Abstract
Carcino-embryonic antigen-like cellular adhesion molecules (CEACAMs), members of the immunoglobulin superfamily, are responsible for cell-cell interactions and cellular signaling events. Extracellular interactions with CEACAMs have the potential to induce phagocytosis, as is the case with pathogenic Neisseria bacteria. Pathogenic Neisseria species express opacity associated (Opa) proteins, which interact with a subset of CEACAMs on human cells, and initiate the engulfment of the bacterium. We demonstrate that recombinant Opa proteins reconstituted into liposomes retain the ability to recognize and interact with CEACAMs in vitro, but do not maintain receptor specificity compared to Opa proteins natively expressed by Neisseria gonorrhoeae. We report that two Opa proteins interact with CEACAMs with nanomolar affinity and we hypothesize that this high affinity is necessary to compete with the native CEACAM homo- and heterotypic interactions in the host. Understanding the mechanisms of Opa protein-receptor recognition and engulfment enhances understanding of Neisserial pathogenesis. Additionally, these mechanisms provide insight into how human cells that are typically non-phagocytic can utilize CEACAM receptors to internalize exogenous matter, with implications for the targeted delivery of therapeutics and development of imaging agents.
Keywords: Pathogen–Host interactions, protein–protein interactions, Opa proteins, CEACAMs, Neisseria gonorrhoeae
Graphical abstract
CEACAMs (Carcino-Embryonic Antigen-like Cellular Adhesion Molecules) are a subgroup of the immunoglobulin superfamily involved in many cellular processes such as cell adhesion, proliferation, differentiation, and tumor suppression.1 Related to these various functions, CEACAM dysregulation is often observed in implantation of circulating tumor cells2 and tumor angiogenesis.3 Twelve different CEACAM variants have been identified in humans, with differential expression in various cell types.1, 4 For instance, CEACAM1 expression is widely distributed and is found in leukocytes, epithelial and endothelial tissue, and T cells, while CEACAM3 is found exclusively on human granulocytes.1
The extracellular regions of all CEACAMs consist of one highly conserved amino-terminal immunoglobulin variable (IgV)-like domain (NCCM, Figure 1A), and one to six immunoglobulin constant (IgC)-like domains.5, 6 NCCM is implicated in homotypic and heterotypic interactions.2, 7–11 Many bacterial pathogens express proteins that interact with NCCMs, especially CEACAM1, 3, 5, and 6.12–17 Additionally, NCCMs are known to interact specifically with fimbrial structures such as Dr adhesins.9 Interactions with NCCMs may occur through carbohydrate moieties on the NCCM, as is the case for CEACAM1 binding to enterobacteria, such as Escherichia coli and Salmonella strains.1 CEACAMs also interact through a non-glycosylated region of NCCM, as is observed for Haemophilus influenzae, Moraxella catarrhalis, and the pathogenic Neisseria species, Neisseria gonorrhoeae (Gc) and Neisseria meningitidis (Nm).18
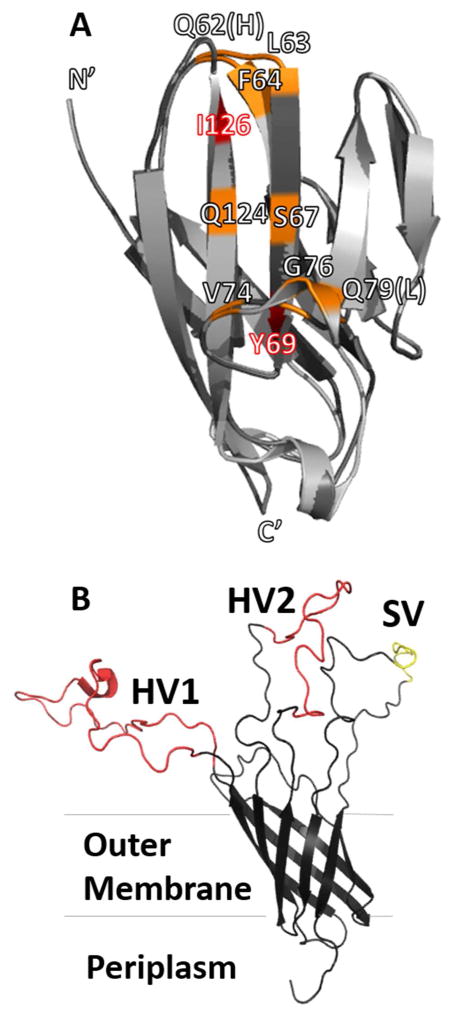
Figure 1.
(A) Structure of NCCM1 (PDB ID 2GK242, light gray) and homology model of NCCM3 (generated with SWISS-MODEL60–63, dark gray). Residues that bind to all OpaCEA proteins are colored red, while residues that only bind specific Opa variants are colored orange. All amino acids on the binding face of NCCM1 are conserved in NCCM3, except Gln62 (His in NCCM3), and Gln79 (Leu in NCCM3). (B) Structure of Opa60 (PDB ID 2MLH)30. The conserved regions of the protein are shown in black, hypervariable regions (HV1 and HV2) are colored red, and semi-variable region (SV) is colored yellow.
Interactions between CEACAM and the pathogenic Neisseria not only allow the bacteria to adhere to and colonize human cells, but can also trigger engulfment of the bacteria. These interactions have been studied in detail in epithelial cells and neutrophils (polymorphonuclear leukocytes). Epithelial cells express the pathogen binding CEACAMs 1, 5, and 6, as well as the non-pathogen binding CEACAM7.19, 20 Primary human neutrophils express CEACAMs 1, 3 and 6, as well as the non-pathogen binding CEACAMs 4 and 8.21 Of particular interest are CEACAMs 1 and 3; both contain cytoplasmic domains involved in signaling that can lead to the internalization of the bacterium, but often trigger opposing cell responses. CEACAM1 contains two immunoreceptor tyrosine-based inhibition motifs (ITIMs) which upon activation triggers the recruitment of the phosphatase SHP-1 to suppress phosphotyrosine-based signaling cascades.22–24 CEACAM3 contains an immunoreceptor tyrosine-based activation motif (ITAM), which recruits kinases (Src family kinases, such as Syk) upon activation to propagate pro-inflammatory signaling cascades.25–28 CEACAM3, which is expressed exclusively on human neutrophils and other granulocytes, is thought to have evolved as innate immune protection, as it has no known endogenous ligand, but interacts specifically with proteins expressed on the surface of human-specific bacterial pathogens such as Neisseria.1 These CEACAM3 interactions mediate uptake of the pathogen, which induces the oxidative burst as well as toxic granule release to effect pathogen killing.1, 29
CEACAM receptors mediate Gc and Nm engulfment by binding to Neisserial opacity-associated (Opa) proteins. Opa proteins are found in the bacterial outer membrane and consist of a membrane spanning eight-stranded β-barrel with four extracellular loops (Figure 1B and Figure S1).30 Within the Opa family, the barrel of the protein and the short extracellular loop 4 has a highly conserved sequence (approximately 70% sequence identity).31 Extracellular loop 1 has a region that exhibits some sequence diversity (the semi-variable loop, or SV, Figure 1B in yellow), while extracellular loops 2 and 3 have regions of high sequence diversity (the hypervariable loops, HV1 and HV2, respectively, Figure 1B in red).5 Receptor specificity is determined primarily by HV1 and HV2.32 To date, 26 SV sequences, 97 HV1 sequences, and 127 HV2 sequences were identified in the 345 unique opa alleles sequenced. Opa sequence diversity is primarily generated from recombination events.33 Of these sequences, receptor specificity has been determined for ~30 Opa variants.34–41
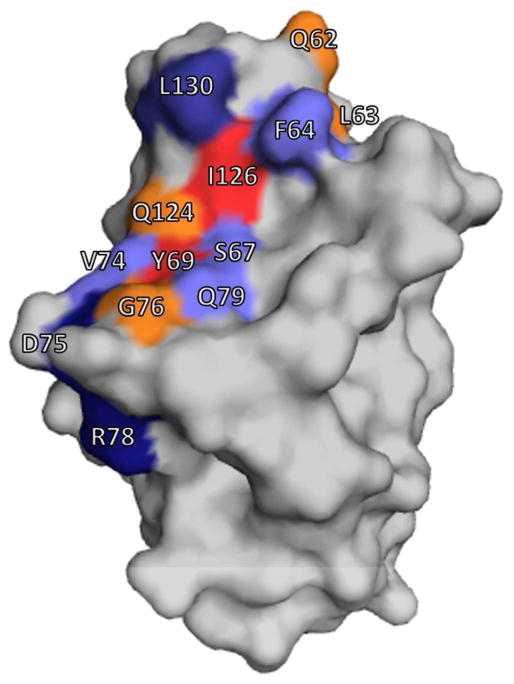
Of the Neisserial Opa proteins investigated, most interact with CEACAMs (OpaCEA). All OpaCEA proteins interact with the non-glycosylated face of the IgV-like domain of CEACAMs.42 However, OpaCEA proteins vary in their specificity of interactions with CEACAMs.12 Ten CEACAM residues were identified which mediate binding to Opa proteins; of these important residues, only Tyr69 and Ile126 (residue numbers of CEACAM1, UniProt ID P13688-1) interact with all studied Opa variants, and are highly conserved on all CEACAMs.43 Of the other eight CEACAM residues involved in binding to Opa proteins, six are conserved between CEACAM1 and 3 (Figure 1A and S2). Opa sequence motifs that determine receptor specificity have not been identified. Multiple sequence alignment of Opa HV regions does not reveal consensus motifs. A specific combination of HV1 and HV2 is required for CEACAM binding. OpaCEA chimeras that contained an HV1 sequence from one OpaCEA and an HV2 sequence from a different OpaCEA do not bind any CEACAMs.32 Thus, individual Opa protein HV primary sequences alone are insufficient for receptor recognition, and unique combinations of HV1 and HV2 sequences are necessary for interactions with receptors.
We seek to understand how Neisseria competes with host CEACAM interactions and utilizes CEACAM signaling for its own advancement. In this manuscript, we demonstrate that recombinant Gc Opa proteins reconstituted into small unilamellar vesicles retain their ability to interact with CEACAMs, but do not display the same selectivity. Using these Opa proteoliposomes, we determined that two OpaCEA proteins (Opa60 from Gc strain MS11 and OpaD from Gc strain FA1090) have high affinity (nM) for NCCM1 and 3. We hypothesize that this tight interaction is necessary for competing with the homotypic (CEACAM – CEACAM) and heterotypic (CEACAM – other adhesion molecule) interactions.
EXPERIMENTAL PROCEDURES
Expression and purification of the N-terminal domain of CEACAMs 1 and 3
The procedure for CEACAM expression and purification was adapted from Fedarovich et al.42 E. coli MC1061 cells transformed with a modified pGEX-2T plasmid (pGEX-2V) containing the N-terminal D1 domain of human ceacam1 gene (amino acids 35-141 (MW = 11.8 kDa) of the mature protein and referred to as NCCM1, Figure S2) were generously provided by Rob Nicholas (University of North Carolina, Chapel Hill, NC.). The N-terminal domain of human ceacam3 gene (amino acids 35-142 (MW = 12.2 kDa) of the mature protein and referred to as NCCM3, Figure S2) was synthesized and cloned into a pGEX-2T vector (Bio Basic Inc., Ontario, Canada). To maintain a construct similar to that of NCCM1, a linker region between the GST and NCCM3 was designed to incorporate a tobacco etch virus protease (TEV) cleavage site (ENLYFQ | PG) in the resulting fusion protein. Additionally, the amino acids SGA were added as a spacer immediate following the TEV cleavage site and before the first amino acid of NCCM3. NCCM cysteine mutations were introduced using PIPE mutagenesis.44
MC1061 E. coli cells with CEACAM plasmid were grown in Luria-Bertani (LB) media supplemented with streptomycin and ampicillin (50 μg/mL each) at 37°C until an OD600 ≈ 0.6 was reached. Cell cultures were cooled to 25°C and protein expression was induced with 1 mM isopropyl β-thio-D-galactoside (IPTG) overnight with constant shaking (200 rpm) at the same temperature. Cells from 1 L culture were harvested by centrifugation (4,500 × g, 20 min, 4°C), resuspended in 15 mL lysis buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 2 mM ethylenediaminetetraacetic acid (EDTA), 2 mM dithiothreitol (DTT), 10% glycerol, half of a Complete protease inhibitor tablet (Roche)), and lysed using a microfluidizer (Microfluidics model 110L, Newton, MA). Cell debris was removed by centrifugation (18,000 × g, 1 h, 4°C), and proteins were precipitated from the supernatant by the addition of ammonium sulfate to 55% saturation with constant stirring for 1 h at 4°C. Precipitated proteins were harvested by centrifugation (12,000 × g, 30 min, 4°C), pellets were resuspended in 30 mL lysis buffer, and the lysate was added to a glutathione resin column previously equilibrated with equilibration buffer (20 mM Tris, pH 7.3, 150 mM NaCl, 2 mM DTT, and 10% glycerol) at 4°C. After loading, the column was washed with 10 column volumes of equilibration buffer, and eluted with 50 mL of the same buffer supplemented with 10 mM reduced glutathione.
Labeling NCCM with fluorescent probe
To cleave the N-terminal domain from the GST, TEV (~ 3.5 μM) was added to the eluent containing purified GST-NCCM fusion protein and dialyzed (20 mM Tris, pH 7.3, 150 mM NaCl, 2mM DTT, 10% glycerol; MWCO = 3,500 kDa) overnight at 4°C. The N-terminal domain of CEACAM was purified from GST and TEV by using an HR Sephacryl S-200 gel-filtration column (26/60 mm, GE Healthcare) previously equilibrated with 20 mM Tris, pH 8.0, 500 mM NaCl, 2 mM DTT, and 10% glycerol (supplemental Figure S3). Fractions containing pure NCCM (as assessed by SDS-PAGE) were combined and concentrated to ~42 μM (determined by A280; ε = 14,440 M−1cm−1 for NCCM1 and ε = 15,930 M−1cm−1 for NCCM3) and stored at −80°C. Wild-type NCCMs do not contain any cysteine residues, so in all cases a Cysteine mutant was introduced (H139C, located on the opposite side of the Opa-binding face, supplemental Figure S4), for specific labeling with a fluorophore. In the crystal structure of NCCM142 residue H139 from three NCCM1 subunits is coordinated by a nickel ion; artificial oligomers can be mediated by such divalent interactions. Our unpublished observations suggest the H139C mutant and the addition of EDTA reduces NCCM oligomerization. Protein purity was greater than 95%, as assessed by SDS-PAGE. Purified NCCM1 or NCCM3 H139C was dialyzed overnight at 4°C to remove DTT (20 mM Tris, pH 7.3, 150 mM NaCl, 10% glycerol; MWCO = 3,500 kDa), and concentrated to ~50 μM. A 1 mM stock of the fluorescent dye, 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid, disodium salt (AMS, Life Technologies, Carlsbad, CA), was freshly prepared and added drop wise to the protein to yield a dye: protein molar ratio of 20:1. The reaction was protected from light and carried out under nitrogen overnight at 4°C. Excess dye was removed by extensive dialysis (MWCO = 3,500 kDa) at 4°C. Labeling efficiency was determined to be greater than 95%, as assessed by MALDI-TOF mass spectrometry (supplemental Figure S5).
Cloning and expression of N. gonorrhoeae
FA1090 Opa− and FA1090 OpaDnv Gc, which is phase-locked ON for constitutive OpaD expression (OpaD+), were generated as previously described.45 FA1090 Opa50nv and FA1090 Opa60nv were constructed by transformation of FA1090 Opa− with a plasmid (pST) containing the non-variable signal sequence of OpaD (99bp) immediately followed by sequences corresponding to the mature Opa50 (711bp) or Opa60 (717bp) protein from MS11 Gc, and flanked by 730bp of the genomic sequence 5′ of OpaD and 889bp 3′ of OpaD from Opa− Gc. These constructs were synthesized and ligated into the pST vector by Genewiz Inc (South Plainfield, NJ). Successful Gc transformants were selected by opaque colony morphology and confirmed by PCR, sequencing and immunoblot. Opa protein sequences are provided in supplemental Figure S1.
Cloning, expression and purification of recombinant Opa proteins for liposomes
The opa60, opa50, and opaD genes were sub-cloned into pET28b vectors (EMD chemicals, Gibbstown, NJ) encoding a thrombin cleavable N-terminal His6-tag (MGSSHHHHHHSSGLVPRGSHM). Expression and purification protocols were followed as previously described.30, 46, 47 Briefly, the opa containing plasmids were transformed into a BL21(DE3) E. coli strain. Cell cultures were grown in LB media and expression was induced to the insoluble fraction. Cells were harvested, resuspended in lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, half of a Complete protease inhibitor pellet), and lysed. The insoluble fraction was pelleted. The pellet was resuspended in extraction buffer (lysis buffer with 8 M urea) and the insoluble fraction was removed by centrifugation. Opa proteins were purified using Co2+ immobilized metal affinity chromatography, and eluted (20 mM sodium phosphate, pH 7.0, 150 mM NaCl, 680 mM imidazole, and 8 M urea). The eluted fractions containing Opa were pooled and concentrated (MWCO = 10 kDa) to 0.5 mM, with an expression yield of ~20 mg/L of cell culture. Protein concentration was determined by A280 (ε = 41,830 M−1cm−1 for Opa60, ε = 43,320 M−1cm−1 for OpaD, ε = 40,340 M−1cm−1 for Opa50) and purity was greater than 95%, assessed by SDS-PAGE.
N. gonorrhoeae pull-down assays
Gc were grown on gonococcal medium base (GCB, Difco, Becton-Dickinson, Franklin Lakes, NJ) containing Kellogg’s supplements I and II48 for approximately 8 hours, then grown in rich liquid medium (GCBL) with periodic dilutions to produce uniformly mid-logarithmic cultures, as previously described.49 Using OD550 to calculate, approximately 3 × 108 CFU/mL Gc was suspended in 1–2 mL GCBL. Cultures were spiked with either GST-NCCM1 (28 mg/mL) or GST-NCCM3 (28 mg/mL) and incubated at 37°C for 30 min with rotation. Cultures were centrifuged (500 × g for 20 min) and 800 μL of supernatant added to 200 μL 5X SDS-PAGE loading buffer. Pellets were washed twice by resuspending in 1 mL PBS + 5mM MgSO4 and centrifuging at 10,000 × g for 3 min. Pellets were resuspended in 200 μL 1X SDS-PAGE loading buffer. Pellet lysates and supernatant samples were analyzed by SDS-PAGE and immunoblotting.
Opa proteoliposome production
Opa protein folding and reconstitution was adapted from Dewald et al.46 Lipid stocks of 1,2-didecanoyl-sn-glycero-3-phosphocholine (diC10PC, Avanti Polar Lipids, Alabaster, AL), originally dissolved in chloroform, were dried under a continuous stream of nitrogen and resuspended into borate buffer (10 mM sodium borate, pH 12, 1 mM EDTA) and sonicated for 30 minutes (Q Sonica model Q500, Newtown, CT) with a 1/8 inch micro tip at 40% amplitude. Post-sonication, urea was added to a concentration of 4 M, and purified recombinant unfolded Opa protein was added in 20 μL aliquots, mixing between additions, yielding a final protein to lipid ratio of 1:1160. The folding reaction was incubated at 37°C for 3 days after which folding was assessed by SDS-PAGE (supplemental Figure S6). After folding, Opa proteoliposomes were harvested by ultracentrifugation (142,400 × g, 2 h, 10°C), resuspended in a new lipid mixture in resuspension buffer (30 mM Tris, pH 7.3, 150 mM NaCl), and pulse sonicated (30 s on, 30 s off for a total of 20 min). Less than 20% of the original DiC10PC lipid is present in the pellet after ultracentrifugation.50 Upon resuspension with a new lipid mixture, the amount of DiC10PC present is approximately 1 mol%. The lipid composition used to resuspend the Opa proteins contained 63 mol% 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 16 mol% 1,2-dimyristoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (sodium salt) (DMPG), 16 mol% cholesterol, 5 mol% 1,2-dimyristoyl - sn - glycero - 3 - phosphoethanolamine - N - [methoxy (polyethylene glycol) - 1000] (ammonium salt) (DMPE PEG 1000), with a protein to lipid ratio of 1:234.
Trypsin cleavage of Opa extracellular loops
OpaTrp liposomes were prepared by adding porcine pancreas trypsin (Sigma) to the Opa-liposome solution, 10 μg of trypsin for every 2 μg of Opa. Upon the addition of trypsin to recombinant folded Opa proteins, NMR analysis and SDS-PAGE demonstrate the β-barrel domain stays intact while regions of the extracellular loops are removed.30, 47 The trypsin-liposome solution was incubated at room temperature for ~ 4 h. After incubation, the trypsin-liposome solution was passed over a column containing p-Aminobenzamidine-agarose (Sigma, St. Louis, MO), previously equilibrated with Opa resuspension buffer, to remove trypsin. The flow through was collected for experiments and cleavage was assessed by SDS-PAGE (supplemental Figure S7).
Liposome pull-down assays
Opa protein concentrations were determined with A280 and dye based assays for comparison. There was less than 10% difference between the two methods, and A280 was used for all experiments. 3.4 μmol of the Opa proteoliposome solutions were aliquoted. GST-NCCM was added in excess (10.2 μmol) and samples were incubated for 30 min at room temperature. Proteoliposomes were harvested by ultracentrifugation (142,400 × g, 2.5 h, 10°C), washed once with 2 mL PBS to remove unbound NCCM, and resuspended in 1 mL PBS. Ninety μL of sample was added to 30 μL of 4x SDS loading buffer, and analyzed via SDS-PAGE and immunoblotting.
SDS-PAGE and Immunoblotting
All samples were boiled for 20 min and homogenized by passing through a needle (30 gauge) and syringe ten times. Twenty μL were loaded onto a pre-cast 4–20% acrylamide gel (Bio-Rad, Hercules, CA). After electrophoresis, gels were transferred to 0.2 μM nitrocellulose using the turbo blot system (Bio-Rad), and immunoblotted with the A0115 α-CEACAM antibody (Dako, Carpentaria, CA), or the 4B12 pan-Opa antibody (hybridoma generously provided by Christof Hauck, University of Konstanz, Germany, and antibody purified by the University of Virginia Hybridoma Core) followed by IRDye 800 CW conjugated goat anti-mouse or goat anti-rabbit IgG (LI-COR Biosciences, Lincoln, NE). Blots were visualized with an Odyssey imager (LI-COR Biosciences). NCCM pellet band intensities were measured using ImageJ51 analysis software, and plotted with Origin Pro 7.5. Quantification of NCCM relative to the total concentration of NCCM or Opa proteins is not feasible because of a lack of a loading control and antibody reactivity differences.
Fluorescence polarization binding assays
Fluorescently labeled NCCM1 or NCCM3 (5 nM) was added to 300 nM Opa protein, and serially diluted with buffer (5 nM NCCM, 20 mM Tris, pH 7.3, 150 mM NaCl) across 11 steps, and done in triplicate. Samples were incubated in an opaque 96-well plate for 30 min, and then the fluorescence polarization was measured for each sample. Fluorescence polarization spectra were collected with a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA). The excitation wavelength was 323 nm and emission spectra were collected at 411 nm, with a wavelength cutoff of 325 nm. Polarization was measured and converted into the fraction of CEACAM bound to the proteoliposomes at varying concentrations of Opa protein. Data was processed and analyzed using Origin Pro 7.5, using the following equation:
| eq. 1 |
Where fB is the fraction bound, Kd is the ligand dissociation constant, RT is the receptor concentration, and LT is the total fluorescent ligand concentration. This equation takes ligand depletion into account, as described by Veiksina et al.52
RESULTS AND DISCUSSION
Recombinant NCCM1 and 3 interact with Opa60 and OpaD+ Gc
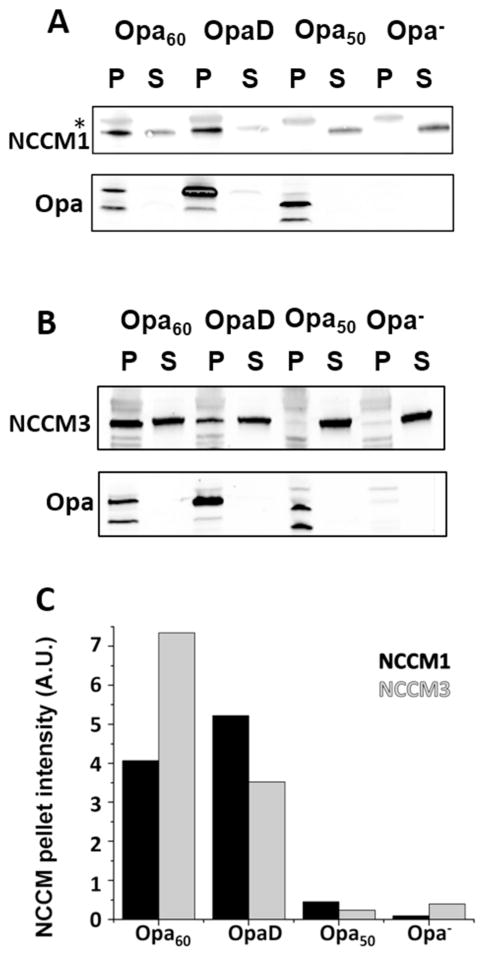
Gc that are phase ON for expression of Opa60 have previously been reported to interact with both CEACAM1 and CEACAM3, in cells transfected to express a specific CEACAM as well as recombinant CEACAM fusion products.39, 53 Here we used Gc constitutively expressing only Opa60, OpaD, or Opa50 (a heparan sulfate proteoglycan binding Opa), along with Opa-deficient (Opa−) bacteria, to assess the ability of Gc to associate with recombinant NCCM1 and 3.45 In the case of GST cleaved NCCMs, precipitation was observed at concentrations greater than ~25 nM; therefore, the GST-NCCM fusion was used for all pull-down assays. To confirm that the GST fusion did not interfere with binding, NCCM1 and GST-NCCM1 binding to Opa expressing Gc were compared and found to be similar (compare supplemental Figure S8 to Figure 2). Since there is little difference between the binding of GST-CCM1 and NCCM1, it is unlikely that there would be a difference between GST-CCM3 and NCCM3 as well. Purified GST-NCCM1 or GST-NCCM3 (referred from here on as NCCM1 and NCCM3) was incubated with each strain of Gc, and the cells with bound NCCM were collected using centrifugation. The fraction of NCCM bound (pellet) and unbound (supernatant) to Gc were determined using SDS-PAGE and immunoblotting. As expected, supernatants from all Gc-NCCM combinations reacted with a pan-CEACAM antibody, and all pellets containing Opa+ bacteria reacted with a pan-Opa antibody (Figure 2). NCCM1 and NCCM3 associated with both Opa60 and OpaD expressing Gc, as assessed by the presence of NCCM in the bacterial pellet. In comparison, Opa50 and Opa− Gc showed negligible association with both NCCMs. These findings validate the use of the pull-down assay for investigating the specificity and selectivity of OpaCEA – NCCM interactions, based on previous reports with MS11 Opa50 and Opa60.39, 53, 54 Furthermore, we report that OpaD expressing Gc binds to NCCM3, as well as NCCM1.
Figure 2.
CEACAM receptors interact specifically with OpaCEA proteins expressed in Gc. Bacteria expressing OpaD, Opa50, or Opa60, or Opa− Gc, were incubated with NCCM1 (A) or NCCM3 (B), and samples were centrifuged for pellet (P) and supernatant (S) immunoblot assessment. Folded Opa proteins migrate at a lower apparent molecular weight than unfolded Opa proteins46, 47; therefore, the two bands correspond to folded (lower band) and unfolded (upper band) protein in the Opa immunoblots. *Higher molecular weight bands in the Gc pellet samples of the CEACAM blots indicate nonspecific CEACAM antibody reactivity with antigens on the surface of Gc. The intensity of NCCM pellet bands were quantified with ImageJ and plotted for each Opa – NCCM combination (C).
Recombinant Opa60 and OpaD reconstituted in liposomes interact with recombinant NCCM1 and 3
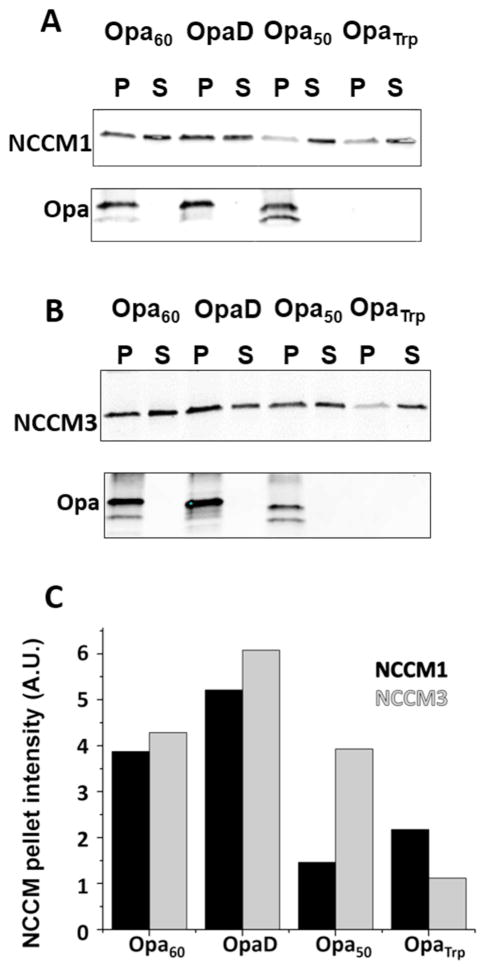
To determine if Opa proteins retain their ability to interact with receptors in vitro, pull-down assays were performed with Opa proteins reconstituted into small unilamellar liposomes (less than 100 nm).46 Bound NCCM was evaluated by comparing the supernatant and pellet after ultra-centrifugation of the Opa proteoliposome – NCCM mixtures, using SDS-PAGE and immunoblotting. While liposomes that do not contain Opa protein would be the optimal negative control, they are too buoyant to be pelleted by centrifugation. Instead, trypsin treated Opa60 (OpaTrp; supplemental Figure S7) was used as a negative control. Trypsin treated Opa60 has the extracellular loops removed, but the barrel remains unperturbed (supplemental Figure S7).47 The supernatants from each proteoliposome – NCCM mixture reacted with the CEACAM antibody, while all of the pellets reacted with the Opa antibody (except for OpaTrp, which likely does not contain the Opa antibody epitope) (Figure 3). Opa60 and OpaD proteoliposome pellets contained both NCCM1 and 3, indicating that these Opa proteins retain their ability to interact with both NCCM1 and 3 when recombinantly expressed and reconstituted into liposomes. For NCCM1, weaker bands are observed for Opa50 and OpaTrp compared to Opa60 and OpaD, indicating that there is some non-specific interaction of NCCM1 with the liposomes. For NCCM3, a band is also observed in the pellet with both OpaTrp and Opa50, indicating non-specific interactions are occurring. However, Opa50 binds less NCCM than both OpaCEA proteins, but more than OpaTrp, which may indicate that Opa50 has different selectivity in vitro compared to that observed in Gc. Together, these results suggest that Opa proteins maintain their ability to interact with receptors in an in vitro liposome environment, but they may not maintain selectivity between CEACAMs.
Figure 3.
Recombinant OpaCEA proteins retain their ability to interact with CEACAM receptors. Opa proteins were recombinantly expressed, purified, and refolded into liposomes, and then incubated with NCCM1 (A) and NCCM3 (B). Samples were then centrifuged and the pellet (P) and supernatant (S) were assessed for the presence of Opa and NCCM. For Opa immunoblots, the two bands correspond to folded (lower band) and unfolded (upper band) protein. The intensity of NCCM pellet bands were quantified with ImageJ and plotted for each Opa – NCCM combination (C).
Several possible scenarios may be able to explain this apparent lack of CEACAM specificity in the in vitro Opa proteoliposome system. The in vitro liposome system contains only purified recombinant Opa proteins, without any other surface structures present on Gc in vivo. While this is necessary to study the effects due to Opa proteins alone, Opa – receptor selectivity may depend on other cellular factors not present in the proteoliposome system. Neisseria possess lipo-oligosaccharides (LOS) on their outer membrane.55 LOS extends above the cell membrane, which may spatially restrict the motion of the Opa extracellular loops. Interactions between Opa proteins and LOS have been demonstrated, and are purported to be electrostatic in nature and involve the basic amino acid residues on the Opa extracellular loops.30, 56, 57 Although PEGylated lipids incorporated in Opa proteoliposomes may mimic the spatial restrictions due to LOS, the PEG polymer will not capture electrostatic interactions between the Opa extracellular loops and LOS.
The liposomal system utilizes not only recombinant Opa proteins, but recombinant NCCMs as well. While the recombinant NCCMs were used in previous Gc binding studies, other CCM domains may additionally be involved in the interaction with Opa proteins. In support of this possibility, Voges et al observed a dependence of Gc engulfment on the presence of the extracellular IgC2 domain of CEACAM1.58 However, since Gc pull-downs demonstrate NCCM selectivity, this is not likely the origin of the loss of selectivity for Opa proteoliposomes.
NCCM1 and 3 interact with Opa60 and OpaD with nanomolar affinities
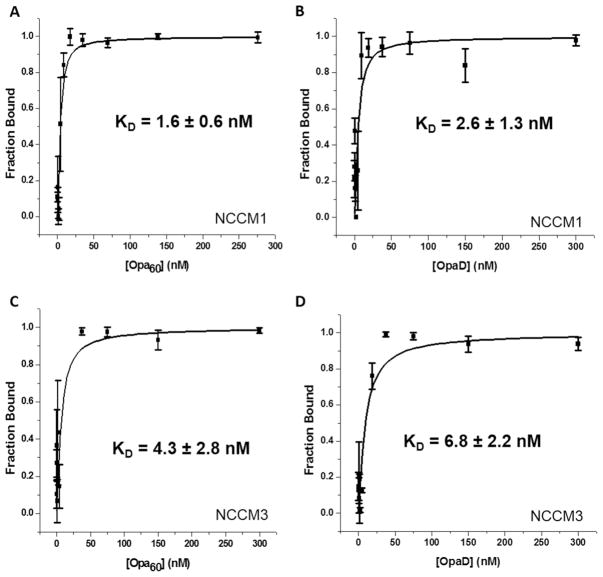
Fluorescence polarization was used to quantify the strength of the interaction between Opa proteins and NCCM1 and 3. These experiments required using NCCMs without the GST tag because of the protein rotational correlation time. The lower concentrations (5 nM) of protein used in the experiment compared to the centrifugal pull-down assays reduced the NCCM oligomerization (unpublished observations). Fluorescently labeled NCCM1 H139C and NCCM3 H139C were incubated with varying concentrations of Opa60 or OpaD proteoliposomes or liposomes without protein, and the fluorescence polarization was measured. The affinities of Opa60 for NCCM1 and NCCM3 were calculated to be 1.6 ± 0.6 nM and 4.3 ± 2.8 nM, respectively, while the affinities of OpaD for NCCM1 and 3 were 2.6 ± 1.3 nM and 6.8 ± 2.2 nM, respectively (Figure 4). To demonstrate the interaction measured was specific, the fluorescence polarization experiments were repeated for Opa60 with a mixture of fluorescently labeled and unlabeled NCCM1 and NCCM3, and the Kd values approximately scaled with the dilution of the fluorescently labeled protein (supplemental Figure S9). Thus, the interactions are high affinity and similar for the combinations of the Opa – NCCM interactions investigated. The affinity of NCCM for liposomes that did not contain any Opa protein was beyond the concentration range investigated (≥ 800 nM) (data not shown).
Figure 4.
OpaCEA proteins in liposomes interact with NCCM receptors with a nanomolar affinity. Varying concentrations of Opa60 (A and C) and OpaD (B and D) were incubated with fluorescently labeled NCCM1 (A and B) or NCCM3 (C and D). Fluorescence polarization was measured and converted into the fraction of CEACAM bound and plotted. Data was fit in OriginPro using eq. 1.
As Opa proteoliposomes are prepared using sonication, we would expect that the Opa proteins are randomly oriented in the liposomes (approximately half with the extracellular loops on the interior of the liposome, and half with the loops on the exterior). However, SDS-PAGE analysis of trypsin treated Opa60 proteoliposomes suggests this is not the case (supplemental figure S7). More than half of the Opa proteins in the liposomes appear to have the extracellular loops cleaved. The addition of the PEGylated lipid in the liposome may force the majority of the Opa proteins to be oriented outward. The interior lipid leaflet of the liposome has a smaller surface area and higher curvature compared to the outer leaflet, and the PEG polymer may sterically restrict the amount of Opa proteins with their loops on the interior. Additionally, the liposomes may be “leaky,” allowing some amount of trypsin to enter the aqueous core of the liposome and cleave the Opa loops. In this case, we would not be able to assess the orientation of the Opa proteins. Therefore, we have analyzed the FP data under the assumption that all of the Opa proteins are oriented with the extracellular loops available for binding, providing a conservative calculation of affinities. Kd values would decrease (higher affinity) if there are fewer Opa proteins available to bind receptors due to an occluded orientation.
In order to effectively compete with the multiple CEACAM interactions, the Opa – CEACAM interaction needs to be high affinity. NCCM is directly involved in cell-cell adhesions, and many of the members of the CEACAM family can be involved in either homotypic or heterotypic interactions.7, 8, 10 Often these heterotypic interactions occur between different CEACAM variants1, but they can also involve other CEACAM domains and other molecules.1, 9, 39 Many of these interactions involve the same binding face of the IgV-like NCCM domain where Opa proteins are known to interact (Figure 5).9, 10, 43 The direct hetero-interaction of NCCM5 and fibronectin was found to have a Kd = 16 nM.2 Abul-Wahid and colleagues calculated the affinity of the A3 soluble IgC-like domain of CEACAM5 to interact with the N-terminal domain with an affinity of 18 nM.2 The hetero-dimerization of NCCM6 with NCCM8 is 2 μM.10 NCCM6 forms a homodimer as well, but with a much lower affinity (60 μM).10 The homo-dimerization of NCCM5 has been reported with two different affinities: approximately 100 nM2 and 1.3 μM10, both of which are tighter than other typical adhesion proteins, such as cadherins.59 The affinity for the homo-dimerization of CEACAM3 is not known; however, the homo-dimerization of NCCM1 is 450 nM.10 Therefore, Opa proteins need to compete with these CEACAM – CEACAM interactions, with many other human proteins that interact with CEACAMs, and potentially with other pathogens, which likely requires a high affinity Opa – CEACAM interaction.
Figure 5.
Surface representation of NCCM1. Residues shown in red are involved in all interactions with OpaCEA proteins. Residues in orange only participate in binding to specific Opa variants. Residues shown in dark blue are involved in NCCM homotypic and heterotypic interactions. Residues highlighted in light blue are also involved in homotypic and heterotypic interactions, and interactions with specific Opa proteins. Residue numbering corresponds to CEACAM1 sequence, UniProt ID P13688-1.
SUMMARY AND CONCLUSION
We report high affinities (nanomolar range) of Neisserial Opa proteins for human CEACAM receptors. While such tight affinities may not be essential for Gc to productively infect host cells, they may be critical for Opa proteins to compete with the multitude of other tight interactions that CEACAMs are capable of forming. A variety of OpaCEA proteins prepared in liposomes maintain their ability to interact with CEACAMs compared to Opa proteins expressed in vivo (in Gc) suggesting a conservation of structure and function independent of the cellular environment. However, the Opa proteoliposomes do not recapitulate the CEACAM selectivity observed with Opa expressing Gc. The in vivo/in vitro toolkit we have developed to study Opa protein interactions with receptors will allow us to explore what drives Opa – CEACAM affinity, specificity, and selectivity.
Supplementary Material
Acknowledgments
Funding Sources: This research was supported by NIH RO1 GM087828 (L.C.), Cottrell Scholar Award, RCSA (L.C.), UVA nanoSTAR (L.C. and A.K.C), NIH R00 TW008042 and R01 AI097312 (A.K.C), and University of Virginia Research and Development Seed Award (A.K.C.). J.N.M was supported in part by NIH T32 GM008136.
We would like to thank Dr. Christopher Stroupe for use of the fluorescent plate reader and technical assistance, Dr. George Bloom and Dr. Antonia Silva for use of the Odyssey imager and technical assistance, and Dr. Rob Nicholas for the NCCM construct.
ABBREVIATIONS
- CEACAM
CCM, carcino-embryonic antigen-like cell adhesion molecule
- IgV
immunoglobulin variable
- IgC
immunoglobulin constant
- NCCM
N-terminal CEACAM domain
- Gc
Neisseria gonorrhoeae
- Nm
Neisseria meningitidis
- ITIM
immuno-tyrosine inhibition motif
- ITAM
immuno-tyrosine activation motif
- Opa
Opacity-associated protein
- SV
semi-variable
- HV
hypervariable
- OpaCEA
a CEACAM binding Opa
- GST
glutathione S-transferase
- GST-NCCM
glutathione S-transferase and N-terminal domain of CEACAM fusion protein
- AMS
4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid, disodium salt
- Opa−
Gc with opa genes deleted
- OpaTrp
trypsinized Opa proteoliposomes
Footnotes
Supporting materials may be accessed free of charge online at http://pubs.acs.org.
Sequence alignment of several Opa proteins (S1), Sequence alignment of human NCCMs (S2), SEC chromatogram of NCCM1 (S3), Structure of NCCM1 H139 (S4), MALDI spectra of fl-NCCMs (S5), SDS-PAGE analysis of Opa folding (S6), SDS-PAGE analysis of OpaTrp (S7), immunoblot of pull-down assay using GST cleaved NCCM1 with Gc (S8), and competition FP assays with Opa60 (S9).
References
- 1.Kuespert K, Pils S, Hauck CR. CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol. 2006;18:565–571. doi: 10.1016/j.ceb.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdul-Wahid A, Huang EHB, Cydzik M, Bolewska-Pedyczak E, Gariépy J. The carcinoembryonic antigen IgV-like N domain plays a critical role in the implantation of metastatic tumor cells. Mol Oncol. 2014;8:337–350. doi: 10.1016/j.molonc.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R, Kujawski M, Pan H, Shively JE. Tumor angiogenesis mediated by myeloid cells is negatively regulated by CEACAM-1. Cancer Res. 2012;72:2239–2250. doi: 10.1158/0008-5472.CAN-11-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zebhauser R, Kammerer R, Eisenried A, McLellan A, Moore T, Zimmermann W. Identification of a novel group of evolutionarily conserved members within the rapidly diverging murine Cea family. Genomics. 2005;86:566–580. doi: 10.1016/j.ygeno.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Sadarangani M, Pollard AJ, Gray-Owen SD. Opa proteins and CEACAMs: pathways of immune engagement for pathogenic Neisseria. FEMS Microbiology Reviews. 2011;35:498–514. doi: 10.1111/j.1574-6976.2010.00260.x. [DOI] [PubMed] [Google Scholar]
- 6.Dehio C, Gray-Owen SD, Meyer TF. The role of neisserial Opa proteins in interactions with host cells. TIM. 1998;6:489–495. doi: 10.1016/s0966-842x(98)01365-1. [DOI] [PubMed] [Google Scholar]
- 7.Öbrink B. CEA adhesion molecules: multifunctional proteins with signal-regulatory properties. Curr Opin Cell Biol. 1997;9:616–626. doi: 10.1016/S0955-0674(97)80114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006;6:433–446. doi: 10.1038/nri1864. [DOI] [PubMed] [Google Scholar]
- 9.Korotkova N, Yang Y, Le Trong I, Cota E, Demeler B, Marchant J, Thomas WE, Stenkamp RE, Moseley SL, Matthews S. Binding of Dr adhesins of Escherichia coli to carcinoembryonic antigen triggers receptor dissociation. Mol Microbiol. 2008;67:420–434. doi: 10.1111/j.1365-2958.2007.06054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonsor DA, Günther S, Beadenkopf R, Beckett D, Sundberg EJ. Diverse oligomeric states of CEACAM IgV domains. Proc Natl Acad Sci U S A. 2015;112:13561–13566. doi: 10.1073/pnas.1509511112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y-H, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen B-S, Melum E, Pertel T, Clayton KL, Raab M, Chen Q, Beauchemin N, Yazaki PJ, Pyzik M, Ostrowski MA, Glickman JN, Rudd CE, Ploegh HL, Franke A, Petsko GA, Kuchroo VK, Blumberg RS. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517:386–390. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCaw SE, Liao EH, Gray-Owen SD. Engulfment of Neisseria gonorrhoeae: Revealing Distinct Processes of Bacterial Entry by Individual Carcinoembryonic Antigen-Related Cellular Adhesion Molecule Family Receptors. Infection and immunity. 2004;72:2742–2752. doi: 10.1128/IAI.72.5.2742-2752.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leusch HG, Drzeniek Z, Markos-Pusztai Z, Wagener C. Binding of Escherichia coli and Salmonella strains to members of the carcinoembryonic antigen family: differential binding inhibition by aromatic alpha-glycosides of mannose. Infection and immunity. 1991;59:2051–2057. doi: 10.1128/iai.59.6.2051-2057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virji M, Evans D, Griffith J, Hill D, Serino L, Hadfield A, Watt SM. Carcinoembryonic antigens are targeted by diverse strains of typable and non-typable Haemophilus influenzae. Mol Microbiol. 2000;36:784–795. doi: 10.1046/j.1365-2958.2000.01885.x. [DOI] [PubMed] [Google Scholar]
- 15.Hill DJ, Virji M. A novel cell-binding mechanism of Moraxella catarrhalis ubiquitous surface protein UspA: specific targeting of the N-domain of carcinoembryonic antigen-related cell adhesion molecules by UspA1. Mol Microbiol. 2003;48:117–129. doi: 10.1046/j.1365-2958.2003.03433.x. [DOI] [PubMed] [Google Scholar]
- 16.Berger CN, Billker O, Meyer TF, Servin AL, Kansau I. Differential recognition of members of the carcinoembryonic antigen family by Afa/Dr adhesins of diffusely adhering Escherichia coli (Afa/Dr DAEC) Mol Microbiol. 2004;52:963–983. doi: 10.1111/j.1365-2958.2004.04033.x. [DOI] [PubMed] [Google Scholar]
- 17.Barnich N, Carvalho FA, Glasser A-L, Darcha C, Jantsheff P, Allez M, Peeters H, Bommelaer G, Desreumaux P, Colombel JF, Darfeuille-Michaud A. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. Journal of Clinical Investigation. 2007;117:1566–1574. doi: 10.1172/JCI30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauck CR, Agerer F, Muenzner P, Schmitter T. Cellular adhesion molecules as targets for bacterial infection. Eur J Cell Biol. 2006;85:235–242. doi: 10.1016/j.ejcb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Frängsmyr L, Baranov V, Hammarstrom S. Four carcinoembryonic antigen subfamily members, CEA, NCA, BGP and CGM2, selectively expressed in the normal human colonic epithelium, are integral components of the fuzzy coat. Tumor Biol. 1999;20:277–292. doi: 10.1159/000030075. [DOI] [PubMed] [Google Scholar]
- 20.Schölzel S, Zimmermann W, Schwarzkopf G, Grunert F, Rogaczewski B, Thompson J. Carcinoembryonic Antigen Family Members CEACAM6 and CEACAM7 Are Differentially Expressed in Normal Tissues and Oppositely Deregulated in Hyperplastic Colorectal Polyps and Early Adenomas. Am J Pathol. 2000;156:595–605. doi: 10.1016/S0002-9440(10)64764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarantis H, Gray-Owen SD. Defining the Roles of Human Carcinoembryonic Antigen-Related Cellular Adhesion Molecules during Neutrophil Responses to Neisseria gonorrhoeae. Infection and immunity. 2012;80:345–358. doi: 10.1128/IAI.05702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HSW, Ostrowski MA, Gray-Owen SD. CEACAM1 Dynamics during Neisseria gonorrhoeae Suppression of CD4+ T Lymphocyte Activation. J Immunol. 2008;180:6827–6835. doi: 10.4049/jimmunol.180.10.6827. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Chen L, Qiao S-W, Nagaishi T, Blumberg RS. Carcinoembryonic Antigen-Related Cell Adhesion Molecule 1 Inhibits Proximal TCR Signaling by Targeting ZAP-70. J Immunol. 2008;180:6085–6093. doi: 10.4049/jimmunol.180.9.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hauck CR, Gulbins E, Lang F, Meyer TF. Tyrosine phosphatase SHP-1 is involved in CD66-mediated phagocytosis of Opa(52)-expressing Neisseria gonorrhoeae. Infection and immunity. 1999;67:5490–5494. doi: 10.1128/iai.67.10.5490-5494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sintsova A, Sarantis H, Islam EA, Sun CX, Amin M, Chan CHF, Stanners CP, Glogauer M, Gray-Owen SD. Global Analysis of Neutrophil Responses to Neisseria gonorrhoeae Reveals a Self-Propagating Inflammatory Program. PLoS Pathog. 2014;10:15. doi: 10.1371/journal.ppat.1004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCaw SE, Schneider J, Liao EH, Zimmermann W, Gray-Owen SD. Immunoreceptor tyrosine-based activation motif phosphorylation during engulfment of Neisseria gonorrhoeae by the neutrophil-restricted CEACAM3 (CD66d) receptor. Mol Microbiol. 2003;49:623–637. doi: 10.1046/j.1365-2958.2003.03591.x. [DOI] [PubMed] [Google Scholar]
- 27.Schmitter T, Agerer F, Peterson L, Münzner P, Hauck CR. Granulocyte CEACAM3 Is a Phagocytic Receptor of the Innate Immune System that Mediates Recognition and Elimination of Human-specific Pathogens. The Journal of Experimental Medicine. 2004;199:35–46. doi: 10.1084/jem.20030204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauck CR, Meyer TF, Lang F, Gulbins E. CD66-mediated phagocytosis of Opa52 Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signalling pathway. Embo J. 1998;17:443–454. doi: 10.1093/emboj/17.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson MB, Ball LM, Daily KP, Martin JN, Columbus L, Criss AK. Opa+ Neisseria gonorrhoeae exhibits reduced survival in human neutrophils via Src family kinase-mediated bacterial trafficking into mature phagolysosomes. Cell Microbiol. 2015;17:648–665. doi: 10.1111/cmi.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox DA, Larsson P, Lo RH, Kroncke BM, Kasson PM, Columbus L. Structure of the Neisserial Outer Membrane Protein Opa60: Loop Flexibility Essential to Receptor Recognition and Bacterial Engulfment. JACS. 2014;136:9938–9946. doi: 10.1021/ja503093y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malorney B, Morelli G, Kusecek B, Kolberg J, Achtman M. Sequence Diversity, Predicted Two-Dimensional Protein Structure, and Epitope Mapping of Neisserial Opa Proteins. Journal of bacteriology. 1998;180:1323–1330. doi: 10.1128/jb.180.5.1323-1330.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bos MP, Kao D, Hogan DM, Grant CC, Belland RJ. Carcinoembryonic antigen family receptor recognition by gonococcal Opa proteins requires distinct combinations of hypervariable Opa protein domains. Infection and immunity. 2002;70:1715–1723. doi: 10.1128/IAI.70.4.1715-1723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bilek N, Ison CA, Spratt BG. Relative Contributions of Recombination and Mutation to the Diversification of the opa Gene Repertoire of Neisseria gonorrhoeae. Journal of bacteriology. 2009;191:1878–1890. doi: 10.1128/JB.01518-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Jonge MI, Vidarsson G, van Dijken HH, Hoogerhout P, van Alphen L, Dankert J, van der Ley P. Functional activity of antibodies against the recombinant OpaJ protein from Neisseria meningitidis. Infection and immunity. 2003;71:2331–2340. doi: 10.1128/IAI.71.5.2331-2340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callaghan MJ, Jolley KA, Maiden MC. Opacity-associated adhesin repertoire in hyperinvasive Neisseria meningitidis. Infection and immunity. 2006;74:5085–5094. doi: 10.1128/IAI.00293-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muenzner P, Dehio C, Fujiwara T, Achtman M, Meyer TF, Gray-Owen SD. Carcinoembryonic antigen family receptor specificity of Neisseria meningitidis Opa variants influences adherence to and invasion of proinflammatory cytokine-activated endothelial cells. Infection and immunity. 2000;68:3601–3607. doi: 10.1128/iai.68.6.3601-3607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen T, Belland RJ, Wilson J, Swanson J. Adherence of pilus- Opa+ gonococci to epithelial cells in vitro involves heparan sulfate. The Journal of Experimental Medicine. 1995;182:511–517. doi: 10.1084/jem.182.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen T, Grunert F, Medina-Marino A, Gotschlich EC. Several Carcinoembryonic Antigens (CD66) Serve as Receptors for Gonococcal Opacity Proteins. The Journal of Experimental Medicine. 1997;185:1557–1564. doi: 10.1084/jem.185.9.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popp A, Dehio C, Grunert F, Meyer TF, Gray-Owen SD. Molecular analysis of Neisserial Opa protein interactions with the CEA family of receptors: identification of determinants contributing to the differential specificities of binding. Cell Microbiol. 1999;1:169–181. doi: 10.1046/j.1462-5822.1999.00017.x. [DOI] [PubMed] [Google Scholar]
- 40.Gray-Owen SD, Lorenzen DR, Haude A, Meyer TF, Dehio C. Differential Opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. Mol Microbiol. 1997;26:971–980. doi: 10.1046/j.1365-2958.1997.6342006.x. [DOI] [PubMed] [Google Scholar]
- 41.Bos MP, Grunert F, Belland RJ. Differential recognition of members of the carcinoembryonic antigen family by Opa variants of Neisseria gonorrhoeae. Infection and immunity. 1997;65:2353–2361. doi: 10.1128/iai.65.6.2353-2361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fedarovich A, Tomberg J, Nicholas RA, Davies C. Structure of the N-terminal domain of human CEACAM1: binding target of the opacity proteins during invasion of Neisseria meningitidis and N. gonorrhoeae. Acta Crystallogr Sec D-Biol Crystallogr. 2006;62:971–979. doi: 10.1107/S0907444906020737. [DOI] [PubMed] [Google Scholar]
- 43.Virji M, Evans D, Hadfield A, Grunert F, Teixeira AM, Watt SM. Critical determinants of host receptor targeting by Neisseria meningitidis and Neisseria gonorrhoeae: identification of Opa adhesiotopes on the N-domain of CD66 molecules. Mol Microbiol. 1999;34:538–551. doi: 10.1046/j.1365-2958.1999.01620.x. [DOI] [PubMed] [Google Scholar]
- 44.Klock HE, Lesley SA. The Polymerase Incomplete Primer Extension (PIPE) method applied to high-throughput cloning and site-directed mutagenesis. Methods in molecular biology. 2009;498:91–103. doi: 10.1007/978-1-59745-196-3_6. [DOI] [PubMed] [Google Scholar]
- 45.Ball LM, Criss AK. Constitutively Opa-expressing and Opa-deficient Neisseria gonorrhoeae strains differentially stimulate and survive exposure to human neutrophils. Journal of bacteriology. 2013;195:2982–2990. doi: 10.1128/JB.00171-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dewald AH, Hodges JC, Columbus L. Physical determinants of beta-barrel membrane protein folding in lipid vesicles. Biophys J. 2011;100:2131–2140. doi: 10.1016/j.bpj.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fox DA, Columbus L. Solution NMR resonance assignment strategies for β-barrel membrane proteins. Protein Sci. 2013;22:1133–1140. doi: 10.1002/pro.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kellogg DS, Jr, Peacock WL, Jr, Deacon WE, Brown L, Pirkle DI. Neisseria gonorrhoeae Ivirulence genetically linked to clonal variation. Journal of bacteriology. 1963;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Criss AK, Seifert HS. Neisseria gonorrhoeae suppresses the oxidative burst of human polymorphonuclear leukocytes. Cell Microbiol. 2008;10:2257–2270. doi: 10.1111/j.1462-5822.2008.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dewald AH. Chemistry. University of Virginia; Virginia: 2012. Folding and biophysical characterization of Neisserial outer membrane Opacity-associated (Opa) proteins in lipid vesicles; p. 217. [Google Scholar]
- 51.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Meth. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veiksina S, Kopanchuk S, Rinken A. Fluorescence anisotropy assay for pharmacological characterization of ligand binding dynamics to melanocortin 4 receptors. Anal Biochem. 2010;402:32–39. doi: 10.1016/j.ab.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 53.Kuespert K, Weibel S, Hauck CR. Profiling of bacterial adhesin — host receptor recognition by soluble immunoglobulin superfamily domains. Journal of Microbiological Methods. 2007;68:478–485. doi: 10.1016/j.mimet.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Fulcher NB. The role of Neisseria gonorrhoeae opacity proteins in host cell interactions and pathogenesis. Department of Microbiology and Immunology University of North Carolina; Chapel Hill, NC: 2004. [Google Scholar]
- 55.Criss AK, Seifert HS. A bacterial siren song: intimate interactions between Neisseria and neutrophils. Nat Rev Micro. 2012;10:178–190. doi: 10.1038/nrmicro2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blake MS, Blake CM, Apicella MA, Mandrell RE. Gonococcal opacity: lectin-like interactions between Opa proteins and lipooligosaccharide. Infection and immunity. 1995;63:1434–1439. doi: 10.1128/iai.63.4.1434-1439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Minor SY, Gotschlich EC. The genetics of LPS synthesis by the gonococcus. In: Goldberg JB, editor. Genetics of Bacterial Polysaccharides. CRC PRess; 1999. pp. 111–131. [Google Scholar]
- 58.Voges M, Bachmann V, Naujoks J, Kopp K, Hauck CR. Extracellular IgC2 constant domains of CEACAMs mediate PI3K sensitivity during uptake of pathogens. PLoS ONE. 2012;7:e39908. doi: 10.1371/journal.pone.0039908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vendome J, Posy S, Jin X, Bahna F, Ahlsen G, Shapiro L, Honig B. Molecular design principles underlying β-strand swapping in the adhesive dimerization of cadherins. Nat Struct Mol Biol. 2011;18:693–700. doi: 10.1038/nsmb.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Cassarino TG, Bertoni M, Bordoli L, Schwede T. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:W252–W258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 62.Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2009;37:D387–D392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guex N, Peitsch MC, Schwede T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective. Electrophoresis. 2009;30:S162–S173. doi: 10.1002/elps.200900140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.