Abstract
Previous work using transcranial magnetic stimulation (TMS) demonstrated that the right presupplementary motor area (preSMA), a node in the fronto‐basal‐ganglia network, is critical for response inhibition. However, TMS influences interconnected regions, raising the possibility of a link between the preSMA activity and the functional connectivity within the network. To understand this relationship, we applied single‐pulse TMS to the right preSMA during functional magnetic resonance imaging when the subjects were at rest to examine changes in neural activity and functional connectivity within the network in relation to the efficiency of response inhibition evaluated with a stop‐signal task. The results showed that preSMA‐TMS increased activation in the right inferior‐frontal cortex (rIFC) and basal ganglia and modulated their task‐free functional connectivity. Both the TMS‐induced changes in the basal‐ganglia activation and the functional connectivity between rIFC and left striatum, and of the overall network correlated with the efficiency of response inhibition and with the white‐matter microstructure along the preSMA–rIFC pathway. These results suggest that the task‐free functional and structural connectivity between the rIFCop and basal ganglia are critical to the efficiency of response inhibition. Hum Brain Mapp 37:3236–3249, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: concurrent, TMS, fMRI, task‐free, stop‐signal
INTRODUCTION
The ability to stop an ongoing action quickly when it is no longer appropriate is an important part of the human executive control function [Logan and Cowan, 1984; Miyake and Friedman, 2012]. This ability may be significantly impaired with brain disorders and lesions that involve the frontal and basal ganglia system [Aron et al., 2003; Nachev et al., 2007; Sumner et al., 2007; Correa et al., 2010; Dalley et al., 2011; Smith et al., 2011; Sebastian et al., 2012; Benis et al., 2014]. Accumulating evidence indicates that such rapid stopping of an ongoing action relies on the fronto‐basal‐ganglia network, including the right inferior frontal cortex (rIFC) and the right presupplementary motor area (preSMA) [Miller and Cohen, 2001; Sumner et al., 2007; Chambers et al., 2009; Aron, 2011].
Recent work has made significant effort in identifying specific functional roles of the nodes/regions within this inhibitory network. A number of studies have used transcranial magnetic stimulation (TMS), a noninvasive brain stimulation technique [Dayan et al., 2013], to tease apart the causal role of several cortical regions, particularly, the preSMA, and the rIFC during the stopping process [Chambers et al., 2006; Chen et al., 2009; Verbruggen et al., 2010; Cai et al., 2012; Obeso et al., 2013]. These studies typically used a variant of the stop‐signal task (SST), a well‐established experimental paradigm for measuring the ability of stopping an ongoing response (i.e., response inhibition) [Logan and Cowan, 1984; Logan et al., 1984]. In the SST, participants are instructed to respond as quickly as possible to the primary (or “go”) stimuli, but stop/withhold a response when a stop‐signal (either an auditory tone or a visual cue) appeared shortly after the onset of the “go” stimulus in a small proportion of the trials. The efficiency of response inhibition is estimated by the stop‐signal response time (SSRT) (Verbruggen and Logan, 2008]. However, the observed TMS effects on response inhibition are unlikely to be limited to the targeted regions [Obeso et al., 2013; Zandbelt et al., 2013; Watanabe et al., 2015]. Studies applying TMS to the right preSMA or rIFC induced changes in cortical excitability of the primary motor cortex (M1) assessed by motor‐evoked potentials [Mars et al., 2009; Neubert et al., 2010]. Using repetitive TMS (rTMS) over the rIFC or preSMA prior to fMRI scans (i.e., offline rTMS), Zandbelt et al. [2013] and Watanabe et al. [2015] showed that the stimulation altered activation patterns in the basal ganglia and the supplementary motor complex (SMA), and that significant changes in these regions were predictive of the SSRT during the stop‐signal task performance. Although these studies have shown that offline TMS (i.e., stimulation over minutes prior to fMRI) induced changes in network properties over time, to what extent TMS induces immediate changes in patterns of neural activity and task‐free functional connectivity within the fronto‐basal‐ganglia network remains unknown.
The objective of this study was to examine immediate online changes in neural activity and task‐free functional connectivity within the fronto‐basal‐ganglia network induced by TMS modulation of the right preSMA and the relation of these changes to the efficiency of response inhibition. In addition, we examined whether these changes may, in part, reflect differences in anatomical connectivity that can account for the efficiency of response inhibition [King et al., 2012; Rae et al., 2015]. We applied single‐pulse TMS during fMRI scans while the subjects were at rest (i.e., online or concurrent TMS‐rfMRI). Concurrent TMS‐rfMRI offers a window for the observation of changes in neural activity independent of task performance and without compensatory neural adjustments as likely induced in offline rTMS studies [Siebner et al., 2009; Bestmann and Feredoes, 2013]. It allows not only the observation of immediate changes in neural activity induced by TMS in remote regions, but also the extent to which TMS affects the network properties including functional connectivity (i.e., temporal coupling of activation) between distant regions [Horwitz, 2003]. We applied single pulse TMS at three different intensities (i.e., high = 120%, medium = 80%, and low = 40% of the individual motor threshold) to the right preSMA, a crucial node in the network. The efficiency of response inhibition was assessed using a stop‐signal task separately from the concurrent TMS‐rfMRI session (see Fig. 1 and detailed descriptions of the task and TMS setup in Methods and Materials).
Figure 1.
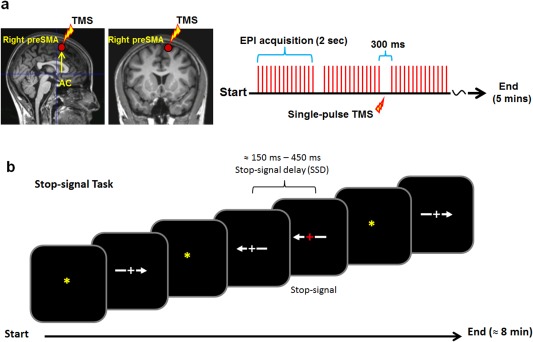
(a) Localization of the TMS target (i.e., the right preSMA) and a schematic illustration of the timing of the single‐pulse TMS relative to the EPI acquisition sequence during the scans. Single‐pulse TMS was delivered 150 ms after the onset of the silence period (300 ms). The rfMRI scans were acquired using a gradient echo‐planar imaging (EPI) sequence with a TR of 2000 ms and a scanner silence period of 300 ms at the end of each TR. (b) Stop‐signal task applied in the study. The stop‐signal delay (SSD) was dynamically controlled such that it increased 50 ms for every successful stopping (stop‐inhibit) response and decreased 50 ms for each failed‐to‐stop (stop‐respond) response.
We expected that changes in the neural activity and the strength of functional connectivity within the network under high‐intensity TMS would be correlated with the ability to stop an ongoing response (SSRT). We also expected that TMS‐induced changes in functional connectivity would likely be correlated with individual differences in anatomical connectivity that can account for response inhibition efficiency.
MATERIALS AND METHODS
Twenty‐two healthy subjects (10 males and 12 females) were enrolled in this study. Five subjects were excluded due to significant scan artifacts and data acquisition problems. Seventeen healthy subjects (7 males and 10 females; mean age = 23.7 [±2.7]) were included in the final data analysis. All participants had a normal structural MRI, neurological examination, and were right‐handed based on the evaluation with the Edinburgh Handedness Inventory [Oldfield, 1971]. All subjects gave their written informed consent to participate in the study, which was approved by the Combined Neuroscience Institutional Review Board at the National Institutes of Health (NIH) and in accordance with the Declaration of Helsinki. Participants received monetary compensation for their time participating in the study.
Apparatus and procedure
The fMRI scans were performed on a 3.0 T PET/MRI scanner (Biograph mMR software VB17P, Siemens, Erlangen, GER) while the participants were at rest (henceforth, rfMRI). TMS was applied using a Magstim Super Rapid2 magnetic stimulator (Magstim Company Limited, Whiteland, UK). A Magstim MRI‐compatible 70 mm TMS coil was mounted on an in‐house built MR compatible TMS‐coil holder and connected to the Magstim stimulator outside the scanner room through an RF waveguide and with a custom‐made ferrite sleeve. The TMS‐coil holder included a 10‐inch‐diameter birdcage fitted with two multielement matrix MR coils (mMR Body TIM Coils) as the MR signal receiver. Each of the matrix coils included six coil elements with an integrated preamplifier. Four rfMRI scans (156 volumes per scan) were acquired using a gradient echo‐planar‐Imaging (EPI) sequence with a volume TR of 2000 ms followed by a 300 ms pause at the end of each TR (other parameters: TE = 25 ms, flip angle = 90°, phase encoding = P ‐> A; FOV = 24 cm, acquisition matrix = 64 × 64, slice thickness = 4 mm, and 34 axial slices with interleaved acquisition). Single‐pulse TMS was delivered 150 ms after the onset of the 300 ms pause period (Fig. 1a). To monitor any potential shift of TMS‐coil position throughout the scan session, three radiographic markers were placed on each subject's head in addition to head restraints (subject's head was strapped to the TMS‐coil holder to insure a direct contact with the TMS coil and minimize head movement). A short (<15 s) marker‐alignment scan (TR = 330 ms, TE = 1.33 ms, flip angle = 15°, FOV = 22 cm, slice thickness = 2 mm, slices = 80 per slab, acquisition matrix = 256 × 256) was acquired immediately before and after the four EPI scans. These marker scans were used to provide an additional estimate of the shift in the head position relative to the TMS‐coil at the end of the scan session (see Supporting Information, Fig. 1 for examples of marker locations and images of the marker‐alignment scans from a representative participant. Also see Table for MR signal quality indexes). On average, the subjects' head movement was minimum (translation: x < 0.1 mm [±0.2], y < 0.3 mm [±0.5], z < 0.2 mm [±0.6]; and rotation: <0.01 mm [±0.01] in x, y, z directions). A gradient echo EPI fieldmap and a high‐resolution (1 × 1 × 1 mm) T1‐weighted anatomical images were also acquired (TR = 2900 ms, TE = 3.03 ms, TI = 1100 ms, FOV = 256 mm, flip angle = 7°, acquisition matrix = 256 × 256, slices = 176 per 3D slab, slice thickness = 1 mm) for unwarping and normalizing the EPI images to a template brain. In addition, diffusion tensor imaging (DTI) data were acquired for each participant with the following scan parameters: TR = 1700 m, TE = 98 ms, FOV = 256 mm, acquisition matrix = 128 ×128, slice thickness = 2 mm, 90 slices without gap, acceleration factor = 2, 10 volumes of b value = 0 s/mm2, 10 diffusion directions with b value = 300 s/mm2, and 60 diffusion directions with b value = 1100 s/mm2. In addition, a T2‐weighted scan with Fast Spin Echo sequence and fat suppression (TR = 5000 ms, TE = 83 ms, FOV = 220 mm, acquisition matrix = 256 × 204, flip angle = 120°, slices = 90, slice thickness = 2 mm) was acquired for each subject at 1.7 mm isotropic voxels to be used as a structural target in postprocessing.
The TMS stimulation site (i.e., right preSMA) was determined for each participant based on the participant's own T1 anatomical MR images using a stereotactic navigation system (Brainsight by Rogue research, Inc., Montreal, Canada). It has been shown that preSMA stimulation induces little discomfort and minimal facial muscle movement relative to other regions (e.g., IFC) [Sandrini et al., 2011]. The center of the TMS coil was placed over the right preSMA, 1 cm anterior to the vertical line from the anterior commissure (AC) perpendicular to the anterior–posterior commissure line in the sagittal plane (see Fig. 1a and also Tremblay and Gracco, 2009]. The localization of the center of the TMS coil to the target (the right pre‐SMA in native space: x = 10, y = 10) was carried out using the subjects' own MR T1 structural image and the stereotaxic neuronavigation system “Brainsight.” The distance between the TMS target location on the scalp and the vertex (Cz) was also calculated for each subject (mean distance = 4.5 cm [±0.14]) and used to mark the TMS target site on a swim cap worn by participants during the concurrent TMS‐rfMRI session. This distance (>4 cm) is consistent with previous studies showing the approximate distance between the vertex and the pre‐SMA [Picard and Strick, 1996; Mars et al., 2009; Arai et al., 2011]. The TMS coil was oriented in line with the longitudinal fissure and with the coil handle pointed posteriorly. Prior to the experiment, the resting motor threshold (rMT) of each participant was determined using the same MRI compatible TMS coil. The individual rMT was set as the lowest intensity of TMS stimulation applied over the left primary motor cortex that was capable of evoking a visible contraction in the relaxed right first dorsal interosseous muscle on at least 5 out of 10 consecutive stimulations [Pridmore et al., 1998]. The average rMT was 66.5% of the maximum stimulator output. To examine TMS‐specific effects on the BOLD signal change, three different stimulation intensities were used during the TMS‐rfMRI scans: 40%, 80%, and 120% of each participant's own rMT. Thirty single‐pulse TMS (10 for each intensity) were delivered semirandomly with a jittered interstimulus interval (ISI range: 9.2–13.8 s) during each rfMRI scan. Four scan runs (<6 min each) were acquired for each subject with a total of 120 TMS pulses (10 × 4 pulses per TMS intensity).
Behavioral task and data analysis
Subjects performed in a separate experimental session, at least 24 h prior to the TMS‐rfMRI session, a variant of the stop‐signal task (SST) used in a previous study [Xu et al., 2015]. They were instructed to stop their response when a visual cue (i.e., a stop‐signal) appeared after the response (“go”) stimulus onset. The stimulus was either a left or right pointing arrow with a “+” sign in the middle (Fig. 1b). Participants were instructed to make a response (i.e., a “go” response) as quickly as they could according to the arrow direction by pressing either the left or the right key on a response box. For 25% of the trials, the “+” sign (i.e., the stop‐signal) turned red after the stimulus onset with a short delay (i.e., the stop‐signal delay or SSD). The SSD was dynamically controlled based on whether a successful (stop‐inhibit) or an unsuccessful (stop‐respond) response was made [Verbruggen and Logan, 2008]. The SSD was set at 100 ms (the shortest SSD) for the first Stop trial and, then a staircase tracking method was implemented such that for every successfully stopped (i.e., Stop‐inhibit) response, the SSD was increased by 50 ms to make it harder to stop on the next trial, and for each fail‐to‐stop (i.e., Stop‐respond) trial, the SSD decreased by 50 ms. The longest possible SSD was 450 ms. The stop‐signal response time (SSRT), a measure of the efficiency of response inhibition, was estimated for each participant by subtracting the mean SSD from the n th (where n is the percentile corresponding to the probability of the stop‐respond trials) fastest RT of the primary “go” responses [Logan, 1994]. The SSRT was then correlated with the TMS‐induced rfMRI BOLD activation, the strength of functional connectivity of the fronto‐basal‐ganglia network, and with the DTI white‐matter microstructure indexes (i.e., the fractional anisotropy [FA], fiber track counts, and averaged fiber length). Recent studies have shown that these microstructure indexes including fiber bundles and fiber length may be associated with cognitive functions [Marner et al., 2003; Behrman‐Lay et al., 2014]. One participant had unusually short SSRT (88 ms) although all other scores were in the normal range. Therefore, the SSRT of this participant was excluded in all correlation/regression analyses to avoid statistical bias.
MRI data processing and analysis
The rfMRI data were processed and analyzed using the SPM8 software (the Wellcome Department of Imaging Neuroscience, University College London, UK). All images were EPI distortion corrected with a gradient echo EPI fieldmap collected during the concurrent TMS‐rfMRI session, and slice‐timing corrected, realigned, and coregistered with the subject's own high‐resolution T1 anatomical image. All subjects' T1 images were combined to generate a T1 template using the DARTEL software and procedures, and normalized to the MNI (Montreal Neurological Institute, Canada) template. The normalization parameters from each subject were then applied to the normalization of the subject's own EPI images. The normalized EPI images were smoothed using an 8 × 8 × 8 mm FWHM kernel. At the first level analysis, the design matrix included four scan runs/sessions and three TMS intensity conditions (low [40%], mid [80%], and high [120%]) plus six motion parameters as confounds. The fMRI activation was modeled using the canonical hemodynamic response function (HRF) with temporal and dispersion derivatives. The data were high‐pass filtered at 128 Hz and the epoch/event duration was set at 1 s. Contrasts (i.e., t‐tests: Low‐baseline, Mid‐baseline, and High‐baseline) from the first level individual analysis were fed into the second (group) level analysis using one‐way within‐subject ANOVAs. Analyses with the whole brain and a priori regions of interest (ROIs) with a binary mask that included the fronto‐basal‐ganglia inhibitory network (i.e., the SMA, preSMA, right IFC, and the basal ganglia) were performed (the ROI mask was created in the MNI template space using the WFU PickAtlas software by the Functional MRI Laboratory at the Wake Forest University School of Medicine, NC). Additional contracts (t tests) were performed to examine the extent of changes in distal activation induced by the three TMS intensities with focus on brain regions showing a monotonic increase or decrease in BOLD signal. All statistical contrasts were corrected for multiple comparisons using the topological false‐discovery rate (FDR) [Chumbley and Friston, 2009; Chumbley et al., 2010] and all reported significant voxels survived a corrected threshold of p < 0.01. Voxels showing a significant monotonic change in the preSMA, SMA proper, rIFC, and the basal ganglia of the network were extracted (8 mm diameter sphere centered on the peak of each cluster) to further examine the relationship between the TMS‐induced BOLD signal change and the efficiency of response inhibition (i.e., the SSRT).
To examine the effect of TMS intensity on functional connectivity within the inhibitory network and the extent to which the connectivity strength may be associated with the efficiency of response inhibition, we performed regional functional connectivity analyses. Partial least square regression (PLSR) analysis [McIntosh and Lobaugh, 2004; Krishnan et al., 2011] was performed to estimate the coupling of the BOLD signals between seven regions in the inhibitory network: the right preSMA, rIFC opercularis (rIFCop), right striatum (rStri), left striatum (lStri), left pallidum (lPal), right pallidum (rPal), and bilateral subthalamic nuclei (STN) (Fig. 4a). The right preSMA was defined as a sphere (8 mm in diameter) centered in the TMS targeted region (MNI xyz = 10, 10, 50). The remaining regions were defined using binary masks created in the MNI template space using the WFU PickAtlas software. The connectivity analysis between two additional regions outside the network (i.e., the right dorsal lateral prefrontal cortex [rDLPFC], and the right inferior‐parietal cortex [rIPC]) was also included as a control. The control region, rDLPFC (8 mm sphere, MNI coordinate: xyz = 37, 33, 32), was determined based on previous studies showing its functional connection with the rIPC in executive control processes [Cieslik et al., 2013]. It is adjacent to the fronto‐basal‐ganglia network and anatomically connected with the right preSMA [Nachev et al., 2008], but its connection with the rIPC is not response inhibition specific. We expected that TMS‐induced changes in the connectivity between the rDLPFC and rIPC, if any, would not be predictive of the efficiency of response inhibition. For each subject, trial‐based regression coefficients (i.e., beta series) [Rissman et al., 2004] from each voxel were extracted from the first level analysis for each TMS intensity level. PLSR was then used to estimate the connectivity between the right preSMA and the other regions as well as between rIFCop and the ROIs in the basal‐ganglia (i.e., rStri, lStri, rPal, lPal, and STN), and the connectivity of the control connection between the rDLPFC and rIPC. The regression coefficient between the first extracted PLS temporal components for each analysis was used as an index of inter‐regional connectivity. The low‐intensity TMS condition served as a baseline control for nonspecific effects of TMS as done in previous concurrent TMS‐fMRI studies [Feredoes et al., 2011; Heinen et al., 2014]. The analysis of the effect of TMS intensity on changes in connectivity focused on the maximal difference between the High and Low TMS conditions using planned t‐tests with the standard Fisher's z transformed correlation coefficients of the connectivity index. Linear regression analyses were also performed between the averaged connectivity index for the two TMS intensity conditions and the efficiency of response inhibition (SSRT) to examine the extent to which the task‐free connectivity within the network was predictive of the SSRT.
Figure 4.
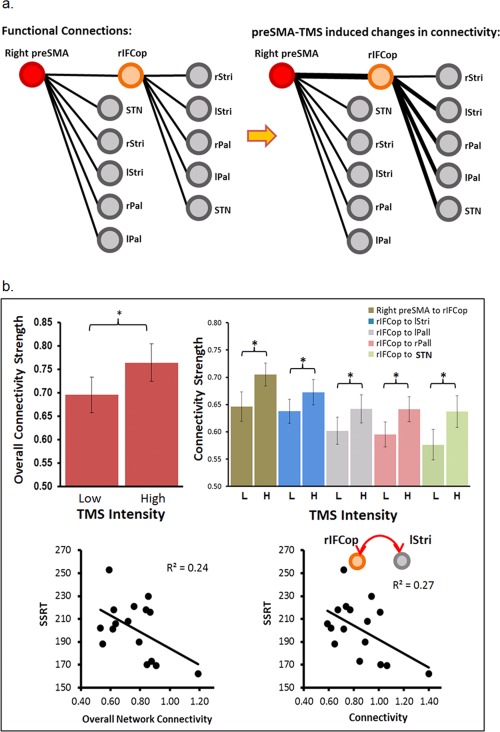
Results of the analyses of functional connectivity. (a) Schematic illustration of the functional connections included in the study and the functional connectivity change (thicker lines) induced by the high TMS condition: right inferior‐frontal cortex opercularis (rIFCop), right striatum (rStri), left striatum (lStri), right pallidum (rPal), left pallidum (lPal), and the subthalamic Nuclei (STN). (b) (1) Overall connectivity (all connections combined) in the high TMS relative to the low TMS condition; (2) TMS‐induced connectivity change in five connections: right preSMA–rIFCop, rIFCop–lStri, rIFCop–lPal, rIFCop–rPal, and rIFCop–STN. The figures at the bottom show negative correlations between the functional connectivity and the SSRT.
The DTI data were preprocessed with the TORTOISE software (by the Pediatric Neuroimaging Diffusion Tensor MRI Center at the National Institutes of Health, http://www.tortoisedti.org) for volume realignment, eddy current correction, EPI distortion correction, and nonlinear tensor fitting [Basser et al., 1994; Pierpaoli et al., 2010]. The preprocessed FA maps were normalized to the MNI (Montreal Neurological Institute, CA) template space using the TBSS (Tract‐Based Spatial Statistics) nonlinear registration procedure of the FSL software (by the FMRIB Analysis Group, University of Oxford, UK). Deterministic tractography was performed using the Diffusion Toolkit (DTK) software (by the TrackVis.org, Martinos Center for Biomedical Imaging, Massachusetts General Hospital) with normalized tensor images in the MNI space. The fiber tacks were determined using the FACT method (fiber assignment by continuous tracking) with the termination angle set at 35° to minimize false positives. Both the FA and DTK track maps were then used to examine the white‐matter microstructure and its relation to the efficiency of response inhibition and the TMS‐induced change in functional connectivity. For the objectives of this study, we focused on the white‐matter regions near the right preSMA (the locus of TMS) and rIFCop that are known to have direct fiber connections between the preSMA, rIFCop, and basal ganglia [Aron et al., 2007; Catani and Thiebaut de Schotten, 2008; Catani et al., 2012; King et al., 2012]. Two seed ROI masks (8mm radius) were created between the right preSMA and rIFCop (Fig. 5) that have been shown to have major fiber bundle connections [Catani et al., 2012; Leunissen et al., 2013]. The two seed ROIs (in MNI space: ROI 1 [near right preSMA] = 12, 16, 50; ROI 2 [near rIFCop] = 30, 8, 22) were placed in the individual DTK track maps. The estimations of fiber counts and fiber length were determined by constraining/including only fibers that originated in both seed ROIs. In addition, two mirror seed ROIs and analyses were applied to the left hemisphere to examine whether there was any hemispheric specificity in relation to the TMS‐induced change of functional connectivity.
Figure 5.
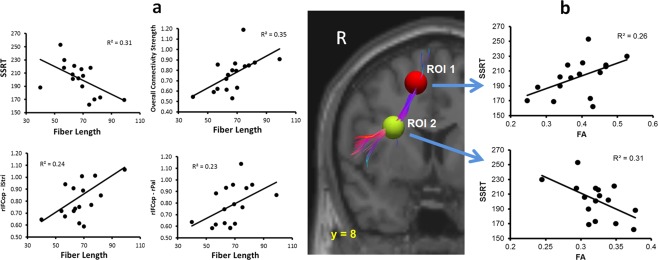
Significant correlations between the white‐mater microstructure (i.e., DTI fiber length and FA values), functional connectivity, and the SSRT. (a) Relationship between the fiber length and the SSRT or task‐free functional connectivity. (b) Correlations between the FA values and the SSRT. The FA values were extracted from the individual normalized FA maps (in the MNI space) in two regions (4 mm diameter: ROI 1 [near right preSMA] = 12, 16, 50; ROI 2 [near rIFCop] = 30, 8, 22) centered on the seed ROIs used in the tractography and the origin of the fiber tracks. The track map in the figure was from a representative participant.
RESULTS
Behavioral performance of the stop‐signal task
On average, the participants made 49% (± 4.7%) of the stop‐inhibit (i.e., successfully stopped) responses, the mean SSRT = 195 (±37) ms (within the normal range, see Logan [1994]), the mean SSD = 218 ms (±57), the observed average stop‐respond (i.e., fail‐to‐stop response) RT = 378 (±81) ms, the estimated stop‐respond RT = 412 (±34) ms, and the averaged “go” RT = 415 ms (±35). Consistent with previous studies using the stop‐signal task, the correlation between the SSRT and the “go” RT was not significant (R 2 = 0.18, p < 0.1).
TMS‐rfMRI results
Figure 2 shows the results of the analysis using a priori ROIs within the fronto‐basal‐ganglia network (also see the whole brain results in Supporting Information, Fig. 2). The results showed that multiple regions within the network had a significant (FDR < 0.01) monotonic increase of BOLD signal change as the TMS intensity increased. These regions included the right preSMA, SMA proper, rIFC (opercularis), right caudate, putamen, pallidum, and the left caudate. Except for the right preSMA and the SMA proper which were directly under or very close to the TMS coil, all regions showed a significant monotonic increase in the BOLD signal. Two one‐way within‐subject ANOVAs performed separately for these regions showed a significant main effect of TMS intensity (increase in signal: F (2,32) = 22.1, MSe = 7.8, p < 0.0001; decrease in signal: F (2,32) = 21.2, MSe = 9.5, p < 0.0001). Post hoc Scheffe's F test (p < 0.05) showed that the BOLD signal change (%) under the three TMS‐intensity conditions differed significantly from each other (positive trend: low TMS = −1.32%, mid TMS = 0.16%, high TMS = 1.55%; negative trend: low TMS = 1.42%, mid TMS = −0.56%, high TMS = −3.43%). Separate one‐way within‐subject ANOVAs for each of these a priori ROIs (with TMS intensity as the within‐subject factor) showed a significant main effect of TMS intensity for all these regions. Post hoc F test (p < 0.05) showed significant differences between the TMS conditions for each of these regions within the network (see Fig. 2 for details). Figure 3 further shows that in the high and the mid TMS conditions, the BOLD signal change in the basal‐ganglia regions (the left caudate and the right pallidum) had significant correlations with the SSRT (the right pallidum: high TMS t 14 = −3.18, R 2 = 0.42, p < 0.01; mid TMS t 14 = −2.8, R 2 = 0.36, P < 0.05; and the left caudate: high TMS t 14 = −2.43, R 2 = 0.30, p < 0.05). When all the basal‐ganglia regions were combined, the BOLD signal change again showed significant correlation with the SSRT (high TMS: t 14 = −2.56, R 2 = 0.32, p < 0.03; mid TMS: t 14 = −2.18, R 2 = 0.25, p < 0.05), indicating that, at least in the high and mid intensity conditions, TMS may induce significant change in neuronal activity distal to the stimulation site within the inhibitory network that are predictive of response‐inhibition efficiency.
Figure 2.
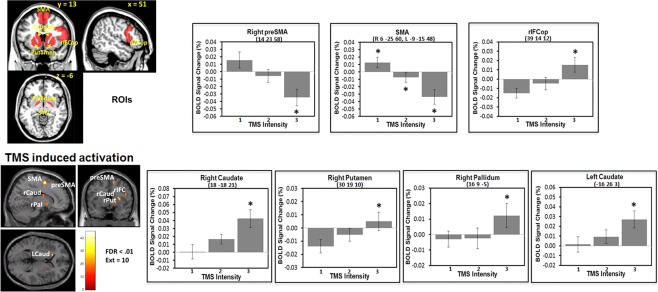
Results of the TMS‐intensity‐induced BOLD signal change with a binary mask that included a priori ROIs of the fronto‐basal‐ganglia network. The top‐left figure shows the ROIs. All reported voxels survived corrections for multiple comparisons using the topological false discovery rate (FDR) with a threshold of p < 0.01. Voxels showing significant differences between the low‐ and high‐TMS conditions were extracted with an 8 mm diameter sphere centered on the peak of each cluster. SMA = supplementary motor area; rIFCop = right inferior‐frontal cortex opercularis; rCaud = right caudate; rPal = right pallidum; rPut = right putamen; * = Scheffe's test, p < 0.05.
Figure 3.
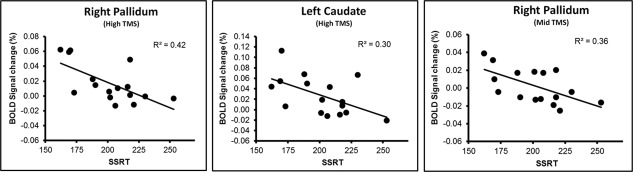
Results of linear regression analyses between the SSRT and the BOLD signal change (%) in the basal ganglia regions that also showed significant monotonic increase of TMS‐intensity‐induced BOLD signal change. All the linear correlations between the SSRT and the BOLD signal change in these regions were statistically significant (p < 0.05). Right pallidum (xyz): 16, 9, −5; left caudate (xyz): −16, 26, 3.
In addition to the TMS intensity effect on the BOLD signal change and its correlation with the SSRT, Figure 4b shows that the overall connectivity of the high TMS condition (0.76) was significantly higher than the low (0.70) TMS condition (paired t‐test: t 16 = 1.95, p < 0.05). Planned t‐tests for each of the connections between the high and low TMS intensities showed a significant difference in the connectivity of preSMA–rIFCop (t 16 = 2.23, p < 0.03), rIFCop–lStri (t 16 = 1.8, p < 0.05), rIFCop–rPal (t 16 = 1.95, p < 0.04), rIFCop–lPal (t 16 = 1.87, p < 0.04), and of rIFCop–STN (t 16 = 1.81, p < 0.05). The connectivity for these connections was significantly stronger in the high TMS condition (0.90, 0.84, 0.77, 0.79, and 0.79) than the low condition (0.79, 0.77, 0.70, 0.71, and 0.68). Separate linear regression analyses using the overall (averaged) connectivity of all connections as the predictor variable showed a significant negative correlation with the SSRT (t 14 = −2.12, R 2 = 0.24, p < 0.05) in the high but not the low TMS condition (R 2 = 0.03). These results indicated a significant relationship between the task‐free network connectivity and the efficiency of rapid response inhibition.
We further examined the relationship between the SSRT and the connectivity of each of the connections that showed significant TMS effect (Fig. 4a, thicker lines). The results of a multiple regression analysis that included all these five connections showed that only the connectivity of rIFCop–lStri accounted for a significant amount of the variance in the SSRT (t 10 = −2.50, R 2 = .38, p < 0.03) (Table 1). Simple regression analysis again showed a significant negative correlation between the SSRT and the rIFCop–lStri connectivity (t 14 = −2.25, R 2 = 0.27, p < 0.05). As the connectivity increased, the SSRT decreased (Fig. 4). None of these connectivity measures was significantly correlated with the “go” RT which is not response inhibition specific. There was also no significant correlation between the SSRT and the control connection rDLPFC–rIPC, a link outside the fronto‐basal‐ganglia network. The high and low TMS intensity did not have significant effect on the strength of this connection either even though the rIPC was sensitive to the TMS intensity (see Supporting Information, Fig. 2 for results from the whole‐brain analysis).
Table 1.
Multiple regression results with SSRT as the dependent valuable and the functional connectivity as the predictor valuables
| Predictor variables | Coefficient | Std error | T | P | Partial R 2 | VIF |
|---|---|---|---|---|---|---|
| Constant | 279.43 | 29.86 | 9.36 | 0.00 | 0.0 | |
| preSMA–rIFCop | −24.7 | 37.83 | −0.65 | 0.53 | 0.04 | 1.7 |
| rIFCop–lStri | −169.1 | 67.72 | −2.50 | 0.03 | 0.38 | 7.4 |
| rIFCop–lPal | 154.2 | 71.84 | 2.15 | 0.06 | 0.32 | 8.6 |
| rIFCop–rPal | −88 | 66.23 | −1.33 | 0.21 | 0.15 | 4.6 |
| rIFCop–STN | 44.52 | 42.36 | 1.05 | 0.32 | 0.10 | 3.0 |
Note: All results in the table are the functional connectivity coefficients (Fisher's Z transformed) in the high TMS condition that showed significant TMS effect. rIFCop = the right inferio‐frontal cortex opercularis; lStri = left striatum; lPal = left pallidum; rPal = right pallidum; STN = subthalamic nuclei.
Based on the results of the TMS‐intensity effect on the functional connectivity and its relation to the response‐inhibition efficiency (SSRT), we further examined individual differences in the white‐matter microstructure (reflected in the fiber counts, fiber length, and FA) and its relation to functional connectivity and response‐inhibition efficiency. Pearson correlations were performed between the fiber counts or length and all the connections (i.e., preSMA–rIFCop, rIFCop–lStri, rIFCop–rPal, rIFCop–lPal, rIFCop–STN, and the overall network connectivity) that showed significant change in functional connectivity under the high TMS condition. The results (Fig. 5a) showed significant positive correlations between the fiber length and functional connectivity of the overall network connectivity (p < 0.01, R 2 = 0.35), rIFCop–lStri (p < 0.05, R 2 = 0.24), and rIFCop–rPal (p = 0.05, R 2 = 0.23). As the fiber length increased, functional connectivity increased. There was also a significant negative correlation between the fiber length and the SSRT (t 14 = −2.53, R 2 = 0.31, p < 0.03), that is, the longer fiber length was associated with more efficient response‐inhibition process (or shorter SSRT). There was no significant correlation between fiber length and the “go” RT (p < 0.2), nor were there significant correlations between the fiber counts and functional connectivity or the behavioral measures. The fiber counts and length indexes from the left hemisphere also did not correlate with the TMS‐induced functional connectivity or the SSRT. Here, we would like to add a caveat of caution in regard to the results of the relationship between the functional and structural connectivity. Although the less stringent statistical correlational analyses revealed significant relationships between the functional and structural connectivity, this study included a relatively small sample of subjects. Therefore, the statistical approach is rather exploratory and, consequently, the results should also be viewed as such. Future studies with larger samples may provide more conclusive analysis.
In addition to the fiber track analysis, we extracted averaged FA values from the same seed ROIs in the right hemisphere using the coregistered and normalized FA maps (Fig. 5b). The results of the linear regression analyses showed significant correlations between the mean FA values of both these ROIs and the SSRT (ROI 1: t 14 = −2.2, p < 0.05, R 2 = 0.26; ROI 2: t 14 = −2.52, p < 0.03, R 2 = 0.31). The FA and the SSRT results indicated a significant relationship between the white‐matter microstructure and the efficiency of response inhibition. Again, no significant correlations were observed between the FA values and the “go” RT.
DISCUSSION
In this study, we applied single‐pulse TMS at three different intensities to the right preSMA during fMRI scans while the subjects were at rest. This task‐free concurrent TMS‐rfMRI revealed, for the first time, immediate effects of TMS on neural activity and task‐free functional connectivity within the fronto‐basal‐ganglia network, and their relation to the efficiency of response inhibition (SSRT) that are not confounded by compensatory neural adjustments or task‐related neural activity.
The results of the study showed TMS‐induced BOLD signal increase in multiple brain regions within the inhibitory network including the rIFC, caudate, putamen, and the right pallidum. The BOLD signal change induced by high‐intensity TMS in the right pallidum and left caudate also correlated with the SSRT, but not with the task response (or “go” response) in general. These results suggest that the widespread effect of preSMA TMS, at least at the suprathreshold level, on the patterns of neural activity beyond the targeted region (i.e., the right preSMA) was immediate and related to the task‐free neural activity associated with response inhibition. Although we cannot rule out completely that the observed effect was not due to subjects' anticipation of the onset of the various TMS pulses, all things being equal, such anticipatory activity would be constant for all three types of stimuli most of the time, at least, with the jittered stimulus presentation timing.
More importantly, our results also showed that relative to the Low TMS condition, High preSMA‐TMS induced immediate changes in the coupling of the rfMRI activation (i.e., functional connectivity) between preSMA and rIFCop, and between the rIFCop and the basal ganglia (i.e., striatum, pallidum, and STN). In the High TMS condition, the SSRT also significantly correlated with the connectivity between the rIFCop and left striatum, and with the mean connectivity of all the connections combined, indicating the impact of preSMA TMS on the task‐free functional connectivity of the network as a whole and the response inhibition process (also see Kahan et al. [2014] for STN stimulation). The preSMA‐TMS effect on functional connectivity appeared to be more specific to response inhibition relative to the “go” response that did not require inhibition. No significant correlations were observed between the functional connectivity and “go” RT. The functional connectivity between the rDLPFC–rIPC, a control link outside the fronto‐basal‐ganglia network, also did not correlate with the SSRT, even though the rIPC activation was sensitive to the TMS intensity.
These results indicate a functional link between the preSMA and remote activation within the network, and between the preSMA and the task‐free functional connectivity of the network. In addition, the preSMA TMS appeared to affect directly its functional connectivity with the rIFCop more than with other regions within the network. However, the change in the task‐free functional connectivity between the preSMA and rIFCop coincided with significant changes in the functional connectivity between the rIFCop and the basal ganglia (i.e., lStri, lPall, rPall, and STN). Although the effect of the preSMA TMS on response inhibition is likely the result of complex interactions among varying levels of altered neuronal activity in multiple regions/nodes within the network, we postulate that functional connectivity between the preSMA and rIFCop itself significantly influences the functional connectivity within the network, particularly, between the rIFCop and the basal ganglia. It is possible that in the context of making a rapid stopping response, preSMA communicates directly with the rIFCop as well as STN, which in turn, induces coordinated neural activity between the rIFCop and the basal ganglia to achieve the rapid stopping response. Consequently, the functional connectivity between the rIFCop and striatum, and the BOLD signal change in the basal ganglia were predictive of the SSRT. This is consistent with previous work showing an interdependent relationship between the preSMA and rIFCop, and the importance of the rIFCop during the inhibition process [Duann et al., 2009; Neubert et al., 2010; Zandbelt and Vink, 2010; Zandbelt et al., 2013; Aron et al., 2014; Picazio et al., 2014].
The observed changes in the task‐free functional connectivity and their relation to the stopping response suggest a possible mechanism underlying the efficiency of response inhibition. We speculate that the strength of the task‐free functional connectivity between the nodes within the network may be critical for regulating the efficiency of the stopping process. Differential effects of TMS loci (e.g., right preSMA vs rIFC) on the task‐free functional connectivity may explain why previous studies applying TMS to the preSMA and the rIFC resulted in inconsistent observations regarding the role of these nodes in the stopping process [Rushworth et al., 2002; Chambers et al., 2007, 2006; Chen et al., 2009; Verbruggen et al., 2010; Cai et al., 2012; Obeso et al., 2013; Zandbelt et al., 2013]. Recent studies showed that suprathreshold TMS could significantly increase the power of the natural frequency of the electrophysiological oscillations associated with neural activity both local and remote to the stimulation site [Rosanova et al., 2009; Pellicciari et al., 2013; Kundu et al., 2014; Pripfl et al., 2014]. Rosanova et al. [2009] reported that single‐pulse TMS over three separate cortical sites (Brodmann areas 19, 7, and 6) of the corticothalamic network induced local and long‐range neural activity with beta, alpha, and gamma oscillations of the natural frequency range differentially associated with these regions. Picazio et al. [2014] also reported that inhibiting a “no‐go” response was associated with frequency oscillations at the beta range from the rIFC relative to the “go” response. Some evidence from intracranial electroencephalography studies indicated that specific neuronal oscillation frequencies (e.g., the beta and gamma band) were directly associated with stopping responses [Swann et al., 2012, 2009]. Swann et al [2012] reported that intracranial electric stimulation of the preSMA in a patient evoked strong local field potentials and an increase in the beta band frequency in the rIFC that was associated with the successful stopping responses. STN stimulation at rest has also been shown to induce beta oscillatory activity in the rIFC and modify effective connectivity (i.e., with causal influence) between multiple regions within the fronto‐basal‐ganglia network [Swann et al., 2011; Kahan et al., 2014]. There is substantial evidence indicating a direct association and cognitive/functional relevance between the BOLD signal change and neuronal synchronization across a wide range of frequencies and frequency power [Scheeringa et al., 2011; Sadaghiani et al., 2012]. Functional connectivity is likely critical to cognitive processes including rapid response inhibition. If the preSMA TMS not only changes patterns of neural activity but also modifies the frequency power associated with the task‐free functional connectivity of remote regions, it is likely that the TMS‐induced activity in regions within the fronto‐basal‐ganglia network would affect the efficiency of response inhibition when the preSMA TMS is applied during experimental tasks. However, it would be important in future studies to determine how stimulation of other nodes in the network (e.g., rIFC) may influence network dynamics.
Related to the influence of task‐free functional connectivity on response inhibition, recent studies have also demonstrated some degree of correspondence between the functional and anatomical connectivity of the human brain [Baird et al., 2005; Rykhlevskaia et al., 2008; Honey et al., 2010; Johansen‐Berg, 2010]. The preSMA‐TMS effect on network activity and response inhibition may also be influenced by the individual differences in the anatomical connectivity. It is known that preSMA has direct white‐matter connections to the striatum and the IFC [Akkal et al., 2007; Nachev et al., 2008; Catani et al., 2012]. All things being equal, cortical connectivity between these regions may influence the effect of stimulation of the preSMA on remote neural activity within the network. As discussed earlier, our results showed that the suprathreshold preSMA TMS induced significant changes in the functional connectivity between the preSMA and rIFCop, and between the rIFCop and the basal ganglia. Our DTI results provided further evidence that the effect of the preSMA TMS on the network may be, in part, attributable to the variability in the white‐matter microstructure (i.e., FA and fiber length). The fact that fiber length and FA values along the DTI fiber tracks between the right preSMA and the rIFC were predictive of the SSRT and that fiber length was significantly correlated with the task‐free functional connectivity between the rIFCop and the basal ganglia, and with the connectivity of the network as a whole indicates the behavioral relevance of these white‐matter pathways [also see King et al., 2012; Neubert et al., 2010; Rae et al., 2015]. These results document individual differences in the white‐matter microstructures [also see Behrman‐Lay et al., 2014] underlying the major pathways between the preSMA, rIFC, and the basal ganglia. However, it should be kept in mind the limitations of the DTI measures and DTI‐based tractography methods applied in the study [Thomas et al., 2014; Reveley et al., 2015], particularly the crossing fiber issue that would likely influence the DTI tractography, fiber counts, and FA measurements [Douaud et al., 2011]. Future studies are needed to further disentangle the relationships between functional and structural connectivity and their relation to behavior. We also cannot explain why the higher the FA values of the preSMA ROI was correlated with less efficient stopping (Fig. 5), while the opposite is true with the FA values of the rIFC ROI. It is possible that the microstructure of the white matter underlying the preSMA alone does not help the stopping performance as the preSMA is also connected with the SMA proper which, in turn, highly connected with the motor cortex. It is possible that for the stopping response, higher cross‐talk between the preSMA and SMA may impede rapid stopping of an already‐initiated response. Future studies may further investigate the relationship between structural connectivity and TMS‐induced changes in functional connectivity. The relationship between the structural and task‐free functional connectivity is also relevant to the understanding of functional deficiency after traumatic brain injury (TBI) which has been shown susceptible to diffuse axonal injuries in the white matter [Johnson et al., 2013].
This concurrent TMS‐rfMRI study revealed a link between the right preSMA and its task‐free functional connectivity within the fronto‐basal‐ganglia network associated with rapid response inhibition. The preSMA TMS not only induced a widespread activation within the stopping network, but also modified the task‐free functional connectivity within the network, particularly, between the rIFCop and left striatum that was predictive of the efficiency of response inhibition. The efficiency of response inhibition and functional connectivity of the network are also related to individual differences in the white‐matter microstructures. These results showed a complex effect of preSMA TMS on the network activity, suggesting that the task‐free functional and structural connectivity between the rIFCop and basal ganglia are critical to the efficiency of response inhibition.
Supporting information
Supporting Information
ACKNOWLEDGMENT
The authors thank Dr Gang Chen at the National Institute of Mental Health for helpful input.
REFERENCES
- Akkal D, Dum RP, Strick PL (2007): Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J Neurosci 27:10659–10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai N, Muller‐Dahlhaus F, Murakami T, Bliem B, Lu MK, Ugawa Y, Ziemann U (2011): State‐dependent and timing‐dependent bidirectional associative plasticity in the human SMA‐M1 network. J Neurosci 31:15376–15383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR (2011): From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry 69:e55–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA (2007): Triangulating a cognitive control network using diffusion‐weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci 27:3743–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW (2003): Stop‐signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci 6:115–116. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA (2014): Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 18:177–185. [DOI] [PubMed] [Google Scholar]
- Baird AA, Colvin MK, Vanhorn JD, Inati S, Gazzaniga MS (2005): Functional connectivity: integrating behavioral, diffusion tensor imaging, and functional magnetic resonance imaging data sets. J Cogn Neurosci 17:687–693. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D (1994): Estimation of the effective self‐diffusion tensor from the NMR spin echo. J Magn Reson B 103:247–254. [DOI] [PubMed] [Google Scholar]
- Behrman‐Lay AM, Usher C, Conturo TE, Correia S, Laidlaw DH, Lane EM, Bolzenius J, Heaps JM, Salminen LE, Baker LM, Cabeen R, Akbudak E, Luo X, Yan P, Paul RH (2014): Fiber bundle length and cognition: a length‐based tractography MRI study. Brain Imag Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benis D, David O, Lachaux JP, Seigneuret E, Krack P, Fraix V, Chabardes S, Bastin J (2014): Subthalamic nucleus activity dissociates proactive and reactive inhibition in patients with Parkinson's disease. Neuroimage 91:273–281. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Feredoes E (2013): Combined neurostimulation and neuroimaging in cognitive neuroscience: past, present, and future. Ann N Y Acad Sci 1296:11–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, George JS, Verbruggen F, Chambers CD, Aron AR (2012): The role of the right presupplementary motor area in stopping action: two studies with event‐related transcranial magnetic stimulation. J Neurophysiol 108:380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Dell'acqua F, Vergani F, Malik F, Hodge H, Roy P, Valabregue R, Thiebaut de Schotten M (2012): Short frontal lobe connections of the human brain. Cortex 48:273–291. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M (2008): A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44:1105–1132. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Gould IC, English T, Garavan H, McNaught E, Kamke M, Mattingley JB (2007): Dissociable mechanisms of cognitive control in prefrontal and premotor cortex. J Neurophysiol 98:3638–3647. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH, Morris AP, Mattingley JB (2006): Executive “brake failure” following deactivation of human frontal lobe. J Cogn Neurosci 18:444–455. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA (2009): Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev 33:631–646. [DOI] [PubMed] [Google Scholar]
- Chen CY, Muggleton NG, Tzeng OJ, Hung DL, Juan CH (2009): Control of prepotent responses by the superior medial frontal cortex. Neuroimage 44:537–545. [DOI] [PubMed] [Google Scholar]
- Chumbley J, Worsley K, Flandin G, Friston K (2010): Topological FDR for neuroimaging. Neuroimage 49:3057–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbley JR, Friston KJ (2009): False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage 44:62–70. [DOI] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Langner R, Laird AR, Fox PT, Eickhoff SB (2013): Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co‐activation‐based parcellation. Cereb Cortex 23:2677–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa A, Trivino M, Perez‐Duenas C, Acosta A, Lupianez J (2010): Temporal preparation, response inhibition and impulsivity. Brain Cogn 73:222–228. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW (2011): Impulsivity, compulsivity, and top‐down cognitive control. Neuron 69:680–694. [DOI] [PubMed] [Google Scholar]
- Dayan E, Censor N, Buch ER, Sandrini M, Cohen LG (2013): Noninvasive brain stimulation: from physiology to network dynamics and back. Nat Neurosci 16:838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G, Jbabdi S, Behrens TE, Menke RA, Gass A, Monsch AU, Rao A, Whitcher B, Kindlmann G, Matthews PM, Smith S (2011): DTI measures in crossing‐fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer's disease. Neuroimage 55:880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duann JR, Ide JS, Luo X, Li CS (2009): Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci 29:10171–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feredoes E, Heinen K, Weiskopf N, Ruff C, Driver J (2011): Causal evidence for frontal involvement in memory target maintenance by posterior brain areas during distracter interference of visual working memory. Proc Natl Acad Sci USA 108:17510–17515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen K, Feredoes E, Weiskopf N, Ruff CC, Driver J (2014): Direct evidence for attention‐dependent influences of the frontal eye‐fields on feature‐responsive visual cortex. Cereb Cortex 24:2815–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Thivierge JP, Sporns O (2010): Can structure predict function in the human brain? Neuroimage 52:766–776. [DOI] [PubMed] [Google Scholar]
- Horwitz B (2003): The elusive concept of brain connectivity. Neuroimage 19:466–470. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H (2010): Behavioural relevance of variation in white matter microstructure. Curr Opin Neurol 23:351–358. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W (2013): Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136:28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan J, Urner M, Moran R, Flandin G, Marreiros A, Mancini L, White M, Thornton J, Yousry T, Zrinzo L, Hariz M, Limousin P, Friston K, Foltynie T (2014): Resting state functional MRI in Parkinson's disease: the impact of deep brain stimulation on 'effective' connectivity. Brain 137:1130–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AV, Linke J, Gass A, Hennerici MG, Tost H, Poupon C, Wessa M (2012): Microstructure of a three‐way anatomical network predicts individual differences in response inhibition: a tractography study. Neuroimage 59:1949–1959. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Williams LJ, McIntosh AR, Abdi H (2011): Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage 56:455–475. [DOI] [PubMed] [Google Scholar]
- Kundu B, Johnson JS, Postle BR (2014): Prestimulation phase predicts the TMS‐evoked response. J Neurophysiol 112:1885–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunissen I, Coxon JP, Geurts M, Caeyenberghs K, Michiels K, Sunaert S, Swinnen SP (2013): Disturbed cortico‐subcortical interactions during motor task switching in traumatic brain injury. Hum Brain Mapp 34:1254–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD (1994): On the ability to inhibit thought and action: A user's guide to the stop signal paradigm In: Dagenbach D, Carr TH, editors. Inhibitory Processes in Attention, Memory and Language. San Diego: Academic; pp 189–239. [Google Scholar]
- Logan GD, Cowan WB (1984): On the ability to inhibit thought and action ‐ A theory of an act of control. Psychol Rev 91:295–327. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA (1984): On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform 10:276–291. [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B (2003): Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol 462:144–152. [DOI] [PubMed] [Google Scholar]
- Mars RB, Klein MC, Neubert FX, Olivier E, Buch ER, Boorman ED, Rushworth MF (2009): Short‐latency influence of medial frontal cortex on primary motor cortex during action selection under conflict. J Neurosci 29:6926–6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Lobaugh NJ (2004): Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage 23:S250–S263. Suppl 1: [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202. [DOI] [PubMed] [Google Scholar]
- A Miyake, NP Friedman (2012): The Nature and Organization of Individual Differences in Executive Functions: Four General Conclusions. Curr Dir Psychol Sci 21:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M (2008): Functional role of the supplementary and pre‐supplementary motor areas. Nat Rev Neurosci 9:856–869. [DOI] [PubMed] [Google Scholar]
- Nachev P, Wydell H, O'Neill K, Husain M, Kennard C (2007): The role of the pre‐supplementary motor area in the control of action. Neuroimage 36 Suppl 2:T155–T163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert FX, Mars RB, Buch ER, Olivier E, Rushworth MF (2010): Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc Natl Acad Sci USA 107:13240–13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso I, Robles N, Marron EM, Redolar‐Ripoll D (2013): Dissociating the role of the pre‐SMA in response inhibition and switching: A combined online and offline TMS approach. Front Hum Neurosci 7:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Pellicciari MC, Cordone S, Marzano C, Bignotti S, Gazzoli A, Miniussi C, De Gennaro L (2013): Dorsolateral prefrontal transcranial magnetic stimulation in patients with major depression locally affects alpha power of REM sleep. Front Hum Neurosci 7:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL (1996): Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 6:342–353. [DOI] [PubMed] [Google Scholar]
- Picazio S, Veniero D, Ponzo V, Caltagirone C, Gross J, Thut G, Koch G (2014): Prefrontal control over motor cortex cycles at beta frequency during movement inhibition. Curr Biol 24:2940–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Walker L, Irfanoglu MO, Barnett A, Basser PJ, Chang L‐C, Koay CG, Pajevic S, Rohde G, Sarlls JE, Wu M (2010) TORTOISE: An Integrated Software Package for Processing of Diffusion MRI Data. Proceedings of the XVIII ISMRM, Stockholm 1597.
- Pridmore S, Fernandes Filho JA, Nahas Z, Liberatos C, George MS (1998): Motor threshold in transcranial magnetic stimulation: a comparison of a neurophysiological method and a visualization of movement method. J ECT 14:25‐27. [PubMed] [Google Scholar]
- Pripfl J, Tomova L, Riecansky I, Lamm C (2014): Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue‐induced nicotine craving and EEG delta power. Brain Stimul 7:226–233. [DOI] [PubMed] [Google Scholar]
- Rae CL, Hughes LE, Anderson MC, Rowe JB (2015): The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. J Neurosci 35:786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reveley C, Seth AK, Pierpaoli C, Silva AC, Yu D, Saunders RC, Leopold DA, Ye FQ (2015): Superficial white matter fiber systems impede detection of long‐range cortical connections in diffusion MR tractography. Proc Natl Acad Sci USA 112:E2820–E2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M (2004): Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage 23:752–763. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M (2009): Natural frequencies of human corticothalamic circuits. J Neurosci 29:7679–7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK (2002): Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol 87:2577–2592. [DOI] [PubMed] [Google Scholar]
- Rykhlevskaia E, Gratton G, Fabiani M (2008): Combining structural and functional neuroimaging data for studying brain connectivity: a review. Psychophysiology 45:173–187. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, Scheeringa R, Lehongre K, Morillon B, Giraud AL, D'Esposito M, Kleinschmidt A (2012): alpha‐band phase synchrony is related to activity in the fronto‐parietal adaptive control network. J Neurosci 32:14305–14310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini M, Umilta C, Rusconi E (2011): The use of transcranial magnetic stimulation in cognitive neuroscience: a new synthesis of methodological issues. Neurosci Biobehav Rev 35:516–536. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Fries P, Petersson KM, Oostenveld R, Grothe I, Norris DG, Hagoort P, Bastiaansen MC (2011): Neuronal dynamics underlying high‐ and low‐frequency EEG oscillations contribute independently to the human BOLD signal. Neuron. 69:572–583. [DOI] [PubMed] [Google Scholar]
- Sebastian A, Gerdes B, Feige B, Kloppel S, Lange T, Philipsen A, Tebartz van Elst L, Lieb K, Tuscher O (2012): Neural correlates of interference inhibition, action withholding and action cancelation in adult ADHD. Psychiatry Res 202:132–141. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Hartwigsen G, Kassuba T, Rothwell JC (2009): How does transcranial magnetic stimulation modify neuronal activity in the brain? Implications for studies of cognition. Cortex 45:1035–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Surmeier DJ, Redgrave P, Kimura M (2011): Thalamic contributions to basal ganglia‐related behavioral switching and reinforcement. J Neurosci 31:16102–16106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner P, Nachev P, Morris P, Peters AM, Jackson SR, Kennard C, Husain M (2007): Human medial frontal cortex mediates unconscious inhibition of voluntary action. Neuron 54:697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, Cai W, Conner CR, Pieters TA, Claffey MP, George JS, Aron AR, Tandon N (2012): Roles for the pre‐supplementary motor area and the right inferior frontal gyrus in stopping action: electrophysiological responses and functional and structural connectivity. Neuroimage 59:2860–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, Poizner H, Houser M, Gould S, Greenhouse I, Cai W, Strunk J, George J, Aron AR (2011): Deep brain stimulation of the subthalamic nucleus alters the cortical profile of response inhibition in the beta frequency band: a scalp EEG study in Parkinson's disease. J Neurosci 31:5721–5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, Tandon N, Canolty R, Ellmore TM, McEvoy LK, Dreyer S, DiSano M, Aron AR (2009): Intracranial EEG reveals a time‐ and frequency‐specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J Neurosci 29:12675–12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Ye FQ, Irfanoglu MO, Modi P, Saleem KS, Leopold DA, Pierpaoli C (2014): Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc Natl Acad Sci USA 111:16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- P Tremblay, VL Gracco (2009): Contribution of the pre‐SMA to the production of words and non‐speech oral motor gestures, as revealed by repetitive transcranial magnetic stimulation (rTMS). Brain Res 1268:112–124. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Aron AR, Stevens MA, Chambers CD (2010): Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proc Natl Acad Sci USA 107:13966–13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD (2008): Models of response inhibition in the stop‐signal and stop‐change paradigms. Neurosci Biobehav Rev 33:647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Hanajima R, Shirota Y, Tsutsumi R, Shimizu T, Hayashi T, Terao Y, Ugawa Y, Katsura M, Kunimatsu A, Ohtomo K, Hirose S, Miyashita Y, Konishi S (2015): Effects of rTMS of presupplementary motor area on fronto basal ganglia network activity during stop‐signal task. J Neurosci 35:4813–4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Levy S, Butman J, Pham D, Cohen LG, Sandrini M (2015): Effect of foreknowledge on neural activity of primary “go” responses relates to response stopping and switching. Front Hum Neurosci 9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandbelt BB, Bloemendaal M, Hoogendam JM, Kahn RS, Vink M (2013): Transcranial magnetic stimulation and functional MRI reveal cortical and subcortical interactions during stop‐signal response inhibition. J Cogn Neurosci 25:157–174. [DOI] [PubMed] [Google Scholar]
- Zandbelt BB, Vink M (2010): On the role of the striatum in response inhibition. PLoS One 5:e13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


