or cutting guides, assessment of continuity defects, and the identification of ideal bone dimensions and shape for reconstruction.7 8 9 10 Regardless of the reconstructive option, this technology has aided the surgeon in reducing operating time and minimizing errors during the surgery.11 12 13 14 In recent years, this technology has also been used in the fabrication of custom-made prostheses.15 In this article, we would like to report on a case in which a custom-made mandibular prosthesis was fabricated to reconstruct a mandibular continuity defect.
Case Presentation
A 63-year-old man was referred to the Discipline of Oral and Maxillofacial Surgery, Jurong Health, for the management of a jaw swelling in the left mandible. Clinical examination revealed a fully edentulous jaw, with a buccal-lingual expansile lesion noted in the left mandible. Radiographic examination showed a multilocular radiolucency extending from the region of the mental foramen to the mandibular ramus, bordering the condyle neck, with focal perforations on the buccal side of the lesion (Fig. 1). Incisional biopsy of the lesion showed a solid ameloblastoma. The surgical management included a partial mandibulectomy (premolar region to condyle) and mandibular reconstruction with either a fibular free flap or free bone graft. However, upon discussion with the patient and his family, owing to concerns with donor site morbidity, these reconstructive options were declined and alternatives were explored. Alternatives included the placement of a reconstructive plate alone or with a custom-made titanium prosthesis. Further discussion resulted in the choice of the latter reconstructive option.
Fig. 1.
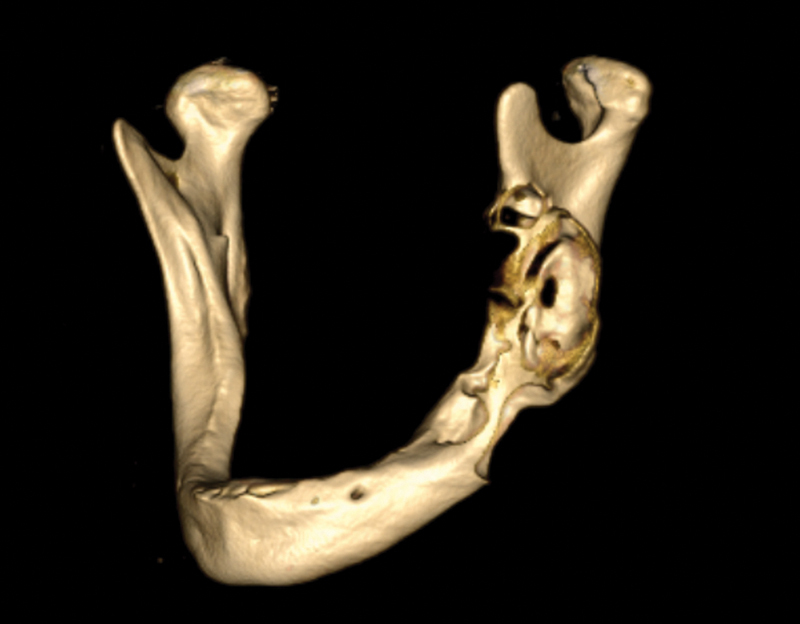
Three-dimensional skull reconstruction showing left mandibular pathology.
Design Sequence
Segmentation of CT scans (3D Doctor, Able Software Corporation, Lexington, MA) was performed to define the extent and margins of the tumor. Together with the biomedical engineers (4T Technologies Pte Ltd, Millenia Tower, Singapore), the resection margins were defined. Once resection margins were determined, virtual surgery was then performed on the 3D-reconstructed image (Fig. 2a). Because of the expansile presentation of the lesion, mirroring of the unaffected side of the mandible was performed instead, and the general shape of the mandibular prosthesis was delineated from this mirrored image. Prosthesis specifications included reduction in the prosthesis height (body) by 10 to 15%, placement of fixation features (using locking screws) on the distal end, placement of suture fixation holes on the ascending part of the proximal end, and the avoidance of any sharp edges. Condyle specifications included reduction in vertical volume of the condylar head by 10% and the retention of the horizontal axis and horizontal length of the patient's condyle. Additional specifications included mirror polishing of the condylar head, a matted body surface, and a smooth transition between the main body and condyle (Fig. 2b).
Fig. 2.
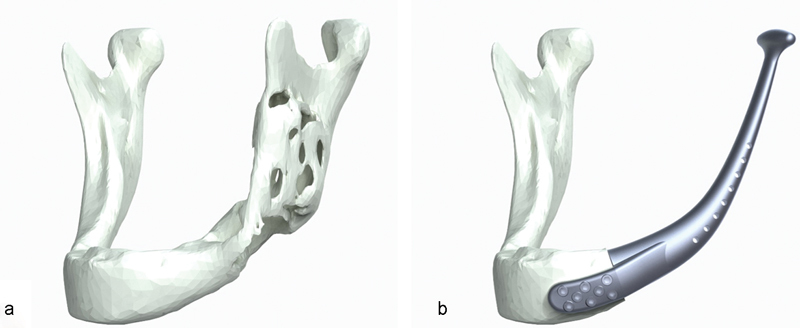
(a) Three-dimensional (3D) image showing left mandibular pathology; (b) 3D image showing mandibular prosthesis prototype design.
The STereoLithography (STL) files were then converted into the CAD software (SolidEdge, Siemens, Nuremberg, Germany) and using additive manufacturing technology (Objet Technologies, Rehovot, Israel), three 3D plastic models (presurgery model, sectioned mandible model, and mandibular prosthesis prototype) were generated (Fig. 3a, b).
Fig. 3.
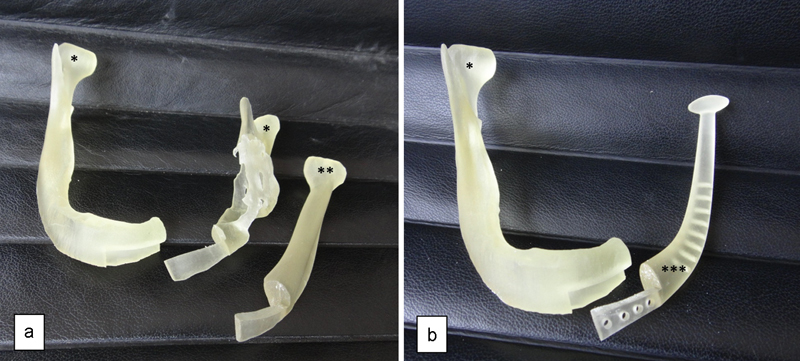
(a) Three-dimensional (3D) plastic models—*Sectioned mandible model; **Mirrored side model. (b) 3D plastic models—*Sectioned mandible model; ***Mandibular prosthesis prototype.
Upon approval of the final prototype, CAD files were inputted into the milling software (Gibbs Cam, Cimatron Technologies Inc., Novi, MI). 5-axis CNC milling (Picomax-95, Fehlmann, Seon, Switzerland) was performed on a titanium blank. Barrel finishing for surface matting with final surface finishing process of blasting and polishing were also done (Fig. 4a, b). Upon completion of the manufacturing process, the titanium mandibular prosthesis was sterilized and packed for use. In addition, surgical resection guides (4T Technologies Pte Ltd) were also constructed from these data to facilitate the accurate fit of the future prosthesis. Fixation screws and instruments (Trinon, Titanium GmbH, Karlsruhe, German) were also sourced for the surgery.
Fig. 4.
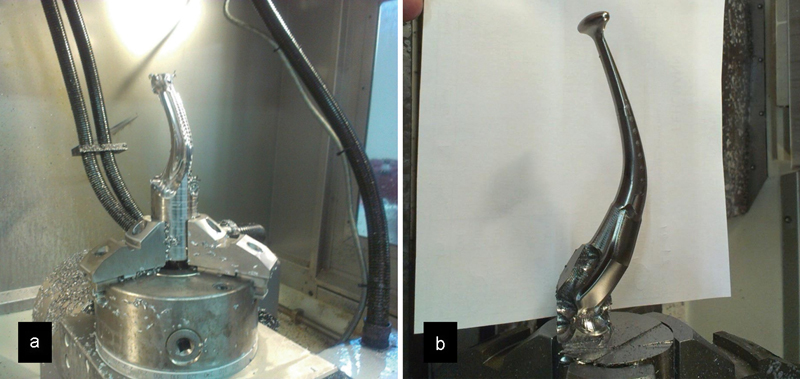
(a) Milling process from a titanium blank; (b) mandibular prosthesis taking shape.
Surgical Sequence
Under general anesthesia, a combined intra- and extraoral approach was undertaken to remove the tumor. Owing to the multiple perforations of the tumor through the bone, a supraperiosteal dissection was performed on the buccal side until the inferior border of the mandible was reached and subsequently connected extraorally. The surgical resection guide was then placed anteriorly to fit (Fig. 5a) and an osteotomy was performed using the guide and a fine sagittal saw. Further posterior and lingual dissection was performed to remove the tumor, with inclusion of the condyle. Following this, the patient's previous dentures were used to place the jaws into the maximum intercuspation.
Fig. 5.
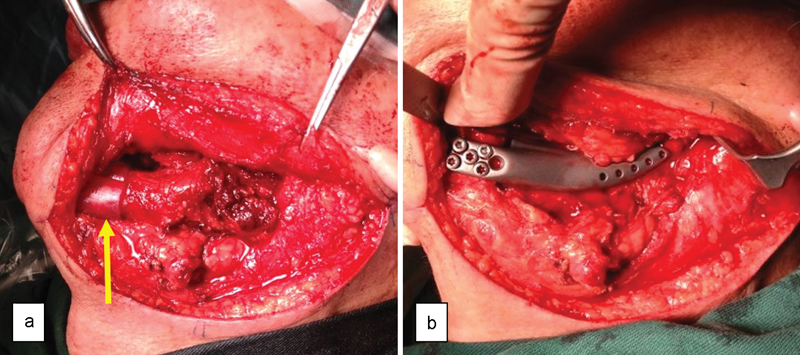
(a) Arrow showing cutting guides in place; (b) anterior fixation of the mandibular prosthesis.
The mandibular prosthesis was then inserted with the condyle portion relocated in the glenoid fossa and was found to be an almost perfect fit. The anterior portion was fixed using 6 locking screws (Fig. 5b). The pterygomasseteric sling was also reattached to the prosthesis using the predesigned suture fixation holes. Finally, a double-layered intra- and extraoral closure was performed. After 5 days, the patient was discharged without complications. Subsequent outpatient reviews were uneventful, with no wound dehiscence or infection. At 2 years review, no tumor recurrence was noted and the patient is functioning well with his new prosthesis (Figs. 6 and 7).
Fig. 6.
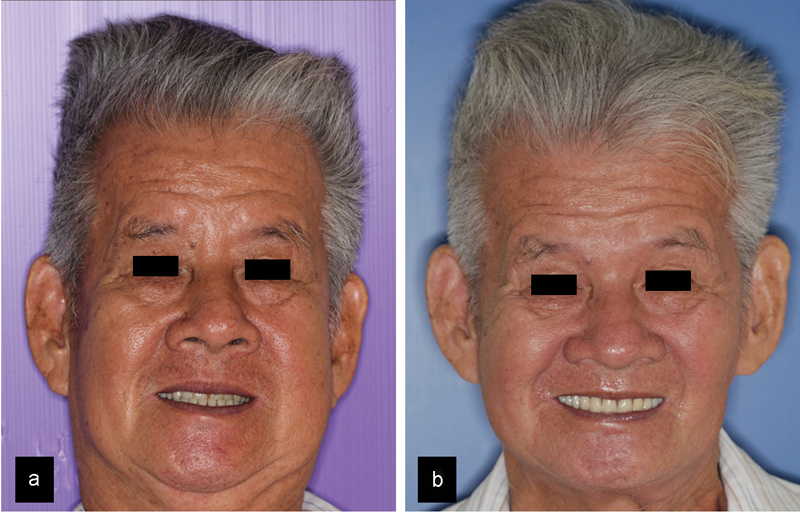
(a) Preoperative facial appearance; (b) postoperative facial appearance.
Fig. 7.
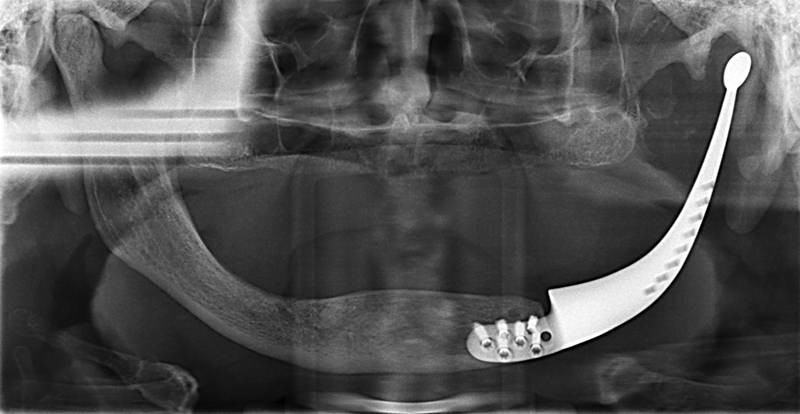
Postoperative radiograph at two years.
Discussion
The use of VSP and CAD-CAM technology has added a new dimension to surgical planning, especially in the areas of craniomaxillofacial trauma, orthognathic surgery, and reconstructive maxillofacial surgery. A recent systematic review of the use of VSP in head and neck oncologic reconstruction reported on the qualitative benefits of VSP, which included increased accuracy of the reconstruction, decreased operative time, decreased flap ischemic time, ease of use, improved predictability of outcomes, improved patient satisfaction, and decreased complications.11 This has a tremendous advantage over traditional reconstructive techniques, from which the accuracy of the reconstruction relies on surgeon skill and experience as well as the anatomy and margins of the recipient site.
The fabrication of a custom-made titanium prosthesis using CAD technology is only recent. Zhou et al reported on six patients who had undergone mandibular resection and reconstruction with a custom titanium tray that was fabricated using CAD, rapid prototyping, and a casting technique.15 The custom trays were packed with autologous particulate bone before being fixed in place. The authors reported that satisfactory facial asymmetry was obtained in six patients. However, at a mean follow-up of 50 months, radiographic findings revealed bone resorption of the grafts. In one patient, dental implants were connected to the fixtures in the tray, but this tray had to be removed due to infection and failure of the graft within the tray. This was attributed to stress shielding of the bone graft by the tray, which was further propagated by the dental implant fixtures.
In our case, the mandibular prosthesis had several design considerations. First, a low-profile body was incorporated so as to minimize the risk of exposure and infection. Intraoperatively, further protection was offered by mobilization of the submandibular gland and rotating it over to the body of the prosthesis. Second, suture fixation holes on the ascending portion of the prosthesis were added to allow the reattachment of the pterygomasseteric sling. Third, with regard to the condyle segment, mirror polishing, a slight reduction in the vertical volume, and maintaining the horizontal axis of the condyle were done to minimize the risk of damage to the glenoid fossa or the risk of perforation. Although some degree of condylar sag was expected and was seen radiographically, there were no clinical implications, as the patient was fully edentulous and able to function well. With the aid of the surgical cutting guide, the positioning and fixation of the prosthesis was accurate and almost flawless, thereby greatly reducing the operating time.
Although this case can be considered a success, there are some limitations to this reconstructive option. Being alloplastic in nature, its use is limited to benign jaw disease, where, unlike malignant disease, there is no risk of exposure from postoperative radiotherapy. In addition, response of the prosthesis under functional loading may vary between patients. We were fortunate that the patient was fully edentulous, which reduced occlusal loading on the prosthesis. At this stage, the placement of ports for dental implant rehabilitation will require further experimental research, and some caution has to be exercised if this has to be applied clinically. Finally, the high cost of the prosthesis may be a limitation for some patients. Although more costly than a microvascular option, it may still be a better option in these cases when the benefits of VSP—such as no donor site morbidity, reduced operating time, and reduced hospital length of stay—are considered.
In conclusion, this case report illustrates the use of VSP and CAD/CAM technology in the fabrication of a custom-made titanium prosthesis for mandibular reconstruction. Although successful in this case, there are limitations in its application and case selection is of vital importance.
Funding
No financial support was received for this study.
References
- 1.Disa J J, Cordeiro P G. Mandible reconstruction with microvascular surgery. Semin Surg Oncol. 2000;19(3):226–234. doi: 10.1002/1098-2388(200010/11)19:3<226::aid-ssu4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 2.Mertens C, Decker C, Engel M, Sander A, Hoffmann J, Freier K. Early bone resorption of free microvascular reanastomized bone grafts for mandibular reconstruction—a comparison of iliac crest and fibula grafts. J Craniomaxillofac Surg. 2014;42(5):e217–e223. doi: 10.1016/j.jcms.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Handschel J, Hassanyar H, Depprich R A. et al. Nonvascularized iliac bone grafts for mandibular reconstruction—requirements and limitations. In Vivo. 2011;25(5):795–799. [PubMed] [Google Scholar]
- 4.Magesh D P, Kumaravelu C, Maheshwari G U. Efficacy of PRP in the reconstruction of mandibular segmental defects using iliac bone grafts. J Maxillofac Oral Surg. 2013;12(2):160–167. doi: 10.1007/s12663-012-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samman N, Luk W K, Chow T W, Cheung L K, Tideman H, Clark R K. Custom-made titanium mandibular reconstruction tray. Aust Dent J. 1999;44(3):195–199. doi: 10.1111/j.1834-7819.1999.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 6.Tideman H, Samman N, Cheung L K. Functional reconstruction of the mandible: a modified titanium mesh system. Int J Oral Maxillofac Surg. 1998;27(5):339–345. doi: 10.1016/s0901-5027(98)80061-1. [DOI] [PubMed] [Google Scholar]
- 7.He Y, Zhu H G, Zhang Z Y, He J, Sader R. Three-dimensional model simulation and reconstruction of composite total maxillectomy defects with fibula osteomyocutaneous flap flow-through from radial forearm flap. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(6):e6–e12. doi: 10.1016/j.tripleo.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Hou J S, Chen M, Pan C B. et al. Immediate reconstruction of bilateral mandible defects: management based on computer-aided design/computer-aided manufacturing rapid prototyping technology in combination with vascularized fibular osteomyocutaneous flap. J Oral Maxillofac Surg. 2011;69(6):1792–1797. doi: 10.1016/j.joms.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 9.Lethaus B, Poort L, Böckmann R, Smeets R, Tolba R, Kessler P. Additive manufacturing for microvascular reconstruction of the mandible in 20 patients. J Craniomaxillofac Surg. 2012;40(1):43–46. doi: 10.1016/j.jcms.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Modabber A, Legros C, Rana M, Gerressen M, Riediger D, Ghassemi A. Evaluation of computer-assisted jaw reconstruction with free vascularized fibular flap compared to conventional surgery: a clinical pilot study. Int J Med Robot. 2012;8(2):215–220. doi: 10.1002/rcs.456. [DOI] [PubMed] [Google Scholar]
- 11.Rodby K A, Turin S, Jacobs R J. et al. Advances in oncologic head and neck reconstruction: systematic review and future considerations of virtual surgical planning and computer aided design/computer aided modeling. J Plast Reconstr Aesthet Surg. 2014;67(9):1171–1185. doi: 10.1016/j.bjps.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 12.Ciocca L, Mazzoni S, Fantini M. et al. A CAD/CAM-prototyped anatomical condylar prosthesis connected to a custom-made bone plate to support a fibula free flap. Med Biol Eng Comput. 2012;50(7):743–749. doi: 10.1007/s11517-012-0898-4. [DOI] [PubMed] [Google Scholar]
- 13.Shen Y, Sun J, Li J. et al. Using computer simulation and stereomodel for accurate mandibular reconstruction with vascularized iliac crest flap. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(2):175–182. doi: 10.1016/j.tripleo.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 14.Metzler P, Geiger E J, Alcon A, Ma X, Steinbacher D M. Three-dimensional virtual surgery accuracy for free fibula mandibular reconstruction: planned versus actual results. J Oral Maxillofac Surg. 2014;72(12):2601–2612. doi: 10.1016/j.joms.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L B, Shang H T, He L S. et al. Accurate reconstruction of discontinuous mandible using a reverse engineering/computer-aided design/rapid prototyping technique: a preliminary clinical study. J Oral Maxillofac Surg. 2010;68(9):2115–2121. doi: 10.1016/j.joms.2009.09.033. [DOI] [PubMed] [Google Scholar]


