Abstract
Background
Understanding the effect of blood donation and iron supplementation on iron balance will inform strategies to manage donor iron status
Study Design and Methods
215 donors were randomized to receive ferrous gluconate daily (37.5 mg iron) or no iron for 24 weeks after blood donation. Iron stores were assessed using ferritin and soluble Transferrin Receptor. Hemoglobin iron was calculated from total body hemoglobin. Total Body Iron (TBI) was estimated by summing iron stores and hemoglobin iron.
Results
At 24 weeks, TBI in donors taking iron increased by 281.0 mg (95% Confidence Interval [CI]: 223.4, 338.6) compared to pre-donation, while TBI in donors not on iron decreased by 74.1 mg (CI: −112.3, −35.9), p<0.0001, iron vs. no iron. TBI increased rapidly following blood donation with iron supplementation, especially in iron depleted donors. Supplementation increased TBI compared to controls during the first 8 weeks after donation: 367.8 mg (CI: 293.5, 442.1) versus −24.1 mg (CI: −82.5, 34.3) for donors with baseline ferritin ≤26 ng/mL; and 167.8 mg (95%CI: 116.5, 219.2) versus −68.1 mg (CI: −136.7, 0.5) for donors with baseline ferritin >26 ng/mL. 88% of the benefit of iron supplementation occurred during the first 8 weeks after blood donation.
Conclusion
Donors on iron supplementation replaced donated iron while donors not on iron did not. Eight weeks of iron supplementation provided nearly all of the measured improvement in TBI. Daily iron supplementation after blood donation allows blood donors to recover the iron loss from blood donation and prevents sustained iron deficiency.
Keywords: Blood donation, Iron depletion, iron supplementation
Introduction
Blood donation is known to reduce body iron stores, and frequent blood donors are often iron depleted.1,2 A number of studies have demonstrated that iron supplementation can reverse the iron depletion and reduce hemoglobin deferrals associated with blood donation.3,4 Our group has recently documented that use of daily iron supplements is necessary for efficient recovery of donor hemoglobin and iron to support blood donation.5 To date studies have separately assessed the effect of blood donation on iron stores, as assessed by serum or plasma ferritin, and hemoglobin, as assessed by venous hemoglobin concentrations. In order to more fully understand the dynamics of iron balance into and out of the body, however, it is necessary to assess these two major iron compartments together. We propose here to use the concept of Total Body Iron (TBI) as a novel and comprehensive model to quantify iron following blood donation. We applied this model to data collected in the Hemoglobin and Iron Recovery Study5 (HEIRS); this allowed us to carefully describe iron balance after blood donation, and the effects of iron supplementation on iron recovery.
Materials and Methods
The Hemoglobin and Iron Recovery Study was conducted, as previously reported, at 4 US blood centers.5 An eligible participant was a volunteer, repeat blood donor ≥18 years who successfully donated a whole blood unit on the day of enrollment, but not in the prior 4 months. Participants agreed not to use iron supplements outside of the study. Participants returned 3–8 days later for their first post-donation visit and classification of index donation ferritin results. Participants were classified for pre-donation iron status as iron-depleted (“low ferritin,” ≤ 26 ng/ml) and iron-replete (“higher ferritin,” > 26 ng/ml) based on the ferritin measurement at enrollment. Randomization was conducted within 8 strata, based on ferritin classification, gender, and age. If a participant met stratification requirements, based on their stratum remaining open for enrollment, they were randomized (in equal numbers within each stratum) to receive either daily oral ferrous gluconate 325 mg containing 37.5 mg of elemental iron for 24 weeks, or no treatment. During a 24 week follow-up period, they provided periodic blood samples.
Participants Studied
215 donors were randomized and followed further; 111 (51 low ferritin and 60 higher ferritin) were assigned to ferrous gluconate, and 104 (50 low ferritin and 54 higher ferritin) were assigned to no supplemental iron. Twelve participants (9 on iron; 3 not on iron) withdrew from HEIRS before completion of all the visits, and 10 additional participants (6 on iron; 4 not on iron) were missing hemoglobin values on 2 or more follow-up visits, leaving 193 participants analyzed for all parameters (96 on iron; 97 not on iron).
Available Samples and Lab Methods
Plasma ferritin (Advia Centaur), and complete blood count (CBC) were measured on EDTA blood samples collected prior to index donation and at day 3–8 and 2, 4, 8, 12, 16, and 24 weeks following donation. Soluble transferrin receptor (sTfR, Roche Tina-quant) was tested at the pre-donation and 24 week visit only.1
Iron Stores
Iron stores (mg/kg bodyweight) were calculated at enrollment and at the 24 week visits, where both ferritin and sTfR were available, from the reported relationship between iron stores and the log ratio of serum transferrin receptor/ferritin: Iron Stores (mg/kg) = − [log(sTfR/ferritin) – 2.8229]/0.1207.6 Plasma ferritin values were increased by 5% to better reflect serum ferritin values required for use in the formula (Assay Manual, ADVIA Centaur, Siemens Healthcare Diagnostics, 2012). Values for the Roche sTfR were converted to the original (“Flowers”)7 sTfR values used in Cook from a previous comparison of the two assays according to the formula: Flowers sTfR =1.5 Roche sTfR + 0.35 mg/L.8
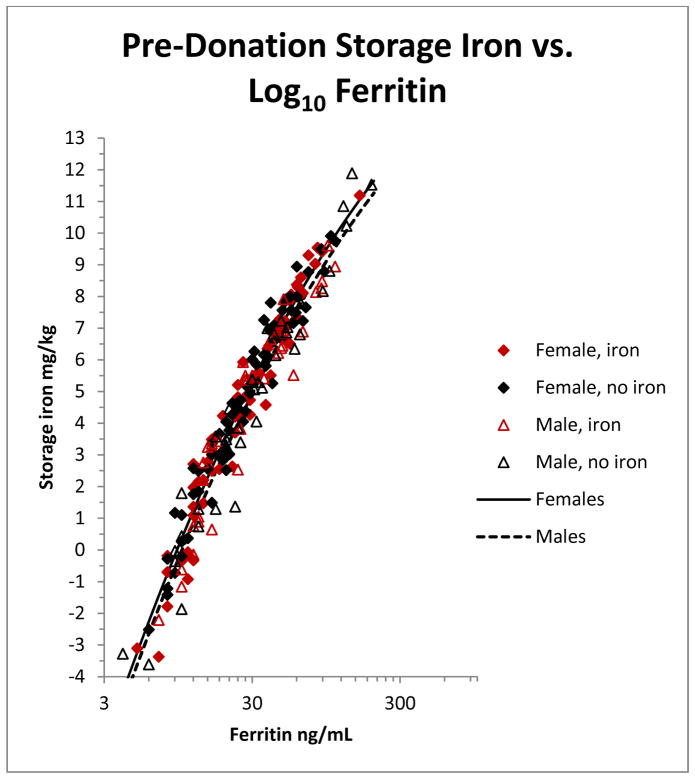
To evaluate TBI at intermediate points during follow-up, we generated estimates of iron stores at all 7 follow-up visits. These were obtained by fitting a regression of iron stores (calculated as above at enrollment) on log10 ferritin using enrollment data from the 193 participants included in the analysis (Figure 1). A similar regression formula derived from the same subjects’ 24-week samples gave nearly identical results (data not shown). Iron stores at all 7 follow-up visits were then estimated from this regression and TBI was calculated as described below.
Figure 1.
Quadratic regression of pre-donation storage iron (mg/kg) against pre-donation ferritin (ng/mL) for 193 subjects enrolled in the HEIRS study. Storage iron was determined from ferritin and sTfR as described in Methods. The male regression is indicated by a solid line and female regression by a dashed line. The HEIRS randomization assignment of the individual donors (see Methods) is shown by the symbols indicated in the Figure.
Total Body Iron (TBI) calculations
We calculated TBI as Total Body Iron (TBI) = red cell iron (mg) +iron stores (mg/kg)*weight (kg). To determine red cell iron (i.e., iron in hemoglobin), we estimated that hemoglobin contained 3.4 mg iron/gm.9 We calculated estimated blood volume (EBV) from height, weight, and gender using the Nadler formula 10 and then calculated red cell iron from EBV and venous hemoglobin (per L) as EBV*Hgb*0.91.11
Changes in Total Body Iron
Changes in TBI were calculated over the entire 24 week period of observation using pre-donation TBI values and comparing them to 24 week TBI values, as well as over three consecutive adjacent intervals: #1: 0 to 8 week sample; #2: 8 week sample to16 week sample; and #3: 16 week sample to 24 week sample. These intervals were chosen in part to reflect the 8 week interdonation interval currently allowed in the US. Using shorter intervals for analysis also led to less precise estimates as these were based on fewer visits, which resulted in an increased proportion of missing data in given intervals. However, because of interest in defining the optimal duration of iron supplementation, a secondary analysis was performed on 4-week intervals during weeks 0–16.
The TBI at the beginning of interval 1 (time 0, i.e., the TBI immediately post-donation) was calculated by taking the pre-donation TBI and subtracting the amount of iron in the donated whole blood. The iron in the donated unit was calculated as pre-donation venous hemoglobin multiplied by donated volume of 525 mL multiplied by 3.4 mg iron/gm hemoglobin.
Statistical Methods and Models Used
Linear models were employed for all statistical comparisons. To examine the effects of treatment (iron or not), baseline ferritin, and other predictors on changes in TBI over the three 8-week intervals we employed a repeated measures model. Reduced forms of the model were employed to analyze subsets of the data, such as the effect of treatment on changes in a single interval. Student’s t-test was employed for comparing two means, such as the mean 24-week change in storage iron in those taking and not taking iron.
We used multiple imputation to account for the manner in which storage iron was calculated from ferritin alone. A separate normally distributed random variate with mean zero and standard deviation 0.80 was added to each estimate of storage iron to account for the residual uncertainty in the estimates. This involved creating 10 data sets with separate imputed values in each, analyzing each of the data sets and then combining the results to obtain final estimates and p-values.
Data analysis took place in SAS/STAT software Version 9.3 of the SAS System for Windows.
Results
Iron in Donated Whole Blood
The iron content of the units of whole blood donated by the 193 participants is shown in Table 1. As expected there is a 9% difference between men and women, reflecting the higher male hemoglobin (men 260 mg iron, women 238.4 mg iron). The median and mean are nearly the same, indicating a symmetric distribution, with a small confidence interval around the mean. Individual donor’s iron losses, however, varied considerably with a range from low to high of nearly 100 mg.
Table 1.
Iron content of whole blood donated by the 193 participants: mg iron/donation
| n | Mean (95% CI) | median | |
|---|---|---|---|
|
|
|||
| Men | 75 | 260.0 (255.1, 264.9) | 260.6 |
| Women | 118 | 238.4 (235.5, 241.3) | 239.2 |
| Total | 193 | 246.8 (243.8, 249.8) | 246.3 |
Total Body Iron – Changes Over 24 Weeks
TBI at enrollment (pre-donation) and at 24 weeks is shown in Table 2. The two treatment groups had nearly identical iron stores and red cell iron pre-donation. As expected, only 15% of TBI was found bound to ferritin as iron stores, with 85% found in the red cell mass. At 24 weeks, TBI in the iron supplemented group increased on average by 281.0 mg (95% Confidence Interval [CI]: 223.4, 338.6) compared to pre-donation. Most of this increase occurred in the iron stores, with a smaller increase in red cell iron. Since on average 247 mg iron was lost through a blood donation (Table 1), these donors not only replenished the hemoglobin iron lost during their donation but added another red cell equivalent to their TBI. In contrast, the group not on iron decreased TBI by 74.1 mg (Mean −74.1 mg; CI: −112.3, −35.9), p<0.0001, iron vs. no iron, the decrease equally divided between iron stores and red cell iron. These donors thus failed to replace the red cell iron lost during their donation, even after 24 weeks.
Table 2.
Total Body Iron (mg) at enrollment and change at final visit, Means (95% CI)
| Iron (n=96) | No Iron (n=97) | |||
|---|---|---|---|---|
| pre-donation | 24-week change (Δ) | pre-donation | 24-week change (Δ) | |
|
| ||||
| iron stores | 344.8 | 219.4 | 347.6 | −35.1 |
| 95% CI | (287.1, 402.5) | (179.4, 259.4) | (287.1, 408.0) | (−66.1, −4.1) |
|
|
||||
| red cell iron | 2,038.4 | 61.6 | 2,034.1 | −39.0 |
| 95% CI | (1,943.1, 2,133.6) | (35.7, 87.5) | (1,936.8, 2,131.4) | (−65.7, −12.3) |
|
|
||||
| Total Body Iron | 2,383.1 | 281.0 | 2,381.7 | −74.1 |
| 95% CI | (2,252.8, 2,513.5) | (223.4, 338.6) | (2,251.2, 2,512.1) | (−112.3, −35.9) |
|
|
||||
All Δ values, p<0.0001, iron vs. no iron
Regression for Iron Stores at Interim Visits - Based Solely on Ferritin
In order to track changes in TBI, we developed a regression to estimate iron stores based solely on ferritin, since sTfR was not available at interim bleeds. Figure 1 plots a quadratic regression of iron stores against log10 ferritin where stored iron was calculated from ferritin and sTfR, as described above.
The formula for the regression is:
where S is storage iron in mg/kg, F is ferritin in ng/ml, G=0 for men and 1 for women. The model explained most of the variance in stored iron (r2=0.95). Analysis of residuals showed an excellent fit to the data throughout the range.
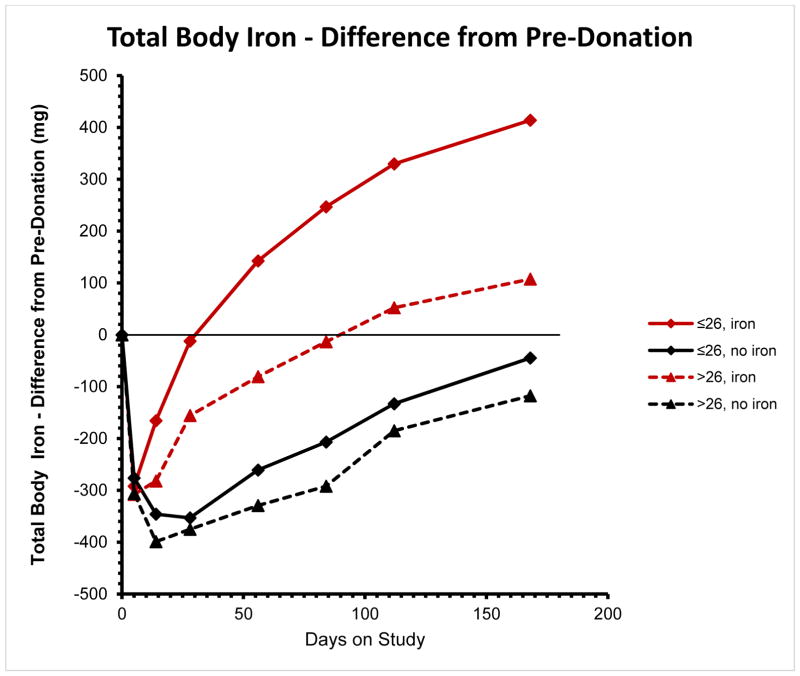
Figure 2 shows the average change in TBI over time in the 4 subgroups (low/high ferritin; iron/no iron treatment) within HEIRS. All groups showed an initial drop at the Day 3–8 sample, reflecting the initial blood donation, with recovery shown by later follow-up samples. Within 4–13 weeks, depending on baseline ferritin levels, donors receiving iron supplements had replaced the donated iron. For donors on iron who were iron depleted before donation, replacement occurred in 4 weeks (to pre-donation iron deficient levels), but then iron absorption continued so that by 24 weeks these donors had absorbed an additional 400 mg of iron (Figure 2). For donors with higher ferritin levels at baseline, recovery to their pre-donation iron replete status was slower but still complete by 13 weeks. Donors not on iron supplementation did not completely recover TBI by 24 weeks – although the low ferritin group nearly did so; however, their “recovery” was to an iron deficient level.
Figure 2.
Change in Total Body Iron (TBI) (mg) from pre-donation TBI at 7 post-donation visits from Day 3–8 to 24 weeks after donation (See Methods). Red lines and symbols represent donors randomized to oral iron supplements. Black lines and symbols represent donors randomized to no iron. Solid lines and diamonds indicate donors whose pre-donation ferritin was ≤26 ng/mL. Dotted lines and triangles represent donors whose pre-donation ferritin was > 26 ng/mL.
Kinetics of Recovery of Total Body Iron after Whole Blood Donation
Table 3 shows the change in TBI over three consecutive adjacent 8 week intervals after blood donation. Subjects receiving iron increased TBI significantly compared to subjects not on iron during the first 8 weeks following blood donation: a 367.8 mg increase (CI: 293.5, 442.1) vs. a decrease of 24.1 mg (Mean change −24.1; CI: −82.5, 34.3) (p < 0.0001, iron vs. no iron) for those with baseline ferritin ≤ 26 ng/mL; and a 167.8 mg increase (CI: 116.4, 219.2) vs. a decrease of 68.1 mg (Mean change −68.1 ; CI: −136.7, 0.5) (p < 0.0001, iron vs. no iron) for donors with baseline ferritin > 26 ng/mL. However, there was no statistically significant effect of iron supplementation compared to no iron in either ferritin group during intervals 2 and 3.
Table 3.
Change in Total Body Iron (mg/interval) at three consecutive adjacent intervals after blood donation: Mean (95% CI) change for donors with at least 6 follow-up hemoglobin values. P: p value for comparing changes among intervals
| Weeks after donation | interval | ≤26 ng/mL | >26 ng/mL | ||
|---|---|---|---|---|---|
|
| |||||
| Iron | No Iron | Iron | No Iron | ||
| 0 to 8 weeks | 1 | 367.8 | −24.1 | 167.8 | −68.1 |
| 95% CI | (293.5, 442.1) | (−82.5, 34.3) | (116.4, 219.2) | (−136.7, 0.5) | |
| 8 to 16 weeks | 2 | 183.9 | 138.3 | 129.8 | 140.9 |
| 95% CI | (132.2, 235.6) | (87.7, 188.9) | (81.8, 177.8) | (75.6, 206.2) | |
| 16 to 24 weeks | 3 | 90.0 | 81.6 | 66.0 | 65.2 |
| 95% CI | (44.1, 135.9) | (27.9, 135.3) | (13.9, 118.1) | (4.6, 125.8) | |
|
| |||||
| P | overall | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| 1 vs 2 | 0.0001 | 0.0003 | 0.38 | 0.0006 | |
| 2 vs 3 | 0.0309 | 0.19 | 0.13 | 0.17 | |
| 1 vs 3 | <0.0001 | 0.0124 | 0.0064 | 0.0060 | |
| Time Since Donation | interval | <26 ng/mL | >26 ng/mL |
|---|---|---|---|
| 0 to 8 weeks | 1 | <0.0001 | <0.0001 |
| 8 to 16 weeks | 2 | 0.19 | 0.79 |
| 16 to24 weeks | 3 | 0.81 | 0.98 |
For those donors on iron, the increase in TBI was highest in interval 1. For those donors on iron with baseline ferritin ≤ 26 ng/L, TBI in intervals 2 and 3 had smaller increases than in interval 1. For those donors on iron with baseline ferritin > 26 ng/mL, TBI in successive intervals were not statistically different, but the increase in TBI in interval 1 was greater than that in interval 3.
Donors not on iron experienced a decrease in TBI during interval 1 - beyond that already accounted for from the blood donation. This observation is reflected in the TBI recovery curves for these donors shown in Figure 2. TBI continued to drop during the first two weeks after blood donation and then flattened out at 4 weeks, partially recovering only between the 4 and 8 week sample. As shown in Table 3 the decrease in interval 1 was reversed by a substantial increase in TBI in interval 2, which was not statistically different from that observed in Interval 3.
In order to better define the optimal duration of iron supplementation, the above analysis was repeated for successive 4 week intervals over the first 16 weeks: 0–4 weeks, 4–8 weeks, 8–12 weeks, and 12–16 weeks. The benefit of iron decreased in each successive 4 week interval and no benefit was shown during weeks 12–16. (Table 4) A statistically significant improvement in TBI due to iron supplementation could only be demonstrated in the 0–4 week interval. In initially iron-depleted donors 88% of the benefit of iron occurred in the first 8 weeks after blood donation, and 87% in the iron-replete donors.
Table 4.
Benefit of Iron supplementation on TBI over 4-week intervals after blood donation: Benefit from iron is shown as differences in change in Total Body Iron (mg/interval) between iron supplemented and no iron donors at four consecutive 4-week intervals after blood donation.
| Interval | Ferritin ≤26 ng/mL | Ferritin > 26 ng/mL | ||
|---|---|---|---|---|
| Benefit of iron | % of Total Iron Benefit** | Benefit of iron | % of Total Iron Benefit** | |
| 0–4 weeks | 323.1 mg* | 73% | 215.7 mg* | 78% |
| 4–8 weeks | 65.6 | 15% | 24.4 | 9% |
| 8–12 weeks | 53.8 | 12% | 37.1 | 13% |
| 12–16 weeks | (11.8)+ | NA | (46.1) + | NA |
P <0.0001, TBI in iron group compared to no iron group. All others are NS.
Total Iron Benefit is the total of weeks 1–12, and ignores weeks 12–16
TBI increased in the no iron group more than in the iron group
Discussion
Our approach to estimating body iron in blood donors is to focus only on the two major compartments, hemoglobin and storage iron. For convenience we have termed the total of these two compartments Total Body Iron (TBI). While this measurement ignores minor iron compartments such as transferrin, myoglobin, and iron cofactors in enzymes and cytochromes, it is ideally suited to assess changes in body iron over time, since any change in minor compartments is likely to be small in early iron depletion. By combining changes in TBI with a precise estimator of iron lost in a blood donation, it is possible to assess the effect of iron supplements expressed as mg per time interval. This approach allows a dynamic look at iron balance in blood donors.
We have shown that TBI increases from pre-donation levels in iron supplemented donors by an average of 281 mg/dL over 24 weeks. A unit of red cells was found to represent approximately 247 mg of iron. Since this index blood donation needs replacement to maintain TBI at pre-enrollment levels, this means that approximately 500 mg of iron was absorbed from the GI tract (from iron supplements and dietary iron) over and above that necessary to replace insensible iron losses occurring through loss of skin, hair, and GI mucosa. Put another way, the donor absorbed the iron to produce 2 units of red cells, the one donated and another one. In the absence of iron, however, donors lost 74 mg of iron from pre-enrollment levels; put another way, over 24 weeks the non-supplemented donors could only replace 2/3 of the index donation’s iron. Since diet and insensible iron losses would not be expected to differ between the iron supplemented donors and the no iron donors, the differences in TBI between the two groups are explained solely by the iron supplements. By combining the gain of 281 mg in the supplement group with the loss of 74 mg in the no iron group, the difference of 355 mg of iron is shown to be attributable to and a benefit of the iron supplements. This represents 5.6% of the total iron content in the 24 weeks of provided iron supplements.
By plotting TBI over sequential time periods after blood donation, the benefits of supplemental iron in recovery from donation are clear. The recovery curves in Figure 2 resemble the hemoglobin recovery curves that were observed for the same groups in HEIRS,5 which is expected given that the majority of TBI is represented by hemoglobin (Table 2). The more rapid recovery in the initially iron depleted donors is likely due to increased absorption of supplemental iron seen in iron depleted individuals as well as higher erythropoietic drive due to their relative anemia pre-donation. In comparison, recovery of TBI in the no iron groups was delayed and incomplete, even by 24 weeks.
Importantly, by estimating the change in TBI over consecutive adjacent 8 week intervals, we have demonstrated that the benefit of iron supplementation is most clearly shown during the first 8 weeks after donation. (Table 3 and 4) During this period, and especially in the first 4 weeks, the rate of increase in TBI is quite high (e.g., 3–6 mg/day), with higher rates in supplemented donors with pre-enrollment iron deficiency (ferritin ≤26 ng/mL). There was some suggestion from the data in Table 3 that continued iron supplements beyond 8 weeks might benefit some donors. There is a higher rate of TBI increase in Interval 2 (8–16 weeks) versus Interval 3 (16–24 weeks) particularly in the iron-depleted group (p=0.03). To analyze this further, the increase in TBI during successive 4-week intervals was analyzed over the first 16 weeks (Table 4). Perhaps because the statistical power of this shorter interval analysis was reduced compared to the 8-week interval analysis, a statistically significant befit of iron could only be demonstrated during the first 4 weeks after donation. An analysis of the contribution of each successive interval to the overall iron benefit showed that 87–88% of the benefit of iron supplementation occurred during the first 8 weeks after donation.The rapid increase in TBI in iron-supplemented donors contrasts with small decreases in TBI in unsupplemented donors over the first 8 weeks after donation. The reasons for the first 8-week decrease in TBI in this group are not clear. They may relate to changes in iron compartments that are not included in TBI. In this model, TBI is the sum of iron stores and red cell (hemoglobin) iron but does not include minor compartments such as transport (i. e., transferrin), bone marrow, and cytochrome iron. Changes in iron in these minor compartments would not be detected in the observed TBI compartments, thus distorting the apparent changes in TBI shown in Table 3. For example, it is possible that the expected expansion of the red cell precursors in the bone marrow following donation could account for post-donation undercounting of body iron in the TBI calculation. Although this would be expected in all groups, it is possible this effect is overwhelmed by the positive effect on iron supplements, so that it is observed only in the non-supplemented donors. Finally, the observations may be due to other small measurement inaccuracies in the model, which are apparent only in the unsupplemented donors, being hidden by the effect of the iron supplementation in the iron groups. Further validation of this model would require the use of radiolabelled iron studies, which would not be feasible in the context of a large study such as HEIRS. However, the model presented here illustrates the dynamics of iron balance in blood donors and the substantial impact of blood donation on body iron.
Given the delay in recovery of the hemoglobin iron compartment in donors who were not taking a supplement, it is no surprise that studies have reported that donors who are deferred for low hemoglobin (most of whom are iron depleted) 12 improve their chances of successfully donating very little by waiting longer between donations.13 For example, a deferred donor with hemoglobin of 12.0 gm/dl with a 60% chance of passing the fingerstick hemoglobin screen after waiting 1–2 weeks until their next donation attempt only improved to 70% passing by waiting 21–24 weeks between deferral and return. From the data presented here and earlier reported results,5 it is now clear that oral iron supplementation at small, well-tolerated doses is necessary to recover iron and hemoglobin levels promptly following whole blood donation and to maintain iron homeostasis in frequent blood donors. Further, the model provides important data indicating that 8 weeks of iron supplementation following blood donation is an effective and efficient way to replenish iron lost from donation. Since it is increasingly apparent that routine iron supplementation of many or most blood donors is needed, it is very important to understand how long after a whole blood donation oral iron supplementation should be offered or recommended. Our data clearly indicate that no more than 8 weeks of oral iron supplementation is necessary. This information should be useful to US blood centers that have implemented or are currently evaluating how best to implement oral iron supplementation among their donors. However, further blood center operational trials to optimize oral iron supplementation methods and to determine the acceptability of and compliance with various methods for donors are needed.
There are study limitations that may affect the generalizability of our findings. First, whereas HEIRS studied an iron dose of 37.5 mg per day, many center-proposed operational protocols anticipate a daily iron dose of 19 mg, either as a supplement or included in a multivitamin. Second, we found excellent compliance from the paid participants in this study. It is possible that reduced compliance in more routine settings may affect the ingested iron dose over the recommended supplementation period. Consequently it is possible that 19 mg a day should be recommended for longer than 8 weeks in routine donor management. However, the very brisk iron response shown in our study in the first 4 weeks indicates that 8 weeks would likely be an adequate recommended supplementation period, even for the reduced dose of 19 mg per day or a somewhat reduced compliance. Findings from STRIDE16 reinforce the likelihood that an 8 week supplementation period using 19 mg would probably be effective since no difference was noted in response of donor iron and hemoglobin parameters between doses of 19 mg and 38 mg per day, when given to frequent whole blood donors for 8 weeks after a whole blood donation. Finally, we only evaluated responses to iron supplementation after a whole blood donation and did not address iron management following double red cell donation or following multiple plateletpheresis donations. The data presented here do show however, that the response to oral supplemental iron is immediate and brisk, particularly in iron depleted donors, and our findings should be considered in planning and studying iron supplementation interventions in these other populations.
The assessment of Total Body Iron in HEIRS represents a novel approach to studying iron balance which is also applicable to a variety of other clinical and community settings. Most of the literature has focused on the storage iron component of TBI.6, 14 While the storage iron approach works well in populations with relatively stable hemoglobin levels,15 it is not a comprehensive assessment of iron balance in a blood donation setting, where lowering of the hemoglobin is expected, with or without subsequent recovery and where hemoglobin represents the major reservoir of iron in the body. The current TBI approach can be used in other cross-sectional and longitudinal studies of blood donor iron depletion and in trials of iron supplementation. Applying this model to other studies should reveal additional important facets of donor iron management, leading to improved donor well-being in addition to enhancing the blood supply.
Acknowledgments
Financial support: This work was supported by NHLBI contracts HHSN268201100001I, HHSN268201100002I, HHSN268201100003I, HHSN268201100004I, HHSN268201100005I, and HHSN268201100006I
The investigators thank the research staff responsible for donor enrollment, follow up, and data collection at the four REDS-III hubs (Walt Bialkowski, Shanita Thomas, Zoya Udee, Pam D’Andrea, Barb Kletter, Chris Sayre, Anne Guiltinan, Daniel A. Hindes, Monalissa Doane), the staff responsible for protocol and data management at the coordinating center (Christopher Siege, Anne-Lyne McCalla), and the staff responsible for laboratory testing and data management at the central laboratory (BSRI Viral Reference Laboratory and Repository Core) for their commitment to the study and skillful support. All staff received contractual financial support. The study sponsor, NHLBI, participated in the design, approval, and conduct of the study; the collection, management, analysis, and interpretation of the data; and the preparation, review, and approval of the manuscript. RGC and DJB had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The NHLBI Recipient Epidemiology Donor Evaluation Study - III (REDS-III), domestic component, is the responsibility of the following persons:
Hubs
A.E. Mast and J.L. Gottschall, BloodCenter of Wisconsin (BCW), Milwaukee, WI
D.J. Triulzi and J.E. Kiss, The Institute For Transfusion Medicine (ITXM), Pittsburgh, PA
E.L. Murphy and E.W. Fiebig, University of California, San Francisco (UCSF), San Francisco, CA
E.L. Snyder Yale University School of Medicine, New Haven, CT and R.G Cable, American Red Cross Blood Services, Farmington CT
Data coordinating center
D. J. Brambilla and M. T. Sullivan, RTI International, Rockville, MD
Central laboratory
M.P. Busch and P.J. Norris, Blood Systems Research Institute, San Francisco, CA
Publication Committee Chairman
R. Y. Dodd, American Red Cross, Holland Laboratory, Rockville, MD
Steering Committee Chairman
S. H. Kleinman, University of British Columbia, Victoria, BC, Canada
National Heart, Lung, and Blood Institute, National Institutes of Health
S. A. Glynn and A.M. Cristman
Footnotes
Protocol available at: https://reds-iii.rti.org/ref/REDS_III_HEIRS_Protocol.pdf
ClinicalTrials.gov Identifier: NCT01555060
Reprints will not be available from the author
Conflict of Interest: AEM has received honoraria and research grant funding from Novo Nordisk. The other authors have no competing interests.
References
- 1.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: analysis of enrollment data from the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2011;51(3):511–22. doi: 10.1111/j.1537-2995.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cable R, Glynn S, Kiss J, et al. Iron deficiency in blood donors: the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2012;52:702–11. doi: 10.1111/j.1537-2995.2011.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith GA, Fisher SA, Doree C, et al. Oral or parenteral iron supplementation to reduce deferral, iron deficiency and/or anaemia in blood donors. Cochrane Database Syst Rev. 2014;7:CD009532. doi: 10.1002/14651858.CD009532.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radtke H, Tegtmeier J, Rocker L, et al. Daily doses of 20 mg of elemental iron compensate for iron loss in regular blood donors: a randomized, double-blind, placebo-controlled study. Transfusion. 2004;44(10):1427–32. doi: 10.1111/j.1537-2995.2004.04074.x. [DOI] [PubMed] [Google Scholar]
- 5.Kiss JE, Brambilla D, Glynn SA, et al. Oral iron supplementation after blood donation: a randomized clinical trial. Jama. 2015;313(6):575–83. doi: 10.1001/jama.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101(9):3359–64. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]
- 7.Flowers CH, Skikne BS, Covell AM, et al. The clinical measurement of serum transferrin receptor. The Journal of laboratory and clinical medicine. 1989;114(4):368–77. [PubMed] [Google Scholar]
- 8.Pfeiffer CM, Cook JD, Mei Z, et al. Evaluation of an automated soluble transferrin receptor (sTfR) assay on the Roche Hitachi analyzer and its comparison to two ELISA assays. Clinica chimica acta; international journal of clinical chemistry. 2007;382(1–2):112–6. doi: 10.1016/j.cca.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Bernhart F, Skeggs L. The Iron Content of Crystallized Human Hemoglobin. Journal of Biological Chemistry. 1943;147(1):19–22. [Google Scholar]
- 10.Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–32. [PubMed] [Google Scholar]
- 11.Chaplin H, Jr, Mollison PL, Vetter H. The body/venous hematocrit ratio: its constancy over a wide hematocrit range. J Clin Invest. 1953;32(12):1309–16. doi: 10.1172/JCI102859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldman M, Uzicanin S, Scalia V, et al. Iron deficiency in Canadian blood donors. Transfusion. 2014;54(3 Pt 2):775–9. doi: 10.1111/trf.12380. [DOI] [PubMed] [Google Scholar]
- 13.Sayers M, Centilli J. Concerning iron balance in blood donors. Transfusion. 2014;54(11):3010–3. doi: 10.1111/trf.12709. [DOI] [PubMed] [Google Scholar]
- 14.Cogswell ME, Looker AC, Pfeiffer CM, et al. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003–2006. Am J Clin Nutr. 2009;89(5):1334–42. doi: 10.3945/ajcn.2008.27151. [DOI] [PubMed] [Google Scholar]
- 15.Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Am J Clin Nutr. 2011;93(6):1312–20. doi: 10.3945/ajcn.110.007195. [DOI] [PubMed] [Google Scholar]
- 16.Mast AE, Bialkowski W, Bryant B, et al. A randomized, blinded, placebo-controlled trial of education and iron supplementation for mitigation of iron deficiency in regular blood donors. Transfusion. 2016 doi: 10.1111/trf.13469. Article first published online: 26 JAN 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]