Abstract
Craniofacial microsomia (CFM) is a congenital anomaly with a variable phenotype. The most prominent feature of CFM is a predominantly unilateral hypoplasia of the mandible, leading to facial asymmetry. Even after correction of the midline, there is often a remaining hard- and soft-tissue deficiency over the body of the mandible and cheek on the affected side. This clinical report describes the skeletal augmentation of the mandible with a patient-specific implant to treat residual facial asymmetry in two female patients with unilateral CFM. Good aesthetic results were achieved in both patients treated with either a Medpor or polyetheretherketone implant without complications after a follow-up time of 55 and 30 months, respectively.
Keywords: facial asymmetry, patient-specific implant, alloplastic implants, unilateral craniofacial microsomia, reconstruction
Craniofacial microsomia (CFM) is a congenital anomaly with a variable phenotype. Structures involved include the mandible, maxilla, zygoma, external and middle ear, muscles of mastication, trigeminal and facial nerves, and the overlying muscle and soft tissues.1 2 3 The most prominent feature of CFM is a predominantly unilateral hypoplasia of the mandible leading to facial asymmetry.1 2 Common surgical techniques used to correct the skeletal asymmetry of the mandible are distraction osteogenesis (DO), conventional osteotomies, and costochondral grafts.2 4 Frequently, this correction of the midline highlights the remaining hard- and soft-tissue deficiency over the body of the mandible and cheek on the affected side. This remaining volume deficit can be corrected with free vascularized grafts, lipofilling, bone grafts, cartilage, or alloplastic materials.5 6 Free vascularized grafts are useful for large defects, but there is considerable donor-site morbidity.5 6 Lipofilling can correct soft-tissue deficits, but is unable to compensate for (large) hard-tissue defects and needs to be repeated over time.2 5 Bone grafts and cartilage are known to create symmetry in patients with unilateral craniofacial microsomia (UCM), but have drawbacks including donor-site morbidity as well as difficulty in modeling the graft and graft resorption.7 8 9
A more recent technique to correct skeletal asymmetry is the use of alloplastic implants. Disadvantages of silicone implants are resorption of the underlying bone and movement of the implant.10 Porous polyethylene (Medpor [Stryker Co., Kalamazoo, MI] or Synpor [Depuy Synthes, West Chester, PA]) allows fibrous tissue to grow into the implant due to the pore size, which reduces implant migration and resorption of the underlying bone. It shows long-term stability when used in the facial skeleton. However, these porous implants require wider exposure for positioning and can be difficult to remove in case of infection. Reported complications of this implant leading to removal are persistent pain, paresthesia, and infection.10 11 Another alloplastic material is polyetheretherketone (PEEK), a semicrystalline polyaromatic linear polymer that is chemically inert, remains stable at high temperatures, is resistant to chemical and/or radiation damage, and has great strength. It has been used successfully in spinal and orthopedic surgery and cranioplasties as a radiolucent alternative for metallic implants. No specific implant-related complications are reported in this field, but general complications such as infection and pain may occur.12 13 The use of PEEK for maxillofacial reconstruction has been documented in the literature, but the number of patients is limited. Furthermore, these studies have a limited follow-up time, varying from 2 to 31 months.14 15 16 17 18
With both PEEK and Medpor implants, perfect anatomical fit can be achieved with preoperative, three-dimensional (3D) computed tomography (CT) planning and if necessary, adjustments can be made during placement.11 12 This report presents two patients with UCM who had skeletal augmentation of the mandible with either a Medpor or PEEK implant to correct the residual asymmetry.
Patients and Methods
The case reports were approved by the Institutional Review Board of the Erasmus Medical Centre and all participants signed an informed consent agreement.
Preoperative planning consisted of full documentation of the case with recent 3D CT scans (with 1-mm slice thickness) and a complete set of photographs including 3D stereophotography. Subsequently, the CT scan data are sent to the implant manufacturer for processing using computer-aided designed (CAD) and computer-aided manufactured (CAM) technique.16 19 The 3D CT images including the implant model were then sent to the surgeon for review and approval. If satisfactory, the patient-specific Medpor or PEEK implants were fabricated at the manufacturer's facility, delivered and, if needed, sterilized at the authors' hospital facility. We do not have a preference for either a Medpor or PEEK implant. The choice is sometimes guided by the insurance status of the patient. Our first step in the final reconstruction is the hard-tissue reconstruction of the mandible and if desired further touch-up of the soft tissue.
Case 1
A 19-year-old woman suffering from Goldenhar syndrome desired correction of her hypoplastic left side. She was 1 year old when she first presented to the Craniofacial Unit of the Erasmus Medical Center with a hydrocephalus, which was treated with a ventricular drain. Due to recurrent otitis media she had an adenoidectomy and grommet insertion on the right side and a cosmetic correction of the left ear. Besides ear anomalies, the patient had a left-sided Pruzansky–Kaban IIB mandible.19 20 Prior to the skeletal augmentation of the mandible, the patient had the following surgical corrections: DO of the mandible and a Le Fort I osteotomy combined with an osteotomy of the mandible. These procedures had corrected the skeletal asymmetry, but the bone volume of the mandible and the soft-tissue volume of the cheek on the affected side remained insufficient (Fig. 1). At the age of 18 years, the residual facial asymmetry was treated with a patient-specific implant, Medpor (Fig. 2). Preoperatively, 3D CT scans were obtained and the patient-specific Medpor implant was designed and fabricated at the manufacturer's facility. The Medpor implant was placed through an intraoral approach over the left mandibular ramus, body, and angle and was secured with two transbuccal Martin 2.0 screws (9.9 and 4 mm). During placement, the Medpor implant did not need to be trimmed. The soft-tissue was closed in layers with Vicryl 2–0 and 4–0. The patient received perioperative antibiotics.
Fig. 1.

Clinical photo of case 1 at the age of 14 years, postdistraction.
Fig. 2.

Clinical photo of case 1 at the age of 18 years, 1-month post–Medpor implant (and before genioplasty and lipofilling).
One year after the procedure, she still had some aesthetical wishes concerning her chin and the soft-tissue volume of her left cheek. Therefore, an osteotomy of the chin was performed combined with lipofilling of the left cheek. The patient was satisfied with the aesthetic result after this procedure (Fig. 3). No complications were reported after a follow-up at 57 months.
Fig. 3.
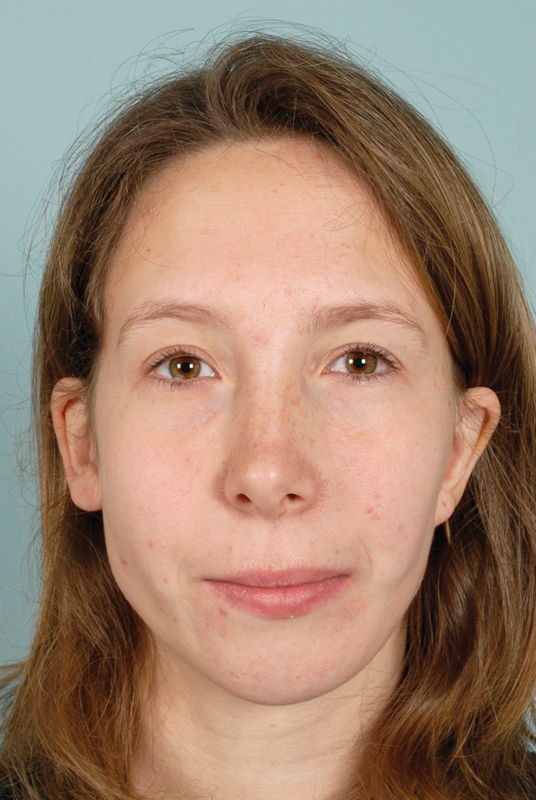
Clinical photo of case 1 at the age of 21 years, 3 years post–Medpor implant, genioplasty, and lipofilling.
Case 2
A 15-year-old woman known with UCM was referred to the Erasmus Medical Center for correction of her facial asymmetry. She had a right-sided Pruzansky–Kaban III mandible. The correction of the asymmetry at another medical center included mandibular DO, reconstruction of the right mandibular condyle with a costochondral rib graft, a Le Fort I osteotomy combined with DO of the mandible, and a coronoidectomy on the right side. At Erasmus Medical Center, another Le Fort I osteotomy in combination with DO of the right mandible was performed to improve the occlusion and further correct the asymmetry. After these procedures, the bone and soft-tissue volume of the mandible and cheek on the affected side remained insufficient (Fig. 4). At the age of 17 years, the patient desired correction of the residual facial asymmetry. 3D CT scans were obtained and subsequently, the PEEK implant was designed and fabricated at the manufacturer's facility. The placement of the PEEK implant was uneventful. It was placed via an intraoral approach on the mandibular corpus and secured with three Synthes screws. The soft tissue was closed with Ethilon. The patient received perioperative antibiotics. The surgery improved the symmetry and support of the lower lip; however, the soft-tissue volume of the right side was still not equal to the left (Fig. 5). We discussed lipofilling with the patient; however, she was satisfied with the result and thus no further surgery was performed (Fig. 6). At the end of follow-up at 32 months, no complications were reported.
Fig. 4.

Clinical photo of case 2 at the age of 15 years, postdistraction.
Fig. 5.

Clinical photo of case 2 at the age of 17 years, 1 month post–PEEK implant.
Fig. 6.

Clinical photo of case 2 at the age of 19 years, 2 years post–PEEK implant.
Discussion
Patients with UCM mainly have a unilaterally underdeveloped mandible, deviating toward the affected side. Following skeletal correction of the asymmetry, both the bone volume of the mandibular ramus and the soft-tissue volume often remain insufficient and facial asymmetry stays disturbingly evident. The ideal material to correct a bony defect is the use of another “hard” material that does not resorb, as replacing “like-with-like” gives the best aesthetic result. Therefore, we chose to correct the hard-tissue deficits with alloplastic implants in both patients. Facial remodeling with alloplastic material has a low incidence of complications in general. A drawback of alloplastic implants is the potential of postoperative infection, which can eventually lead to failure of the implant. In our patients, both implants were placed through an intraorally approach, leading to a better aesthetic result due to the absence of a submandibular scar. No major complications were reported after 57 and 32 months, respectively. The biggest advantage of using biomaterial over bone graft (or other autologous material) is that a perfect anatomical fit can be achieved with preoperative planning.11 12 Furthermore, biomaterial does not resorb over time, which aids stability and gives good aesthetic results.10 12 These two cases show a good aesthetic outcome with the use of patient-specific implants (either Medpor or PEEK) in the correction of facial asymmetry. The surgeon had no personal preference for Medpor or PEEK. A major disadvantage of these patient-specific implants is that they are expensive which may cause difficulties for the insurance companies or for the patients if they have to pay for the implants themselves.
Our experience has shown that both patient-specific PEEK and Medpor implants can provide good results in the correction of the residual hard-tissue facial asymmetry in UCM patients.
References
- 1.Ohtani J Hoffman W Y Vargervik K Oberoi S Team management and treatment outcomes for patients with hemifacial microsomia Am J Orthod Dentofacial Orthop 2012141(4, Suppl):S74–S81. [DOI] [PubMed] [Google Scholar]
- 2.Cousley R RJ, Calvert M L. Current concepts in the understanding and management of hemifacial microsomia. Br J Plast Surg. 1997;50(7):536–551. doi: 10.1016/s0007-1226(97)91303-5. [DOI] [PubMed] [Google Scholar]
- 3.Heike C L, Hing A V. Seattle, WA: University of Washington; Craniofacial microsomia overview. [PubMed] [Google Scholar]
- 4.Pluijmers B I, Caron C JJM, Dunaway D J, Wolvius E B, Koudstaal M J. Mandibular reconstruction in the growing patient with unilateral craniofacial microsomia: a systematic review. Int J Oral Maxillofac Surg. 2014;43(3):286–295. doi: 10.1016/j.ijom.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Guijarro-Martínez R, Miragall Alba L, Marqués Mateo M, Puche Torres M, Pascual Gil J V. Autologous fat transfer to the cranio-maxillofacial region: updates and controversies. J Craniomaxillofac Surg. 2011;39(5):359–363. doi: 10.1016/j.jcms.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Cobb A R, Koudstaal M J, Bulstrode N W, Lloyd T W, Dunaway D J. Free groin flap in hemifacial volume reconstruction. Br J Oral Maxillofac Surg. 2013;51(4):301–306. doi: 10.1016/j.bjoms.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Sbordone L, Toti P, Menchini-Fabris G B, Sbordone C, Piombino P, Guidetti F. Volume changes of autogenous bone grafts after alveolar ridge augmentation of atrophic maxillae and mandibles. Int J Oral Maxillofac Surg. 2009;38(10):1059–1065. doi: 10.1016/j.ijom.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Moon B J, Lee H J, Jang Y J. Outcomes following rhinoplasty using autologous costal cartilage. Arch Facial Plast Surg. 2012;14(3):175–180. doi: 10.1001/archfacial.2012.138. [DOI] [PubMed] [Google Scholar]
- 9.Gui L, Zhang Z, Zang M. et al. Restoration of facial symmetry in hemifacial microsomia with mandibular outer cortex bone grafting combined with distraction osteogenesis. Plast Reconstr Surg. 2011;127(5):1997–2004. doi: 10.1097/PRS.0b013e31820cf485. [DOI] [PubMed] [Google Scholar]
- 10.Yaremchuk M J. Facial skeletal reconstruction using porous polyethylene implants. Plast Reconstr Surg. 2003;111(6):1818–1827. doi: 10.1097/01.PRS.0000056866.80665.7A. [DOI] [PubMed] [Google Scholar]
- 11.Cenzi R, Farina A, Zuccarino L, Carinci F. Clinical outcome of 285 Medpor grafts used for craniofacial reconstruction. J Craniofac Surg. 2005;16(4):526–530. doi: 10.1097/01.scs.0000168761.46700.dc. [DOI] [PubMed] [Google Scholar]
- 12.Kurtz S M, Devine J N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28(32):4845–4869. doi: 10.1016/j.biomaterials.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eppley B L, Kilgo M, Coleman J J III. Cranial reconstruction with computer-generated hard-tissue replacement patient-matched implants: indications, surgical technique, and long-term follow-up. Plast Reconstr Surg. 2002;109(3):864–871. doi: 10.1097/00006534-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Kim M M, Boahene K DO, Byrne P J. Use of customized polyetheretherketone (PEEK) implants in the reconstruction of complex maxillofacial defects. Arch Facial Plast Surg. 2009;11(1):53–57. doi: 10.1001/archfaci.11.1.53. [DOI] [PubMed] [Google Scholar]
- 15.Goodson M L, Farr D, Keith D, Banks R J. Use of two-piece polyetheretherketone (PEEK) implants in orbitozygomatic reconstruction. Br J Oral Maxillofac Surg. 2012;50(3):268–269. doi: 10.1016/j.bjoms.2011.04.077. [DOI] [PubMed] [Google Scholar]
- 16.Scolozzi P. Maxillofacial reconstruction using polyetheretherketone patient-specific implants by “mirroring” computational planning. Aesthetic Plast Surg. 2012;36(3):660–665. doi: 10.1007/s00266-011-9853-2. [DOI] [PubMed] [Google Scholar]
- 17.Guevara-Rojas G, Figl M, Schicho K. et al. Patient-specific polyetheretherketone facial implants in a computer-aided planning workflow. J Oral Maxillofac Surg. 2014;72(9):1801–1812. doi: 10.1016/j.joms.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Gerbino G, Bianchi F A, Zavattero E, Tartara F, Garbossa D, Ducati A. Single-step resection and reconstruction using patient-specific implants in the treatment of benign cranio-orbital tumors. J Oral Maxillofac Surg. 2013;71(11):1969–1982. doi: 10.1016/j.joms.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Kaban L B, Moses M H, Mulliken J B. Correction of hemifacial microsomia in the growing child: a follow-up study. Cleft Palate J. 1986;23 01:50–52. [PubMed] [Google Scholar]
- 20.Pruzansky S. Not all dwarfed mandibles are alike. Birth Defects Orig Artic Ser. 1969;5:120–129. [Google Scholar]


