Abstract
BACKGROUND
Allogeneic hematopoietic stem cell donor selection is based primarily on human leukocyte antigen degree of match and it often occurs without regard to the red blood cell (RBC) compatibility between donor and recipient. When major ABO-mismatched grafts are infused, it is imperative that an accurate determination of the incompatible RBC content is made to ensure that the product is safe for infusion. RBC content determination requires the hematocrit (HCT) parameter which can be obtained via manual (directly measured) or automated (calculated) methods.
STUDY DESIGN AND METHODS
Ninety-seven (97) apheresis hematopoietic progenitor grafts were assessed for HCT by manual testing and by 4 commercially available automated hematology analyzer instruments. A clinical model was developed to assess the frequency of unnecessary RBC reductions or alteration in standard infusion practice.
RESULTS
Statistically significant (p<0.001) differences were observed where the manual HCT value was markedly lower than automated HCT values. At stringent incompatible RBC threshold of 10 mL, the number of preventable RBC reduction procedures ranged from 18–69%.
CONCLUSION
Accurate determination of RBC content of hematopoietic progenitor grafts is essential for patient safety. Despite the rapidity and convenience offered by automated HCT methods, they significantly overestimate the incompatible RBC content of grafts which may trigger unnecessary RBC reduction procedures or split infusions. In products where automated HCT methods indicate excessive amounts of incompatible RBCs are present, we advise the performance of confirmatory testing with a manual HCT method to ensure that the automated HCT value is not a false positive.
INTRODUCTION
Hematologic malignancies as well as non-neoplastic diseases such as hemoglobinopathies can be treated with allogeneic hematopoietic stem cell transplantation (HSCT)1. Most HSCTs utilize HPC,Apheresis(HPC(A)) mainly due to the convenience and safety of the procedure which typically yields HPC grafts with sufficient number of CD34-positive hematopoietic progenitors to ensure durable engraftment. HPC,Apheresis collection technology relies on the differential density of blood components to enrich the collected product for white blood cells(WBCs). In the process of collection however, other cell types such as red blood cells(RBCs) and platelets are also collected and included in the product.
HPC donor selection is performed using human leukocyte antigen(HLA) type as the primary criteria whereas donor ABO-Rh blood type incompatibility is generally not a major consideration. Early investigations of recipients of ABO-incompatible allogeneic HSCT demonstrated that ABO-mismatched allogeneic HSCT recipients fared as well as ABO-identical transplants but the investigators observed occasional delayed red cell engraftment or hemolysis2, 3, therefore ABO compatibility was not considered an important factor for outcome after HSCT. Approximately 30% to 50% of HSCT recipients receive an HPC(A) product that contains ABO-mismatched blood4, 5. There are no standards for the amount of incompatible RBCs that a transplant recipient may receive, although most transplant centers generally follow blood transfusion guidelines that permit administration of 0.2 to 0.4 mL/kg of incompatible RBCs equating to 15 to 30 mL of packed RBC (in a 75 kg recipient). The consequences of using an ABO-incompatible HPC graft are both immediate and delayed. In the short term, recipients of a major ABO-mismatched graft can experience an acute hemolytic transfusion reaction whereas the delayed consequences may include delayed engraftment and pure red cell aplasia6. In situations where the incompatible RBC content of the graft exceeds the acceptable limits, either the graft must undergo a modification in the form of RBC reduction, or the graft infusion must be prolonged or divided so that the incompatible RBC exposure is reduced for each infusion event. Of note, product manipulation such as RBC reduction has potential drawbacks other than the resources used to perform the procedure, most importantly the risk of CD34-positive cell loss and possible introduction of microbial contamination.
In order to calculate the amount of incompatible RBCs in an HPC(A) product, the hematocrit(HCT) of the product must be determined. The HCT measures the proportion of the blood that consists of packed RBCs. The HCT is expressed as a percentage by volume. Manual methods pack RBC by centrifugation. This information can be obtained by performing either a manual HCT direct measurement method, or by running a product sample on an automated hematology analyzer. A caveat of using an automated platform is that the value is not a direct measurement but rather a calculated parameter which uses sample volume, measured RBC number and measured mean (red) cell volume(MCV)7. The use of an automated analyzer platform is advantageous because of the ability to produce accurate, precise, and rapid results when analyzing peripheral blood for which it was designed. However, analysis of HPC(A) on an automated platform should be performed with caution since the relative proportion of cells types is markedly skewed towards WBC enrichment. In this study we sought to ascertain what, if any, clinically significant difference in HCT values would be obtained when comparing the manual HCT reference method with four automated analyzer HCT results.
MATERIALS AND METHODS
Study Samples
HPC(A) samples (n=97) were analyzed using the manual hematocrit test method and HCT analysis by four different automated hematology analysis platforms: Advia 2120i (Siemens, Tarrytown, NY); XE-2100 (Sysmex, Kobe, Japan); CELL-DYN Sapphire (Abbott, Santa Clara, CA); and AcT Diff (Beckman Coulter, Hialeah, FL).
The method comparison study was approved by the Memorial Sloan Kettering institutional review board.
Manual hematocrit (manual HCT direct measurement)
The manual HCT determination was performed as follows: capillary tubes were 75% filled with HPC(A) product, sealed, and centrifuged for 10 minutes at 12000+/−600 RPM using a microhematocrit centrifuge (C-MH30, Unico, NJ). The centrifuged tubes were measured for the total height of the sample and the height of the packed red cell layer using a capillary micro-hematocrit reader (CMH30, Unico, NJ). The red cell layer height was divided by total sample height to obtain the percent HCT value. This assay satisfies proficiency testing by method comparison with an automated hematology analyzer (Advia 2120i; Siemens, Tarrytown, NY).
Automated Hematology Analyzers HCT
Determination of HCT on automated hematology analyzers is markedly different than the manual HCT method. The HCT is not determined directly, but rather it is a calculated value. The number of RBCs is multiplied by the mean RBC volume (MCV) of the sample RBCs to calculate the volume of the red cell component of the sample. This calculated RBC volume is subsequently divided by the total sample volume to produce a calculated HCT value. Calculated HCT (%) = (RBC × MCV)/10.
Clinical Modeling
To assess the potential clinical consequences of using automated hematology analyzers in place of the manual HCT method, a commonly encountered clinical scenario was devised. This clinical scenario makes the following assumptions: the HPC(A) product is 250 mL and the patient is an adult weighing 70 kg. Several thresholds for incompatible RBC volume in the product were assessed; specifically 10 mL, 15 mL, 20 mL, 30 mL, and 40 mL which would trigger either a RBC reduction procedure or require a change in infusion practice (e.g. split infusion over time).
Data Analyses
The percent difference between the reference method (manual HCT) and the calculated HCT from the automated analyzer was calculated as the difference between the two HCT values divided by the average of the two values.
The descriptive statistics for the HCT values were determined using StatView V5.0.1 (SAS Institute, North Carolina) and Excel 2007 (Microsoft, Washington).
Paired, two-tailed t-tests were performed to ascertain whether there was a significant difference between the manual HCT and automated analyzer HCT results.
Truth tables comparing the manual HCT and the automated analyzer HCT were constructed for the above clinical scenario using different incompatible RBC thresholds to determine the sensitivity, specificity, positive predictive value, negative predictive value, and number of RBC reduction procedures which could be avoided if using the manual HCT method for test confirmation.
RESULTS
In order to determine if there were differences in the determined values of hematocrit (HCT) between the manual HCT method and several commercially available automated hematology analyzers HCT, HPC(A) were tested in parallel (n=97). No interference was observed in HCT determination using the manual method in samples with high nucleated cell count concentration.
The HCT values of the HPC(A) products were plotted in a box-whisker plot to demonstrate the mean HCT values and the spread of the data points (figure 1). The manual HCT method was found to detect the lowest mean HCT value of 0.99 % whereas the Advia HCT, Sysmex HCT, Abbott HCT, and Coulter HCT were markedly higher with a mean difference which ranged from 2.15% to 4.46% (table 1). The standard deviation of the mean of determined HCT values on the automated hematology analyzers was higher in all cases as compared to the manual HCT method (table 1).
Figure 1.
Hematocrit (%) values for HPC(A) products using manual and automated HCT procedures
Table 1.
Descriptive Statistics for various hematocrit determinations.
| Compared to Manual HCT | |||||
|---|---|---|---|---|---|
| Mean | Median | Std. Dev. | Mean Diff. | P-Value | |
| Manual HCT% | 0.99 | 0.50 | 1.55 | ND | ND |
| Advia HCT% | 4.14 | 3.80 | 2.35 | −3.16 | <.001 |
| Sysmex HCT% | 3.13 | 2.20 | 2.59 | −2.15 | <.001 |
| Abbott HCT% | 3.58 | 2.81 | 2.93 | −2.59 | <.001 |
| Coulter HCT% | 5.44 | 4.70 | 2.67 | −4.46 | <.001 |
Paired, two-tailed, t-test analyses were performed to ascertain if the differences in measured HCT values by automated methods as compared to the manual method were significant. In all cases examined, highly statistically significant differences were observed (p<0.001) (table 1).
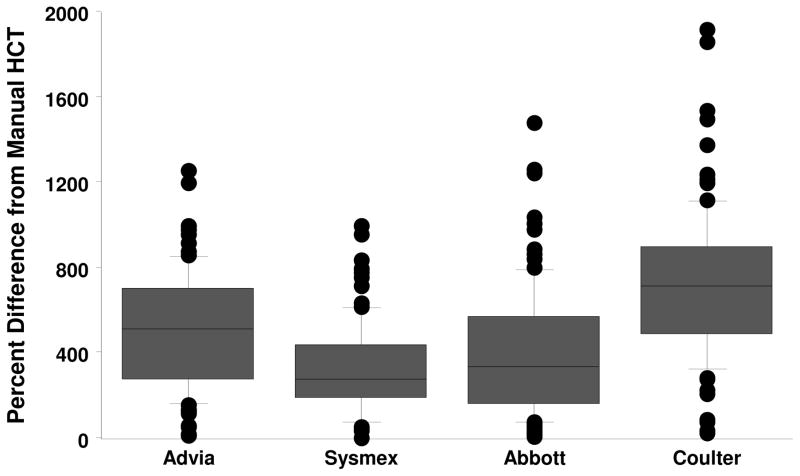
While the mean HCT values between the manual HCT and automated HCT methods were different, percent difference between the values were calculated to represent the relative magnitude of the observed difference (figure 2). As compared to the manual HCT method the mean percent differences were found to be: Advia HCT 508%; Sysmex HCT 323%; Abbott HCT 401%; and Coulter 723%. In all cases, the automated HCT methods produced a statistically higher (p<0.001) HCT value, thus a positive bias was observed.
Figure 2.
Hematocrit percent difference of automated analyzer as compared to the manual HCT test for HPC(A) products
A clinical model for a typical HPC(A) product and various incompatible RBC cut-off thresholds (10, 15, 20, 30, 40 mL) was developed to determine the sensitivity, specificity, positive predictive value, and negative predictive value for the automated HCT methods (table 2).
Table 2.
Performance characteristics of each hematocrit determination method when applied to the clinical model.
| Incompatible RBC Cut-off limit | ||||||
|---|---|---|---|---|---|---|
| 10 mL | 15 mL | 20 mL | 30 mL | 40 mL | ||
| Advia | Sensitivity | 100% | 100% | 100% | ND | ND |
| Specificity | 59% | 89% | 96% | 99% | 99% | |
| Positive Predictive Value | 10% | 23% | 33% | 0% | 0% | |
| Negative Predictive Value | 100% | 100% | 100% | 100% | 100% | |
| Preventable RBC reductions | 39% | 10% | 4% | 1% | 1% | |
| Sysmex | Sensitivity | 100% | 100% | 100% | ND | ND |
| Specificity | 82% | 95% | 98% | 98% | 99% | |
| Positive Predictive Value | 19% | 38% | 50% | 0% | 0% | |
| Negative Predictive Value | 100% | 100% | 100% | 100% | 100% | |
| Preventable RBC reductions | 18% | 5% | 2% | 2% | 1% | |
| Abbott | Sensitivity | 100% | 100% | 100% | ND | ND |
| Specificity | 80% | 91% | 97% | 98% | 99% | |
| Positive Predictive Value | 17% | 27% | 40% | 0% | 0% | |
| Negative Predictive Value | 100% | 100% | 100% | 100% | 100% | |
| Preventable RBC reductions | 20% | 8% | 3% | 2% | 1% | |
| Coulter | Sensitivity | 100% | 100% | 100% | ND | ND |
| Specificity | 28% | 80% | 92% | 96% | 99% | |
| Positive Predictive Value | 6% | 14% | 20% | 0% | 0% | |
| Negative Predictive Value | 100% | 100% | 100% | 100% | 100% | |
| Preventable RBC reductions | 69% | 20% | 8% | 4% | 1% | |
The sensitivity of all of the automated analyzers was 100% for cut off limits of 20 mL and below, however the sensitivity of the analyzers at limits of 30mL and above could not be determined due to the fact that no HPC(A) products tested contained that high an amount of RBCs. Each of the analyzers were shown to have a specificity of 92% or greater when the RBC incompatible cut off limits are established at 20mL and greater. At more stringent RBC cut-offs (10 and 15 mL), there is a marked decrease in the specificity of the automated analyzers with values as low as 28% at the lowest limit of 10mL (Coulter vs manual HCT method). Additionally, if the manual HCT method were used as a confirmatory test for automated HCT values which exceed the incompatible RBC thresholds, the resulting percentage of preventable RBC reduction procedures was calculated (table 2). At the most restrictive threshold (10 mL), the percentage of preventable RBC reduction procedures ranges from 18% up to 69%.
DISCUSSION
Commercially available clinical hematology analyzers for patient testing are used routinely as part of the characterization process for HPC products due to ease of use and rapid results. Because the hematocrit readings are being obtained from analyzers which are not designed for samples with supraphysiologic nucleated cell densities (200–400×10E3 nucleated cells/mcL), there is more inherent inaccuracy in the HCT measurement due to it being a calculated measurement which does not take into account the marked shift in cell population proportions. If a HPC product is found to have an unacceptably high level of ABO-incompatible RBCs then RBC reduction or a change in infusion strategy (e.g. split infusions over 4 hours) should be invoked8, 9. A consequence of RBC reduction is a loss of nucleated cells and thereby HPCs due to the additional manipulations10. Patients may also require additional preparation when receiving an ABO-incompatible product in the form of large volume intravenous hydration before and after the infusion as well as other interventions, all of which may delay the cell infusion.
Accurate RBC quantification is also important for pediatric patients receiving unwashed cryopreserved HPC(A) infusions. Upon a freeze/thaw cycle of HPC(A), considerable amounts of hemolysate can be generated because DMSO/HSA-based cryoprotectants do not adequately protect RBCs in HPC(A). In situations when there is low HPC content, a wash procedure would be avoided by performing a bedside thaw infusion. Thus in pediatric autologous bedside thaw infusions, accurate assessment of RBC content would help to guide clinicians as to the risks associated with infusion of unwashed products for this patient population. Another clinically relevant instance when the RBC content of HPC(A) is of crucial importance is during performance of immunomagnetic cell selection. Our institutional experience shows that products with HCT of >4% by the spun hematocrit method exhibit excessive clumping during various stages of the cell selection process. We cannot attribute this clumping to other “usual suspects” such as high granulocyte content since we have cases where the HCT was >4% with typical granulocyte content which also exhibited clumping.
We observed that the automated clinical hematology analyzers variably but consistently overestimate the HPC product hematocrit especially when the hematocrit is low(0.5–3.0%; figure 1). The overestimation was found to be highly significant in all comparisons with the manual HCT method(table 1). A high nucleated cell count in relation to a very low RBC count results in biased RBC-related parameters when HPC products are analyzed by automated hematology instruments. The markedly lower proportion of RBC content in HPC(A) as compared to whole blood may lead to falsely elevated RBC counts and MCV measurements which results in a positive bias in the calculated HCT value(HCT=RBC*MCV). Conversely, the manual spun HCT method is not affected by the skewed proportions of cells in the HPC product.
To better appreciate the clinical significance of these laboratory findings, a clinical scenario with a fixed volume product(250 mL) was developed to model what the resultant levels of incompatible RBCs would be present in the HPC(A) products. Multiple incompatible RBC cutoff thresholds were tested(10–40 mL). The preventable percentage of RBC reductions was found to be high at the most restrictive threshold of 10 mL, ranging from 18%–69% (table 2), which suggests the greatest variability in nucleated cell analysis and results in a skewed HCT value. With this knowledge, a proposed algorithm for laboratory practice would be to continue determining the HCT for HPC(A) via the more convenient and rapid automated HCT methods, however in cases when the RBC threshold is exceeded, an additional sample of the product should be tested with the manual HCT method to quantify the true RBC content of the product. This tiered approach harnesses the advantages of both methodologies with minimal modification to preexisting work flows. In many cases, as demonstrated in the preventable RBC reduction percentages (table 2), many resource and time intensive procedures could be avoided.
Footnotes
Reprints: Will not be available from the author
Conflicts of Interest: Authors STA and SMM have received honoraria from Abbott Laboratories in association with presenting data from projects unrelated to the content of the manuscript submitted to TRANSFUSION. All other authors have no conflicts of interest relevant to the manuscript submitted to Transfusion.
Sources of Support: None
References
- 1.Wingard JR, Wirk B, Brown R. Hematopoietic stem cell transplantation: An overview. In: McLeod BC, Szczepiorkowski ZM, Weinstein R, Winters JL, editors. Apheresis: Principles and practice. Bethesda: AABB Press; 2010. pp. 465–481. [Google Scholar]
- 2.Buckner CD, Clift RA, Sanders JE, Williams B, Gray M, Storb R, Thomas ED. Abo-incompatible marrow transplants. Transplantation. 1978;26:233–238. doi: 10.1097/00007890-197810000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Bensinger WI, Buckner CD, Thomas ED, Clift RA. Abo-incompatible marrow transplants. Transplantation. 1982;33:427–429. doi: 10.1097/00007890-198204000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Rowley SD, Donato ML, Bhattacharyya P. Red blood cell-incompatible allogeneic hematopoietic progenitor cell transplantation. Bone Marrow Transplant. 2011;46:1167–1185. doi: 10.1038/bmt.2011.135. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez-Aguirre CH, Gomez-De-Leon A, Alatorre-Ricardo J, Cantu-Rodriguez OG, Gonzalez-Llano O, Jaime-Perez JC, Mancias-Guerra C, Flores-Jimenez JA, Gomez-Almaguer D. Allogeneic peripheral blood stem cell transplantation using reduced-intensity conditioning in an outpatient setting in abo-incompatible patients: Are survival and graft-versus-host disease different? Transfusion. 2014;54:1269–1277. doi: 10.1111/trf.12466. [DOI] [PubMed] [Google Scholar]
- 6.Yazer MH, Triulzi DJ. Immune hemolysis following abo-mismatched stem cell or solid organ transplantation. Current opinion in hematology. 2007;14:664–670. doi: 10.1097/MOH.0b013e3282e9a576. [DOI] [PubMed] [Google Scholar]
- 7.Longanbach S, Miers M. Automated blood cell analysis. In: Keohane E, Smith L, Walenga J, editors. Rodak’s hematology: Clinical principles and applications. St. Louis: Elsevier Saunders; 2015. pp. 208–234. [Google Scholar]
- 8.Davis-Sproul J, Haley NR, McMannis JD. Collecting and processing marrow products for transplantation. In: Roback JD, Grossman B, Harris T, Hillyer CD, editors. Aabb technical manual. Bethesda: AABB; 2011. pp. 779–799. [Google Scholar]
- 9.Delaney M, Haspel RL. Thawing and infusing cellular therapy products. In: Areman EM, Loper K, editors. Cellular therapy: Principles, methods, and regulations. Bethesda, MD: AABB; 2009. pp. 375–382. [Google Scholar]
- 10.Crosskell M, Loper K, McKenna DH. Basic cellular therapy manufacturing procedures. In: Areman EM, Loper K, editors. Cellular therapy: Principles, methods, and regulations. Bethesda, MD: AABB; 2009. pp. 303–329. [Google Scholar]