Abstract
Since their inception five decades ago, most antivirals have been engineered to disrupt a single viral protein or process that is essential for viral replication. This approach has limited the overall therapeutic effectiveness and applicability of current antivirals due to restricted viral specificity, a propensity for development of drug resistance, and an inability to control deleterious host-mediated inflammation. As obligate intracellular parasites, viruses are reliant on host metabolism and macromolecular synthesis pathways. Of these biosynthetic processes, many viruses, including Herpes simplex viruses (HSV), are absolutely dependent on the bioavailability of arginine, a non-essential amino acid that is critical for many physiological and pathophysiological processes associated with either facilitating viral replication or progression of disease. To assess if targeting host arginine-associated metabolic pathways would inhibit HSV replication, a pegylated recombinant human Arginase I (peg-ArgI) was generated and its in vitro anti-herpetic activity was evaluated. Cells continuously treated with peg-ArgI for over 48 hours exhibited no signs of cytotoxicity or loss of cell viability. The antiviral activity of peg-ArgI displayed a classical dose-response curve with IC50’s in the sub-nanomolar range. peg-ArgI potently inhibited HSV-1 and HSV-2 viral replication, infectious virus production, cell-to-cell spread/transmission and virus-mediated cytopathic effects. Not unexpectedly given its host-targeted mechanism of action, peg-ArgI showed similar effectiveness at controlling replication of single and multidrug resistant HSV-1 mutants. These findings illustrate that targeting host arginine-associated metabolic pathways is an effective means of controlling viral replicative processes. Further exploration into the breadth of viruses inhibited by peg-ArgI, as well as the ability of peg-ArgI to suppress arginine-associated virus-mediated pathophysiological disease processes is warranted.
Keywords: Herpes Simplex Virus, Arginase, Arginine, Antiviral, Metabolism, HSV-2, HSV-1, Drug-Resistance
1. Introduction
Herpes simplex virus (HSV) type-1 (HSV-1) and HSV type-2 (HSV-2) are closely related members of the human herpesvirus family. The prevalence of HSV-1 is estimated between 50–90% with 70% seropositive by adolescence and near 100% of adults 60 years or older positive for viral DNA (Hill et al., 2008; Xu et al., 2006). Although not as prevalent as HSV-1, HSV-2 is one of the most common sexually transmitted infections (Smith and Robinson, 2002). HSV is associated with genital herpes, herpes labialis, and herpetic keratitis; however, more severe disease manifestations can occur, including neonatal infections and encephalitis (Gnann and Whitley, 2002; Kimberlin, 2005; Whitley, 2002, 2006; Whitley and Gnann, 2002). Following an initial primary infection, HSV establishes a lifelong latent infection that sporadically reactivates leading to episodes of recurrent disease or asymptomatic viral shedding (Koutsky et al., 1990). Therefore, anti-herpetics are often utilized to treat recurrent disease or to prophylactically suppress viral transmission.
Synthetic nucleoside analogues, such as acyclovir, have proven a safe and effective means to inhibit HSV by targeting genomic replication (Dorsky and Crumpacker, 1987). However, the long-term use of these anti-herpetics has resulted in evolution of drug resistant viruses (Field, 1989; Field and Coen, 1986). Although acyclovir resistance was considered rare in immunocompetent individuals (Christophers et al., 1998; Fife et al., 1994; Nugier et al., 1992), some recent studies have shown a higher predilection for resistance within certain groups: children with oral herpetic lesions (≈4%) (Wang et al., 2011); patients with recurrent herpetic keratitis (≈6.5%) (Duan et al., 2009); patients with recurrent genital herpes (Cunningham et al., 2012; Kriesel et al., 2005). The prevalence of drug resistance in immunocompromised depends on the type of immunosuppression, varying between 3.5–7% in HIV patients (Englund et al., 1990; Levin et al., 2004; Reyes et al., 2003; Ziyaeyan et al., 2007) to 25% in allogeneic hematopoietic stem cell transplant recipients (Langston et al., 2002). These increasing trends have generated renewed interest in identifying novel targets that can suppress viral replication and minimize the likelihood of developing future resistance.
As obligate intracellular parasites, viruses are dependent on their host’s metabolism and macromolecular synthesis pathways, often redirecting cellular processes to provide resources necessary for replication (Maynard et al., 2010). Of the numerous biosynthetic processes, many viruses are dependent on arginine bioavailability or de novo biosynthesis (Gonczol et al., 1975; Tankersley, 1964; Wigand and Kumel, 1978). Arginine is a versatile non-essential amino acid that is interconvertible with proline, ornithine, glutamate, and citrulline (Morris, 2006; Wu and Morris, 1998). Biochemically, arginine serves as a precursor for synthesis of protein, nitric oxide, polyamines and nucleotides (Morris, 2007). Physiologically, arginine and its metabolites have critical functions in innate and adaptive immunity, inflammation, wound healing, and vascularization (Morris, 2006, 2007; Wu et al., 2009; Wu and Morris, 1998). However, these normally physiological processes often contribute to severe virus-induced disease. Therefore, arginine bioavailability is a critical determinant of several physiological and pathophysiological processes that are involved both in facilitating viral replication and progression of disease.
Importantly, arginine is essential for replication of many viruses, including HSV (Gonczol et al., 1975; Tankersley, 1964; Wigand and Kumel, 1978). To evaluate the potential of targeting host arginine-associated metabolic pathways for control of HSV, we generated a pegylated human recombinant Arginase I (peg-ArgI). peg-ArgI exhibited no demonstrable toxicity and effectively inhibited HSV replication, infectious virus production, cell-to-cell transmission, and virus-induced cytopathic effects. These findings illustrate that targeting host arginine-associated metabolic pathways is as effective as current nucleoside analog anti-herpetics in its ability to control HSV replication.
2. Materials and Methods
2.1. Viruses and cells
Primary human corneal epithelial cells (HCEC) were from Invitrogen. HSV-1(McKrae) was the parental wildtype HSV-1 strain utilized. The G strain of HSV-2 was obtained from ATCC. The parental HSV-1 (KOS) and its drug resistant derivatives that specified mutations in either the polymerase (PAAr5) or thymidine kinase (tkLTRZ1; tkG7dG.2) genes were a kind gift from Dr. Donald Cohen (Coen and Schaffer, 1980; Fleming and Coen, 1984; Griffiths and Coen, 2003; Horsburgh et al., 1998). All HSV viral stocks were propagated in Vero cells and stored as infectious cell preparations at −80°C.
2.2. Production, pegylation and protein analysis of recombinant human ArginaseI
Recombinant human ArginaseI (ArgI) was expressed in E. coli and purified by AbboMax (San Jose, CA). Protein concentrations were determined by the Pierce BCA Protein Assay kit. To increase enzymatic activity and protein stability, ArgI was covalently conjugated to O-[2-(N-Succinimidyloxycarbonyl)-ethyl]-O’-methylpolyethylene-glycol (PEG) 5000mw (Sigma-Aldrich) (Cheng et al., 2007; Tsui et al., 2009) at a protein:PEG molar ratio of 1:18. Samples were maintained at a constant pH=7.5 using 1N NaOH and stirred at RT for 3h. After PEGylation the peg-ArgI solution was brought to a pH=7.0. The relative purity of ArgI and the extent of protein conjugated to PEG were assessed by SDS-PAGE with Novex SimplyBlue Safe Stain. The specificity of the protein expressed and purified as human Arginase I was assessed by western analysis using an antibody to human Arginase I (Cell Signaling). The extent of PEG covalently incorporated onto ArgI or BSA was assessed by western with an antibody against covalently attached or free PEG molecules (abcam; ab133471).
2.3. Arginase activity assays and determination of arginine and glutamine levels by HPLC
The enzymatic activity of peg-ArgI was determined across a 2-fold dilution series (from 5000–19.5ng) by measuring the production of L-ornithine from L-Arginine, relative to a standard curve of L-ornithine as previously reported (Rodriguez et al., 2004). For determination time-dependent depletion of arginine for each dosage of drug, pre-warmed DMEM, or medium containing 2-fold serial dilutions of peg-ArgI (from 10,000-78ng/ml) was incubated at 37°C. At 5, 10, 20, 40, 60, 180, or 360 minutes post treatment, media was deproteinized with methanol, centrifuged and supernatants were stored at −80°C u ntil analysis. Deprotonated media was derivatized with 0.2 mol/L ophthalaldehyde/β-mercaptoethanol and fifty microliters per sample was analyzed by high-performance liquid chromatography (HPLC)-electrochemical detector on an ESA CoulArray Model 540, with an 80 × 3.2 column with 120A pore size. Standards of Larginine or glutamine in methanol were run with each experiment and the levels of each amino acid in a test sample were determined relative to this standard curve. Experiments for each time point and dosage were repeated at least three times.
2.4. Cytotoxicity and cell viability assays
The cytotoxic effects of peg-ArgI and other anti-herpetics was assessed on Vero cells (according to Clinical Laboratory Standards Institute (CLSI) guidelines) (Sweirkosz, 2004), as well as primary HCEC. 8,000cells/well were seeded to 96-well plates. The next morning, media was aspirated and replenished with media containing either: 1) 10µg/ml of peg-ArgI; 2) 10µg/ml of peg-BSA; 3) Mock treatment; 4) 1% triflouorothymidine (TFT); 5) 50µM Acyclovir (ACV; Sigma Chemical); or 6) 300µM ACV. The relative cytotoxic effect of each compound was determined for 5 replicates at 4h, 24h, and 48h later using the Promega CellTox Green Cytotoxicity Assay according to the manufacturer’s directions on a Tecan Infinite M200 scanning microplate spectrofluorometer. Trypan blue exclusion was utilized to visualize drug toxicity effects over the course of 15 days of continuous drug treatment. Subconfluent Vero cell monolayers were mock treated or treated with: peg-ArgI at 1µg/ml or 10µg/ml; 1% trifluorthymidine (TFT); or ACV at 1µg/ml or 67µg/ml. At 12h, 24h, 48h, 8 days, and 12days post treatment, cell toxicity was assessed by incubating treated cells with trypan blue for 5 minutes and visualizing uptake by fluorescent microscopy using a PI filterset. At day 12 post treatment, media was replaced with fresh media supplemented with their respective drug concentrations. 3 days later, (15 days post initial treatment) cell toxicity was assessed by trypan blue. Phase and fluorescent images of cell monolayers were independently captured using a Zeiss Axiovert fluorescent microscope. Representative images were overlaid within the Zeiss imaging software and compiled without image editing in photoshop.
2.5. Viral yield reduction assay, virus titrations, and inhibition of virus-mediated cytopathic effects
To evaluate the effects of peg-ArgI or other anti-herpetics on reduction of HSV infectious virus yield, Vero cell monolayers in 12-well plates were either left uninfected or infected with HSV-1, HSV-2 or HSV-1 drug resistant viruses at an MOI of 0.1, 1.0 or 5.0. After 2h adsorption at 4°C, virus was removed and cells w ere overlaid with pre-warmed medium or medium containing 2-fold serial dilutions of peg-ArgI (from 10,000-19.5ng/ml). Virus was allowed to penetrate for 2h at 37°C after which ext racellular virus was inactivated by low-pH treatment (Kadeppagari et al., 2012; Sanchez et al., 2012). Cells were subsequently washed twice with complete media and overlaid with media containing peg-ArgI. After incubation at 37°C for 24h, cells and supernatants were frozen and thawed three times to obtain total infectious virus. Viral yield was determined by endpoint titration plaque assays on Vero cells. To evaluate the effects of peg-ArgI and other anti-herpetics on reduction of HSV-mediated cytopathic effects (CPE), Vero cell monolayers in 12-well plates were infected and treated identically to as described above for determination of viral yields. The effects of treatments on HSV-1-induced CPE were visualized at 24hpi by phase-contrast microscopy.
2.6. Time of treatment viral yield assay
The effects of timing of treatment on the ability of peg-ArgI to affect HSV-1 replication was determined using a viral yield reduction assay. Vero cells were mock treated or treated with peg-ArgI (500 or 5,000 ng/ml) or peg-BSA (5,000ng/ml) at 8, 4, 2, and 1 hour prior to infection, at the time of infection (0h), or at 1, 2, 3, 4, 5, 6, 8, 16, 20 and 24 hours post infection. 48h post infection, cells and supernatants were frozen and thawed three times to obtain total infectious virus. Viral yield was determined by endpoint titration plaque assays on Vero cells and drug treatment effects were expressed as fold reduction relative to mock treated controls from the same time point. All experiments were repeated in triplicate and expressed as mean +/− SEM.
2.7. Plaque reduction assay
The HSV-1 plaque reduction assay was performed essentially according to CLSI guidelines (Sweirkosz, 2004). HSV-1 virus was added to 90% confluent Vero cell monolayers in 6-well plates at a titer of ≈250 plaque forming units (PFU)/well. After incubation for 1h, the inoculum was removed and DMEM supplemented with 0.5% carboxymethylcellulose and the indicated concentrations (serial 2-fold dilutions from 10,000-19.5ng/ml) of peg-ArgI was added. After 72h incubation, the number of viral plaques was determined by counting visually under high powered magnification. Any focal point of CPE no matter the size was counted as a plaque. The percentage of inhibition for each treatment condition was determined relative to the average number of plaques in the mock treated controls. Results were log transformed and analyzed in GraphPad by nonlinear regression using the program’s inhibitor response analysis package. The concentration of drug that inhibited a given percentage of the plaque formation in each well (% Inhibitory Concentration; ICx%), as well as the 95% confidence intervals for each IC was determined using the software’s inhibitory dose response analysis package.
3. Results
3.1. Production and characterization of the host-targeted antiviral, peg-ArgI
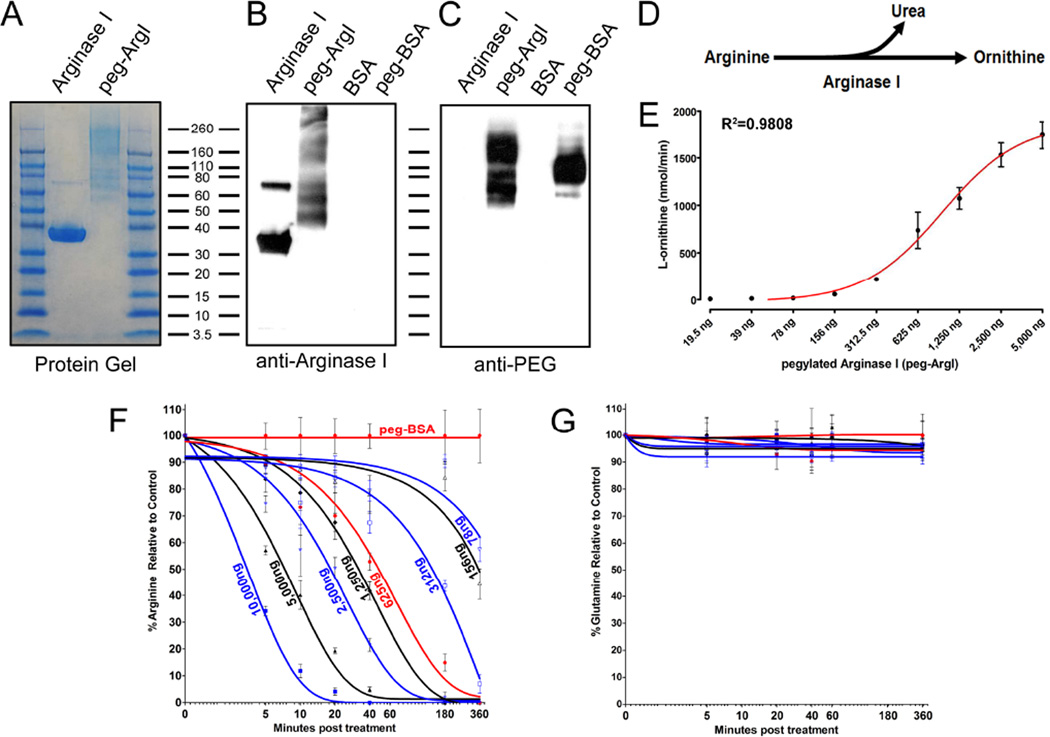
HSV, like many viruses, is critically dependent upon arginine availability for productive replication (Gonczol et al., 1975; Tankersley, 1964; Wigand and Kumel, 1978). To modulate host arginine-associated metabolic pathways, we generated a recombinant human ArgI that was covalently conjugated to PEG. SDS-PAGE and western analysis revealed that the purified recombinant protein was ArgI and specified the appropriate apparent molecular weight of ≈36kDa with an absence of protein contaminates (Fig.1A;B). ArgI and control bovine serum albumin (BSA) was subsequently modified by pegylation to increase its biological stability and enzymatic activity. Following pegylation, ArgI was visualized as a heterogeneous pool of protein products with molecular weights that ranged from ≈70–250kDa (Fig.1A;B). This heterogeneous pool of peg-ArgI was most likely due to variation in the extent of PEG molecules covalently coupled onto each protein molecule. Importantly, there was no native ArgI present following pegylation, indicating that all protein had incorporated some PEG molecules under these conjugation conditions. PEG incorporation onto ArgI and BSA was confirmed by visualizing free and covalently coupled PEG via western analysis (Fig.1C). In agreement with ArgI analysis, pegylated protein was observed from 70–250kDa, with a majority of protein extensively pegylated and greater than 160kDa. Similarly, pegylated-BSA was observed as an approximately heterogeneous product centralized around 110kDa (Fig. 1C).
Figure 1.
Production and evaluation of an enzymatically active pegylated recombinant Arginase I for modulation of host arginine-associated metabolic pathways. Recombinant human ArgI was produced by expression in E. coli, column purified, and covalently conjugated to PEG-5000. (A) The purity of the purified recombinant ArgI, as well as the extent of pegylation were assessed by staining for total protein following SDS-PAGE (A). (B; C) The specificity of the expressed product and the presence of PEG was determined by western analysis for processed ArgI and control BSA using antibodies against either human ArgI (B) or PEG molecules (C). (D) ArgI catalyzes the hydrolysis of Arginine to Urea and Ornithine. (E) The enzymatic activity of 2-fold serially diluted concentrations of peg-ArgI was determined by measuring the conversion of arginine to the end-product L-ornithine. Each point represents the mean +/− the S.E.M. of samples analyzed in triplicate. (F; G) Dose-response and time-dependent effects of peg-ArgI treatment on the relative levels of either arginine (F) or glutamine (G). DMEM media was treated with serial 2-fold dilutions of peg-ArgI from 10µg/ml to 78ng/ml the levels of arginine or glutamine were determined in triplicate by HPLC at the time points indicated.
The ability of recombinant peg-ArgI to modulate host arginine-associated metabolic pathways and control viral replication is dependent on retention of its functional enzymatic activity. ArgI catalyzes the hydrolysis of arginine to urea and ornithine (Fig.1D). Ornithine production was determined across two-fold serial dilutions of peg-ArgI from 5,000-19.5ng (Fig.1E). peg-ArgI exhibited a non-linear dose-dependent conversion of arginine to ornithine, with an 50% effective concentration (EC50) in this assay of 932ng and a 95% confidence interval of 746–1163ng. The kinetics by which varying dosages of peg-ArgI can specifically deplete exogenous arginine was assessed by HPLC (Fig. 1F;G). peg-ArgI exhibited a dose-dependent kinetics of arginine depletion, with high dosages depleting arginine by 50% within minutes of addition. All dosages above 625ng/ml completely depleted arginine to nearly undetectable levels within 180 minutes. However, there was a notable shift in kinetic profiles at dosages below 625ng/ml where a clear transition zone for both kinetics and ability to substantially deplete arginine was visualized (Fig. 1F). Importantly, peg-ArgI treatment had no effect on glutamine levels relative to controls (Fig. 1G). This biologically active peg-ArgI was utilized to assess the feasibility of modulating host arginine-associated metabolic pathways for inhibition of HSV replication.
3.2. Assessment of cell viability and cytotoxicity following treatment with peg-ArgI
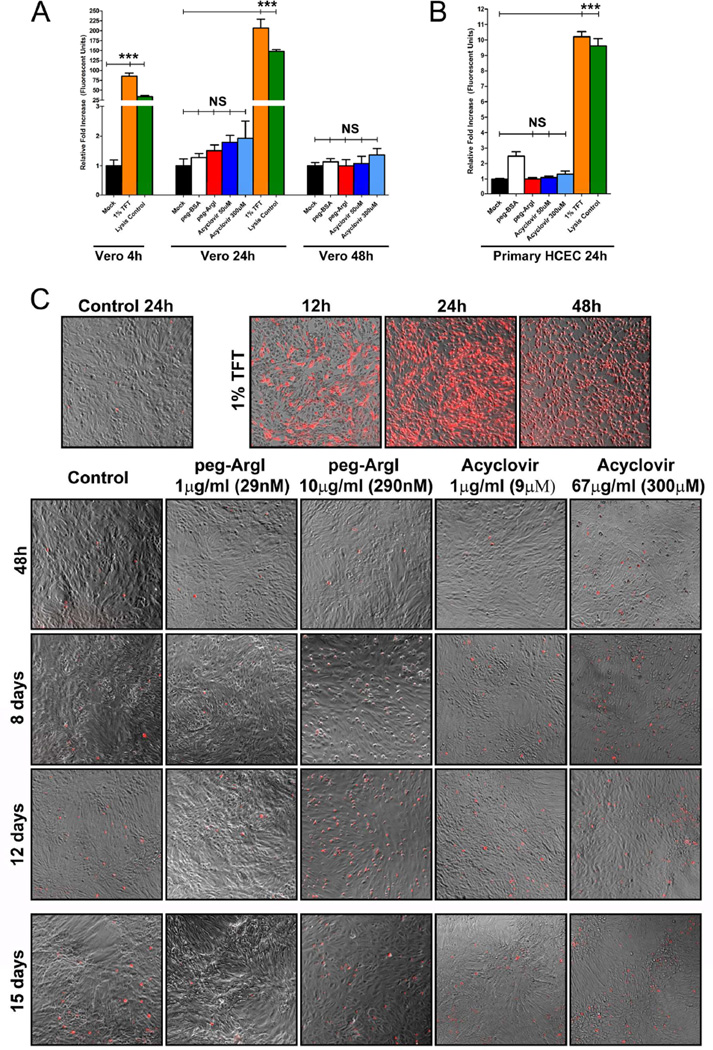
The effects of peg-ArgI on cell viability were assessed on Vero cells (Fig.2A), as well as on primary human corneal epithelial cells (Fig.2B). peg-ArgI treated Vero cells exhibited no deleterious changes in cell viability or pronounced cytotoxic effects at 24h or 48h posttreatment. Similarly, primary HCEC treated with peg-ArgI for 24h had no discernible changes in cell viability compared to mock treated controls. Consistent with its established safety profiles, high dose acyclovir treatment of both Vero and HCEC cells exhibited little to no changes in cell viability relative to control treatments. In contrast, Vero and HCEC cells treated with the ophthalmic anti-herpetic TFT showed markedly decreased cell viability following 4h of exposure and complete death by 24h. This amount of cell death was greater than that observed in detergent lysed cell controls.
Figure 2.
peg-ArgI exhibits low cytotoxicity in both established and primary cells. The cytotoxicity of peg-ArgI (red) relative to the anti-herpetics, Acyclovir (shades of blue) or 1% Trifluorothymidine (TFT; orange), was determined in either CLSI standard (Sweirkosz, 2004) Vero cells (A) or primary human corneal epithelial cells (B) at 4, 24, or 48h post-treatment. Values from mock treated cells were set to 1 and cytotoxicity is expressed as a fold increase in toxicity relative to mock treatment (black). Detergent lysed cells (Lysis Control; green) were used as a positive control. Each bar represents the mean +/− the S.E.M. of five replicates. Experiments were repeated at least 3 times. (C) Comparison of long-term continuous treatment toxicity effects for peg-ArgI versus the anti-herpetics TFT or Acyclovir. Subconfluent Vero cells were continuously treated with individual drugs at the indicated concentrations for 12 days and relative toxicity was visualized at the days indicated by trypan blue exclusion assay and fluorescent microscopy. At day 12, the media was refreshed with new drug and final toxicity was visualized on day 15 post initial treatment.
To assess longer-term consequences of continuous peg-ArgI treatment, trypan blue exclusion assays were utilized to visualize cell viability over a 15 day treatment course (Fig.2C). As expected, TFT treatment induced an immediate loss of cell viability that reached 100% cell death by 24h post-treatment. By contrast, both peg-ArgI and ACV had minimal effects on cell viability over the 15 days examined. Although peg-ArgI treatment at 1µg/ml seemed to have less toxicity than controls, continuous 10µg/ml peg-ArgI exhibited some signs of toxicity starting at day 8 and continuing through day 12. At day 12, all media with corresponding drugs was replaced. This refreshing of media and drug appeared to reinvigorate the 10µg/ml peg-ArgI treated cells, indicating that at high doses of peg-ArgI treatment cells may be shunting other nutrients from the media to compensate for severe/continuous arginine depletion. Taken together, these results indicate that peg-ArgI modulation of host arginine-associated metabolic pathways does not drastically alter cell viability during 48h of continuous treatment and at doses below 10µg/ml, peg-ArgI has little effect on cell viability over a two week treatment period.
3.3. peg-ArgI inhibits HSV-1 viral replication and virus-mediated cytopathic effects
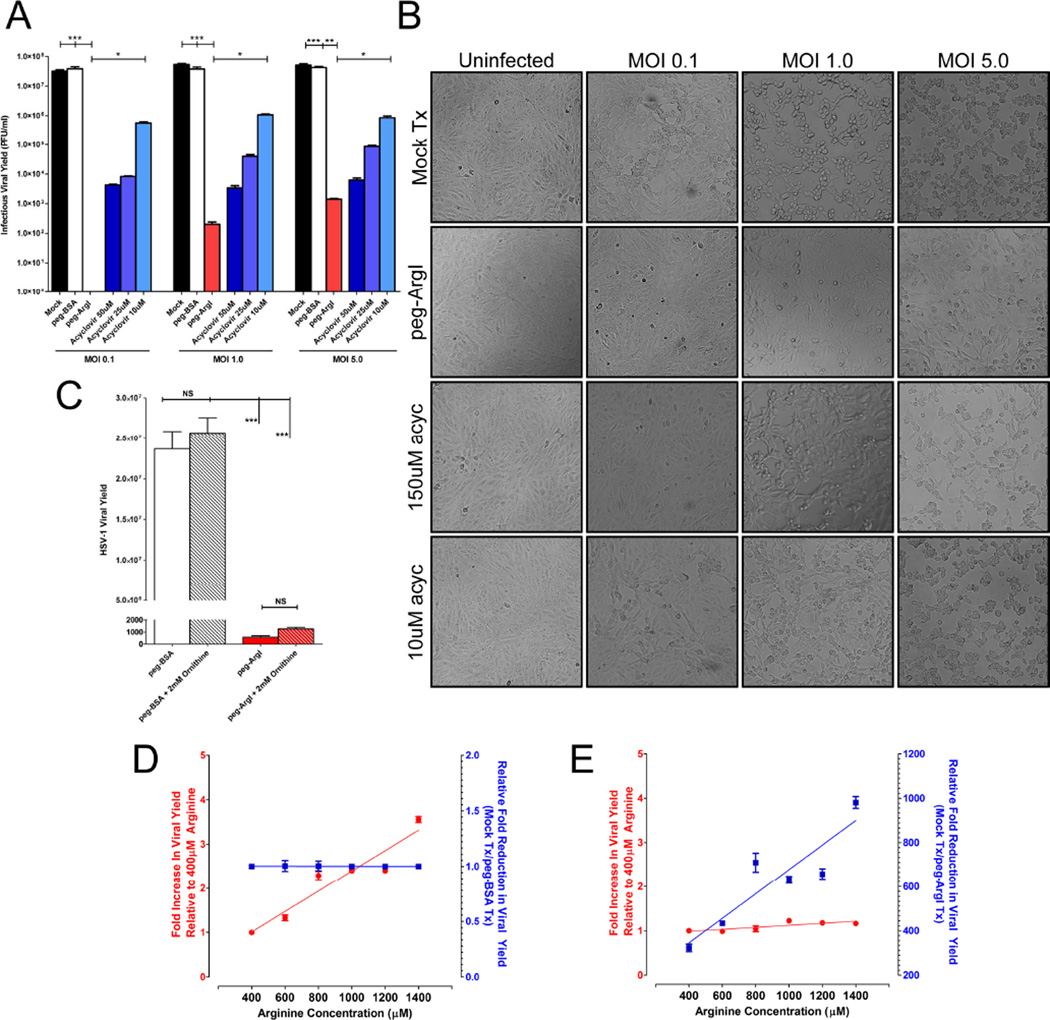
To determine if peg-ArgI possessed antiviral activity, Vero cells were infected at low to high MOIs (0.1; 1.0; 5.0) and the yield of infectious virus (Fig.3A), as well as HSV-mediated CPE (Fig.3B) were assessed 24hpi. Mock and peg-BSA negative control treated HSV-1 infected cells had similar viral yields that were substantially reduced across all MOIs following treatment with either peg-ArgI or ACV. Infectious HSV-1 was undetectable in peg-ArgI treated Vero cells infected at an MOI of 0.1. At MOIs of 1 and 5, peg-ArgI reduced total viral yields by greater than 5 and 4 logs, respectively. By comparison, at all MOIs, 10µM ACV only reduced viral yields by less than 100 fold. Therefore, the antiviral activity of peg-ArgI was significantly greater than 10µM ACV (p<0.01) and substantially reduced infectious HSV production relative to 50µM or 25µM ACV treatment.
Figure 3.
peg-ArgI depletion of arginine potently inhibits infectious HSV-1 viral yield and suppresses HSV-mediated cytopathic effects across a range of multiplicity of infections. (A) Vero cells were infected at a multiplicity of infection (MOI) of 0.1, 1.0 or 5.0 and were subsequently treated with either peg-ArgI (5µg/ml; red) or varying doses of acyclovir (shades of blue). The total HSV-1 infectious viral yield was determined at 24h post-infection by titering lysed cell stocks on Vero cells. Each bar represents the mean +/− the S.E.M. of six replicates. Experiments were repeated at least 3 times. One way analysis of variance (ANOVA) with Kruskal-Wallis post-test was utilized to analyze differences between all treatment groups. Asterisks indicate significant differences between the indicated treatments: *** p<0.0001; ** p<0.001; * p<0.01. The limit of detection for these assays was 50 plaque forming units/ml. (B) peg-ArgI treatment suppresses HSV-mediated cytopathic effects. Vero cells were infected with HSV-1(McKrae) at an MOI of 0.1, 1.0 or 5.0 and subsequently treated with peg-ArgI (1.25µg/ml) or acyclovir (10 or 150µM). The extent of HSV-mediated cytopathic effects were visualized by phase-contrast microscopy at 24h post-infection and digitally photographed. (C) Increased ornithine levels are not responsible for the anti-herpetic effects associated with peg-ArgI mediated conversion of arginine to ornithine. Addition of 2mM ornithine to either normal media (gray bars) or to media treated with peg-ArgI (red bars) did not inhibit replication of HSV-1. (D) Increasing concentrations of arginine above the 400µM baseline results in concomitant increases in infectious HSV-1 viral yield (D; red). Treatment of infected cells with control peg- BSA does not affect viral yields across all doses relative to mock treatments (D; blue). (E) peg- ArgI treatment nullifies the additive replicative effects of increasing arginine concentrations (E; red); thereby, increasing the fold reduction in HSV-1 viral yield relative to mock treatments (E; blue).
At 24 hpi, HSV-1 infected Vero cell monolayers exhibit CPE, indicative of a productive viral infection. The extent of this CPE is dependent upon the initial MOI, as well as the extent of viral replication. At low MOIs (0.1) CPE is present as viral plaques within the cell monolayer due to cell-to-cell spread; whereas at higher MOIs (1 and 5) 100% of the cell monolayer is rounded and detaching (Fig.3B). Although treatment of HSV-1 infected Vero cells with 10µM ACV suppressed infectious viral yield across all MOIs (Fig.3A), there was little, if any, inhibitory effect on HSV-mediated CPE or cell-to-cell spread (Fig.3B). Similarly, although 150µM ACV suppressed CPEs at low MOI, CPE was readily observed at MOIs of 1 and 5. In contrast, peg-ArgI treatment abolished HSV-mediated CPE at MOIs of 0.1 and 1, although some singly rounded cells could be observed at an MOI of 1. Some CPE was apparent in peg-ArgI treated Vero cells that were infected at an MOI of 5; however, the extent of cell damage and cell-to-cell spread was significantly lower than in untreated or high-dose ACV treated infections. In addition, consistent with the cytotoxicity data, cells treated with peg-ArgI had no obvious changes in cell morphology or monolayer formation. These antiviral effects were not due to peg-ArgI-mediated metabolism of arginine to ornithine. Addition of 2mM ornithine to HSV-1 infected cell cultures that either were cultured in media that had arginine present or were depleted of arginine elicited no significant effect on yield of infectious HSV-1 (Fig.3C). By contrast, in the absence of peg- ArgI, increasing levels of arginine within cultured media concomitantly increased HSV-1 viral yield (Fig3D; red). These results indicate that increasing arginine levels directly influenced HSV-1 replication. Increased levels of arginine in the presence of 500ng/ml peg-ArgI had no significant effect on HSV-1 replication, indicating that this concentration of peg-ArgI was effective at metabolizing the additional arginine (Fig. 3E; red), thereby increasing the overall fold reduction in viral yield relative to controls (Fig.3E; blue). As expected, treatment with control peg-BSA had no effect on reducing viral yield (Fig. 3D; blue). Taken together, this data demonstrates that peg-ArgI effectively inhibits HSV-1 replication directly through its effects on exogenous arginine availability and that depletion of exogenous arginine does not deleteriously affect host cell viability.
3.4. Assessment of effects of varying time of peg-ArgI treatment relative to infection
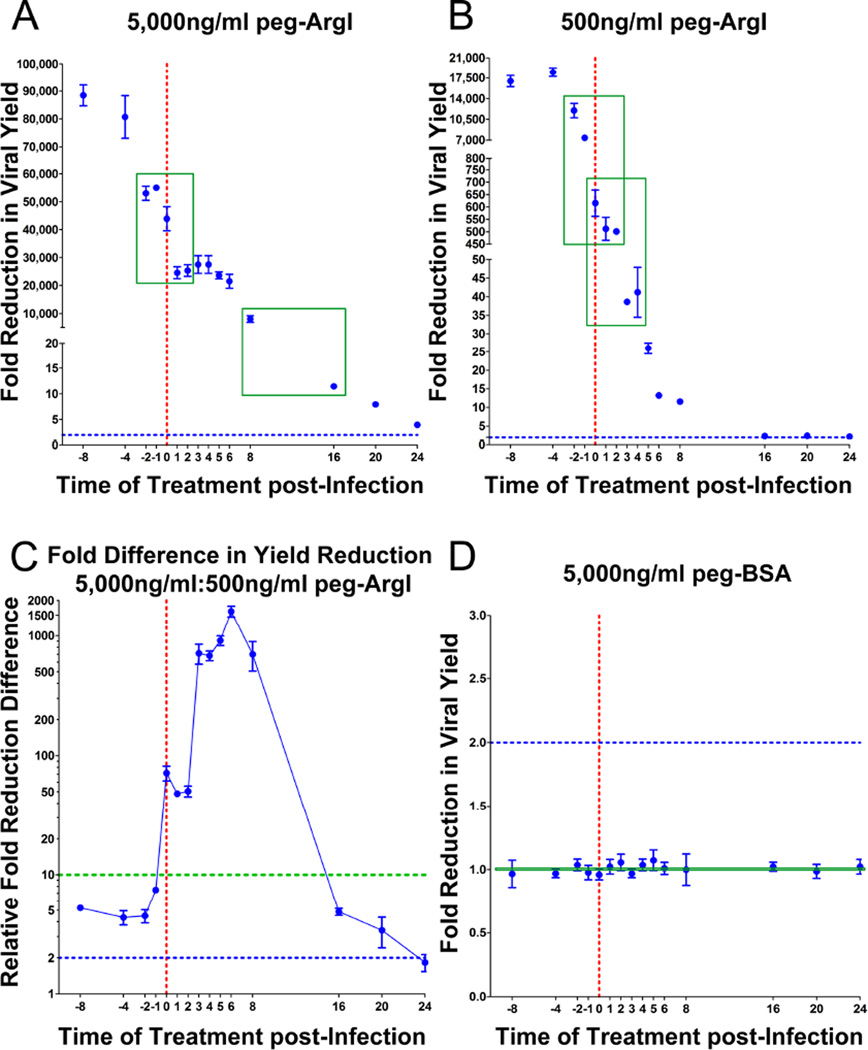
Determination of antiviral effects of a drug across a time course of HSV infection can yield insights into its potential mechanisms of action, as well as provide information as to when it is most effectively delivered relative to time of infection. Therefore, HSV-1 infected cells were treated with 5,000ng/ml or 500ng/ml peg-ArgI at the indicated time points and the fold reduction in viral yield relative to mock treated controls was determined (Fig.4A;B). In both dosages, treatment of cells prior to infection yielded the greatest increase in antiviral activity, maxing out approximately 4 hours prior to infection. However, treatment of cells with 5,000ng/ml peg-ArgI reduced viral yield by greater than 7,000 fold for up to 8h post infection and less than 2 fold loss of antiviral activity for up to 6h post infection (Fig.4B). By contrast, 500ng/ml peg-ArgI exhibited a marked decrease in effectiveness between 2 and 3h post infection (Fig.4B). Comparisons of fold reduction differences between these two dosages indicated that treatment with 5,000ng/ml had compounding antiviral effects that could not be simply accounted for by a 10-fold difference in concentration (Fig.4C). As expected, treatment with control peg-BSA, did not reduce viral yield across all time points (Fig.4D). Taken together, these results were a bit unexpected given that we had previously observed that arginine levels were reduced to nearly undetectable within a short treatment window for both of these dosages, yet the degree to which the individual dosages inhibit viral replication and the timing at which treatment was effective were markedly different.
Figure 4.
The ability of peg-ArgI to significantly affect HSV-1 viral yields is dependent on time of treatment and dose. (A;B) Vero cells were treated with peg-ArgI at 5,000ng/ml (A) or 500 ng/ml (B) at indicated time points relative to the time of HSV-1 infection (0 hour; red dotted line) and the fold reduction in viral yield relative to mock treatments were determined. Two windows where significant changes in peg-ArgI’s ability to control viral yield were observed are highlighted (green boxes). A baseline two-fold change is indicated on each graph as a blue dotted line (A–D). (C) The relative fold difference in viral yield reduction between treatments with 5,000ng/ml versus 500ng/ml for each time point is depicted. Differences above the 10 fold increase in peg-ArgI concentration are identified by points above the green dotted line. (D) Treatment of infected cells with peg-BSA had no effect on HSV-1 viral yields independent of time of treatment.
3.5. Determination of effective inhibitory concentrations for peg-ArgI-mediated antiviral activities
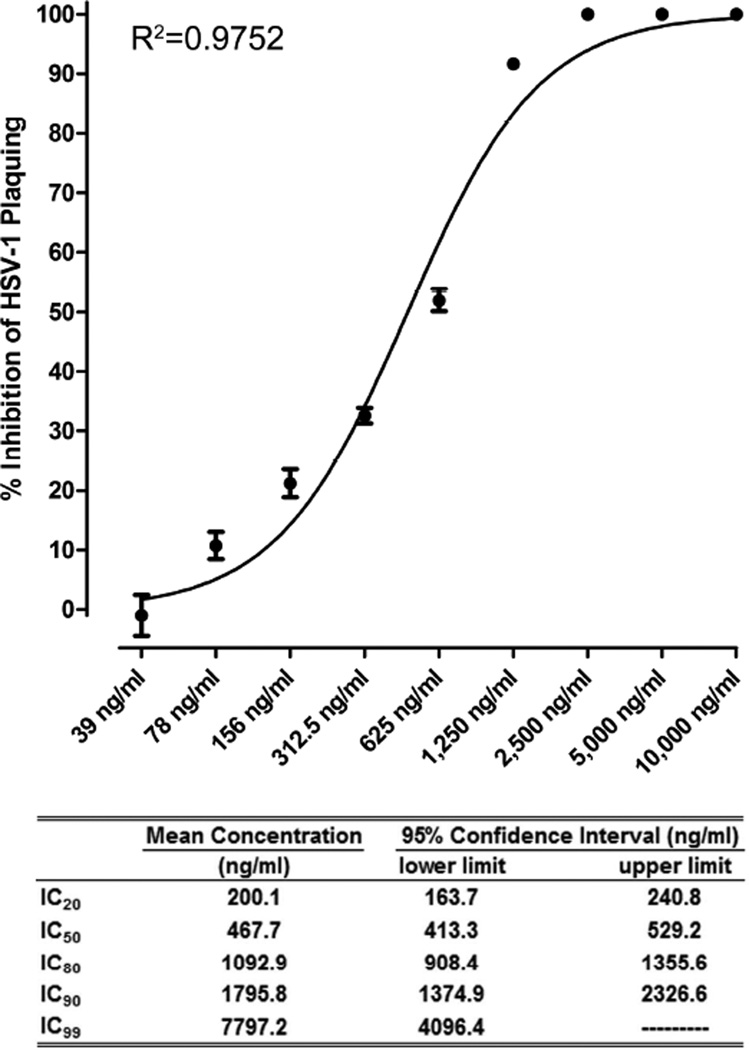
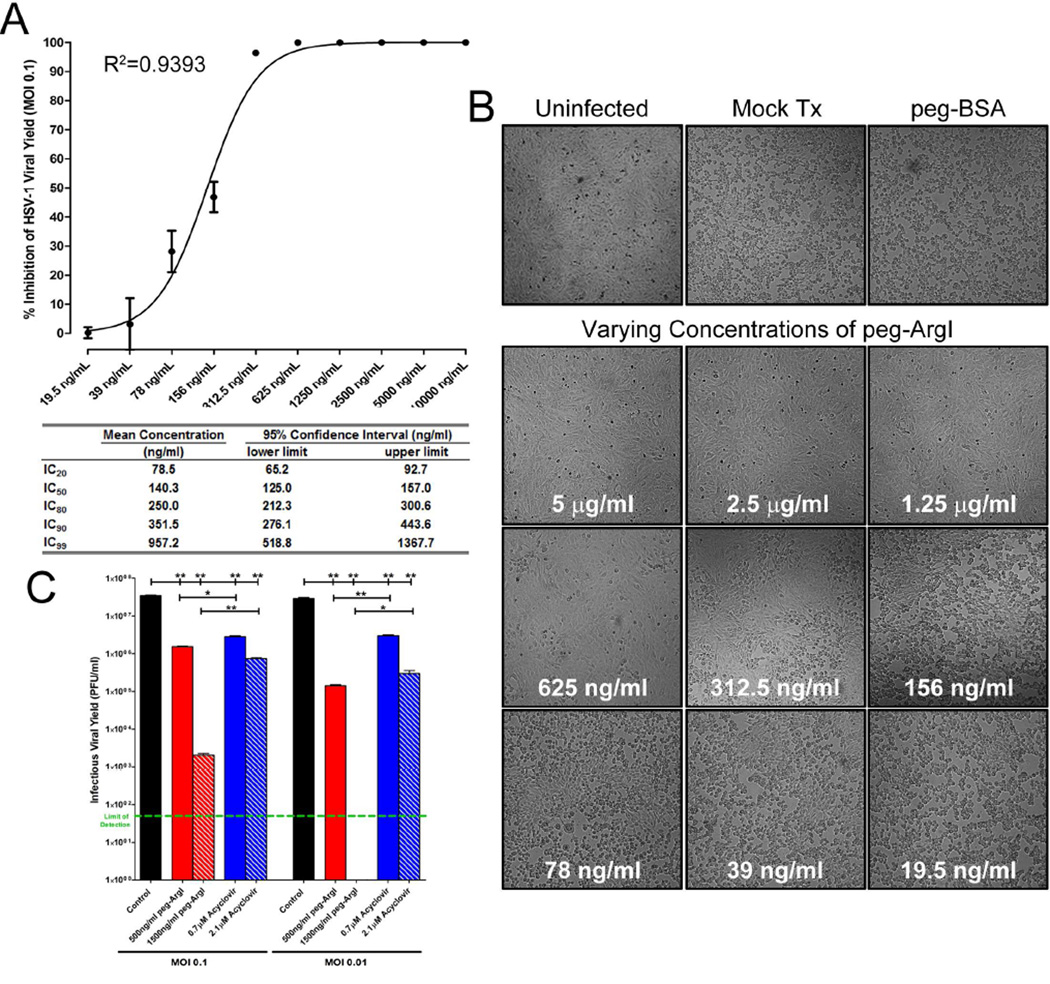
The CLSI has defined a standardized plaque reduction assay for establishment of HSV antiviral drug susceptibility (Sweirkosz, 2004). Vero cells were infected with approximately 250PFU of HSV-1 and treated with serial two-fold dilutions of peg-ArgI (10µg/ml to 39ng/ml). Log transformed dose-inhibition response analysis demonstrated a non-linear dose response effect for peg-ArgI-mediated inhibition of HSV-1 plaquing, with concentrations greater than 2,500ng/ml completely ablating the appearance of HSV-mediated CPE (Fig.5). The concentration of peg-ArgI resulting in 50% inhibition of HSV PFU (IC50) was 467.7ng/ml with a 95% confidence interval between 413.3–529.2ng/ml. Other calculated percent inhibition calculations are shown in Fig.5. A similar dose response analysis was performed on HSV-1 viral yield following infection of Vero cells at an MOI of 0.1 (Fig.6A). This analysis showed a similar non-linear dose response for inhibiting infectious HSV-1 production, with peg-ArgI concentrations greater than 1250ng/ml completely abolishing detection of any infectious virus (Fig.6A). The calculated percent inhibitory concentrations for inhibition of HSV-1 viral yield, as well as their respective 95% confidence intervals are shown in Fig.6A. Concurrent with this analysis, the ability of peg-ArgI to inhibit HSV-mediated CPE and HSV cell-to-cell spread was examined (Fig.6B). Mock or peg-BSA treated HSV-infected Vero cells had nearly 100% CPE 24h post-infection. In contrast, cells treated with 5, 2.5, or 1.25mg/ml peg-ArgI exhibited no apparent signs of viral infection or drug mediated cytotoxicity when compared to uninfected Vero cells. Consistent with findings from viral yield analysis (Fig.6A), peg-ArgI showed a dose-dependent inhibition of HSV-mediated CPE and cell-to-cell spread from 625-19.5ng/ml (Fig.6B). The ability of peg-ArgI and acyclovir to suppress HSV-1 viral yield were directly compared at concentrations that approximated each drug’s IC50 (500ng/ml for peg-ArgI; 0.7µM for ACV) and IC90 (1500ng/ml for peg-ArgI; 2.1µM for ACV) as determined by plaque reduction assays (Fig. 6C). Although drugs significantly reduced HSV-1 viral yield at two different MOIs, the IC90 dose of peg-ArgI markedly suppressed HSV-1 viral yield compared to ACV, especially at lower multiplicities of infection. These marked differences were not as apparent at comparable IC50 concentrations. Collectively, these data illustrate that peg-ArgI effectively inhibits HSV-1 replication, plaquing, CPE, and cell-to-cell spread in a dose dependent manner and that incremental increasing in peg-ArgI dosages can significantly impact antiviral activity.
Figure 5.
peg-ArgI inhibits HSV-1(McKrae) replication in a dose-dependent manner. The percent inhibitory concentrations of peg-ArgI that abrogated HSV-1 replication were determined using the CLSI standard plaque reduction assay. Cells were infected with 250 PFU per well and treated with replicate serial 2-fold dilutions of peg-ArgI from 10µg/ml to 39ng/ml. Percent inhibition of viral plaque formation for each treatment was determined relative to a mock treated control. Each point represents the mean +/− the S.E.M. of samples analyzed in triplicate. The indicated percent inhibitory concentrations were determined by log transformed nonlinear fit analysis of the data.
Figure 6.
peg-ArgI inhibits infectious HSV-1 virus production, viral spread, and virus-mediated cytopathic effects in a dose-dependent manner. Vero cells were infected at an MOI of 0.1 and treated with replicate serial 2-fold dilutions of peg-ArgI from 10µg/ml to 19.5ng/ml. (A) At 24h post-infection, the yield of infectious virus was determined and the percent inhibition of viral replication was calculated relative to a mock treated control. Each point represents the mean +/− the S.E.M. of six replicates. The indicated percent inhibitory concentrations were determined by log transformed nonlinear fit analysis of the data. (B) Prior to preparation of viral stocks for viral yield determination, the ability of peg-ArgI to inhibit HSV-mediated cytopathic effects and viral spread were visualized by phase-contrast microscopy. (C) Comparison of peg-ArgI and Acyclovir antiviral effects on HSV-1 viral yield at concentrations that approximate each drug’s IC50 and IC90 from plaque formation assays. The limit of detection of 50 plaque forming units is shown as a green dashed line.
3.6. peg-ArgI inhibits replication of drug resistant HSV-1 isolates
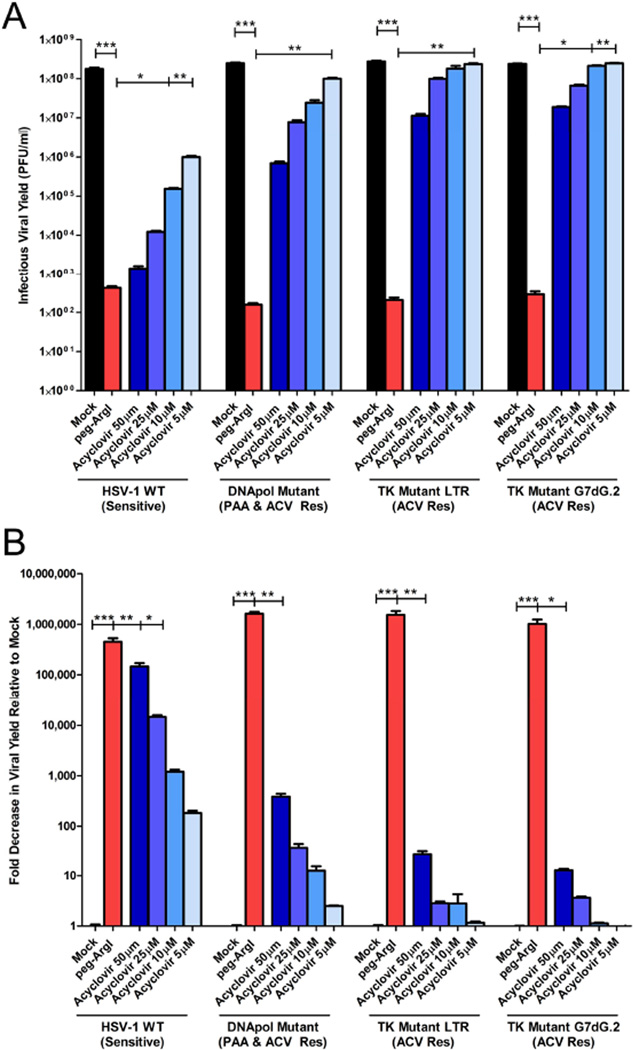
As nucleoside-analogs, all currently licensed anti-herpetics share either common prodrug activation pathways or similar modes of action. Therefore, mechanisms of HSV drug resistance between different therapeutic drugs are often analogous and occur through specific mutation of two key viral genes, the UL30 DNA polymerase or UL23 thymidine kinase genes (Coen and Schaffer, 1980). We therefore examined the antiviral activity of peg-ArgI treatment on ACV and foscarnet-resistant HSV-1 isolates that specified mutations in either the viral thymidine kinase gene (tkLTRZ1; tkG7dG.2) or DNA polymerase gene (PAAr5) (Coen and Schaffer, 1980; Field and Coen, 1986; Griffiths and Coen, 2003). As has been reported previously, all drug resistant viruses replicated well in the presence of ACV (Figs.7A & 7B). Although higher doses of ACV(50µM) had slight effects on replication of drug resistant isolates, the effects on infectious viral yield of normal doses of ACV(5–10µM) were negligible. Even at 50µM ACV, the thymidine kinase resistant viruses showed less than a 100-fold reduction in infectious viral yield compared to the corresponding mock treated infection. In stark contrast, peg-ArgI treatment significantly reduced infectious viral yields of all drug resistant isolates, decreasing production of HSV-1 by more than 500,000-fold, indicating that peg-ArgI may be an effective therapeutic against ACV- and foscarnet-resistant HSV infections, irrespective of the specific mutations that confer drug resistance.
Figure 7.
peg-ArgI inhibits replication of drug resistant HSV-1 isolates. Vero cells treated with either peg-ArgI (1.25µg/ml; red) or acyclovir (50, 25, 10 or 5µM; shades of blue) were infected at an MOI of 1 with either the parental acyclovir sensitive HSV-1(KOS) or viruses that specify mutations in the polymerase gene (PAAr), which confers resistance to foscarnet and ACV or thymidine kinase genes (HRLTR1; TKG7dG.2), which confer resistance to ACV treatment. (A) The total HSV-1 infectious viral yield was determined at 24h post-infection by titering lysed cell stocks on Vero cells. (B) The antiviral activity was expressed as a fold reduction in infectious viral yield relative to mock treatment. Each bar represents the mean +/− the S.E.M. of six replicates. Experiments were repeated at least 3 times. One way analysis of variance (ANOVA) with Kruskal-Wallis post-test was utilized to analyze differences between all treatment groups for each viral isolate. Asterisks indicate significant differences between the indicated treatments: *** p<0.0001; ** p<0.001; * p<0.01. The limit of detection for these assays was 50 plaque forming units/ml.
3.6. peg-ArgI effectively suppresses HSV-2 replication
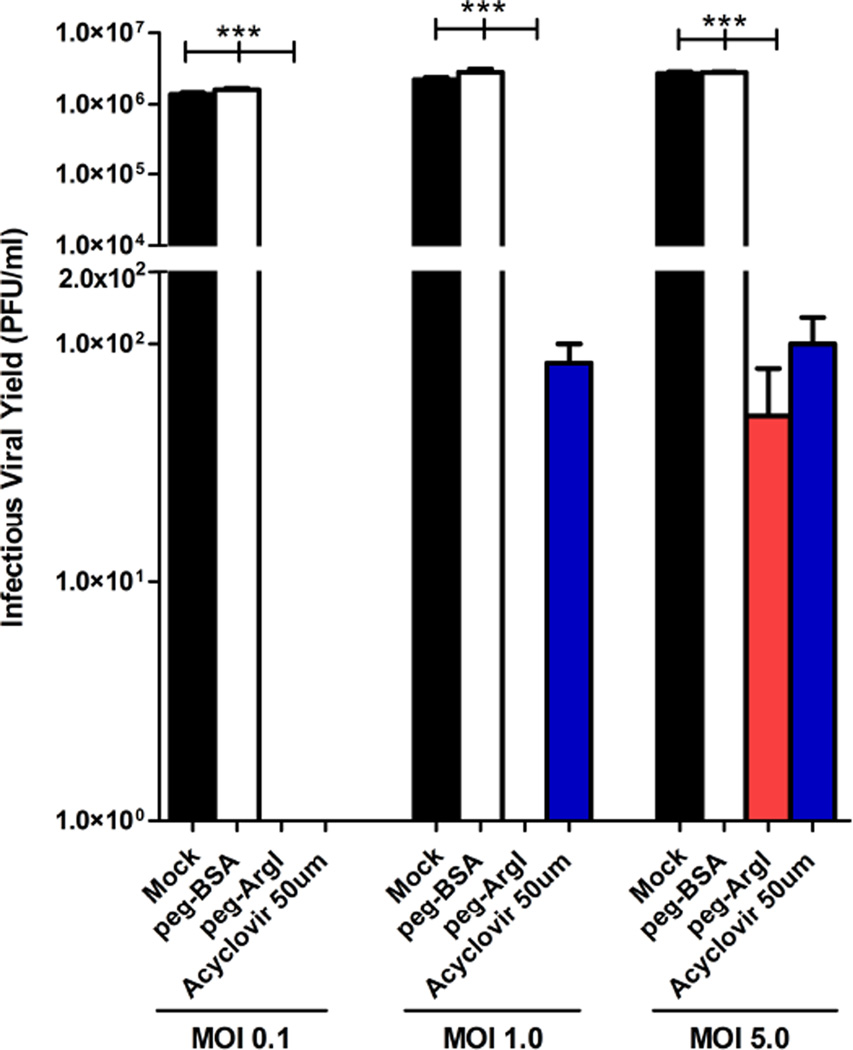
HSV-1 and HSV-2 share common replicative mechanisms. We therefore examined if peg-ArgI could suppress HSV-2 viral replication and inhibit yield of infectious HSV-2. Consistent with peg-ArgI effects on HSV-1, HSV-2 replication and infectious virus production was completely abrogated in Vero cells infected at low MOI. In addition, production of infectious HSV-2 was completely inhibited by peg-ArgI treatment at a MOI of 1.0 and only slightly detectable at an MOI of 5 (Fig.8), representing a 10 to 100-fold greater effect of peg-ArgI on HSV-2 viral yield at these MOIs relative to its effects on HSV-1 (Fig.3A). Moreover, the effects of peg-ArgI treatment on HSV-2 replication were either similar to or better than treatment with high dose acyclovir. Collectively, these data indicate that peg-ArgI possesses broad anti-HSV activity that can effectively inhibit viral replicative processes required for infectious virus production.
Figure 8.
peg-ArgI inhibits replication of HSV-2. Vero cells treated with either peg-ArgI (5µg/ml) or acyclovir (50µM) were infected with HSV-2(G) at a multiplicity of infection (MOI) of 0.1, 1.0 or 5.0. The total HSV-2 infectious viral yield was determined at 24h post-infection by titering lysed cell stocks on Vero cells. Each bar represents the mean +/− the S.E.M. of three replicates. Experiments were repeated at least 3 times. One way analysis of variance (ANOVA) with Kruskal-Wallis post-test was utilized to analyze differences between all treatment groups at a given MOI. Asterisks indicate significant differences between the indicated treatments: *** p<0.0001. The limit of detection for these assays was 50 plaque forming units/ml.
4. Discussion
Despite its limitations, for over four decades acyclovir or its derivatives have been the standard of treatment for HSV infections. As the first systemically deliverable antiviral, acyclovir established a precedence by which all subsequent antiviral development has been measured (Dorsky and Crumpacker, 1987). Rationally selected host-targeted therapeutics offer the potential to overcome the limitations of current virus-targeted therapeutic methods. Most viruses are dependent on arginine-associated metabolic pathways to facilitate viral replication, providing a target that could facilitate control of a broad range of viral pathogens (Gonczol et al., 1975; Rouse and Schlesinger, 1967; Spring et al., 1969; Tankersley, 1964). Modulation of host arginine-associated metabolic pathways can be facilitated by multiple biological enzymes: arginyl-tRNA synthetase, nitric oxide synthases, arginase I, arginase II, argine:glycine amidinotransferase, arginine decarboxylase, and arginine deiminase (Morris, 2007, 2009; Wu and Morris, 1998). Two major enzyme therapeutics, the mycoplasma-derived arginine deiminase (ADI) and human ArgI, are in various stages of clinical development for cancer (Feun and Savaraj, 2006; Glazer et al., 2010; Mussai et al., 2015; Tomlinson et al., 2015; Yau et al., 2013). ADI catalyzes the deamination of arginine to citrulline and ammonia with buildup of its end products associated with toxicity (Feun et al., 2008; Glazer et al., 2010). Unlike ADI, the non-xenogenic and native human ArgI hydrolyzes arginine to ornithine and urea, the latter being non-toxic and excreted in urine (Wu et al., 2009). Therefore, ArgI was selected for its potential to inhibit viral replication without inducing cytotoxic complications. As expected, our data demonstrates that ArgI has the ability to control replication of HSV-1 and HSV-2 without inducing apparent cytotoxicity even with long term depletion of arginine. These low levels of toxicity were comparable to acyclovir and orders of magnitude better than the gold-standard ophthalmic anti-herpetic, TFT, which exhibited severe toxicity after only four hours of exposure.
The antiviral activity of peg-ArgI displays a classical dose-response curve that is dependent upon both the initial levels of arginine and the enzymatic activity of peg-ArgI. The levels of arginine within DMEM (398µmol/L) are approximately five times greater than that found in normal human plasma (77.4+/−18.2µmol/L) and in HSV-infected tissue microenvironments (Armengou et al., 2003; Luneburg et al., 2011), providing relevance of these studies to minimal dosing levels required for inhibition of HSV. peg-ArgI was effective in controlling HSV-1 and -2 replication across a broad range of MOIs, completely abrogating infectious virus production at low MOIs and being superior to acyclovir treatment across all MOIs. Depending on the method of assessment, peg-ArgI exhibited a 50% inhibitory concentration in the low to mid (140–465) ng/ml or equivalent to 4–13.3nM ArgI. peg-ArgI exhibited a 99% inhibition of HSV-1 viral yield and plaquing at approximately 222nM and 27nM ArgI, respectively. Taken together, these results demonstrate that peg-ArgI can effectively control HSV viral replication, infectious virus production, and viral spread/transmission at sub-nanomolar concentrations. Many other viruses, including, Adenovirus, Cytomegalovirus, Varicella-Zoster, Polyomavirus, Vaccinia, and Influenza are critically dependent on arginine availability for efficient replication (Archard and Williamson, 1971; Goldblum et al., 1968; Gonczol et al., 1975; Tankersley, 1964; Wigand and Kumel, 1978). It will be of great interest to determine the breadth of viruses inhibited by peg- ArgI treatment.
In cases of repeated or chronic infections, such as in herpetic infections, misuse or continuous use of antivirals can elicit development of drug resistance. Because all licensed anti-herpetics are nucleoside analogs that target a single viral replicative process, some mutations, especially within the HSV polymerase gene, confer resistance to both first and second-line antivirals (Coen and Schaffer, 1980). Not unexpectedly given its novel host-targeted mechanisms of action, peg-ArgI was effective at inhibiting drug resistant HSV-1. Similar to newly identified helicase-primase anti-herpetic drugs (Betz et al., 2002; Kleymann et al., 2002; Weller and Kuchta, 2013), peg-ArgI was superior to acyclovir in its ability to inhibit infectious viral yield, virus-induced cytopathic effect, and viral spread. However, unlike HSVprotein targeting helicase-primase inhibitors, peg-ArgI modulation of host arginine-associated metabolic pathways minimizes the likelihood that viruses could evolve resistance. This would contrast virus-targeted nucleoside analogs and helicase-primase inhibitors where mutations that confer resistance are easily identified (Biswas et al., 2007; Coen and Schaffer, 1980; Fleming and Coen, 1984; Muylaert et al., 2014; Pelosi et al., 1998).
There are several limitations in targeting arginine metabolic pathways as a potential antiviral approach, especially in the case of treatment of lifelong herpetic infections. Long-term systemic arginine depletion in afflicted patients simply is not feasible nor would the benefits outweigh risks. Because peg-ArgI is a biological, oral administration is most likely precluded, and it is therefore limited to either direct injection or topical delivery. However, modulation of localized acute disease through topical applications of peg-ArgI in the eye or on the skin could prove effective. Similarly, short-term systemic treatments of patients experiencing severe acute viral-associated disease may be tenable. In cases such as herpetic encephalitis, it must be determined if systemic treatments can either reach afflicted sites or if systemic arginine depletion would be sufficient to effectively shut down arginine-related metabolic processes at sites it is incapable of reaching.
When treating severe viral-mediated diseases, the host-targeted antiviral nature of peg-ArgI may be complemented by its effects on other deleterious host-mediated processes that contribute to disease. Virus-mediated diseases are a combination of virus-induced pathology and deleterious host-mediated inflammatory responses. Although antivirals effectively suppress viral replication, virus-associated disease may still proceed due to exacerbated host responses. This is especially apparent in virus-mediated ocular diseases, where infection of the eye by HSV or Adenoviruses can result in severe vision-threatening sequelae, including T cell-mediated inflammation, vascularization of normally avascular ocular tissue, vascular leakage and edema. Suppression of these disease processes can be controlled by steroidal anti-inflammatories; however, their use in contraindicated in herpetic eye infections as they increase ocular pressure and license uncontrolled viral replication.
Arginine availability is an important regulator of T-cell function, inflammation, neovascularization, and vascular integrity (Bansal and Ochoa, 2003; Rodriguez and Ochoa, 2008; Rodriguez et al., 2002; Rodriguez et al., 2003; Scallan et al., 2015; Wu and Meininger, 2000). It has been hypothesized that the eye maintains its immunoprivilege and avascularity by keeping arginine at low levels (Fu et al., 2011). During HSV infection of the eye, disease progression correlates with increased arginine levels (Kahan et al., 1979). In this regard, peg-ArgI has potential to modulate these deleterious disease-associated sequelae. However, unlike steroidals, which can license viral replication, peg-ArgI would have the added benefit of being capable of controlling viral replicative processes while simultaneously suppressing hostmediated disease. These potential combined activities would represent a novel approach with vast therapeutic potential.
Highlights.
-
-
A pegylated recombinant human Arginase I (peg-ArgI) was generated to modulate host arginine-associated metabolic pathways.
-
-
Cell-lines and primary cells treated with peg-ArgI exhibited no cytotoxicity or apparent changes in cell viability.
-
-
Modulation of host arginine-associated metabolic pathways by peg-ArgI potently inhibited replication of HSV-1 and HSV-2.
-
-
peg-ArgI suppressed replication of single and multidrug resistant HSV-1 viruses.
-
-
Modulation of arginine-associated pathways as a broadly active antiviral that can also suppress disease sequelae is discussed.
Acknowledgments
This work is in memory of Dr. James M. Hill, a cherished colleague, friend, and mentor. Without his contributions and continuous support this research would not have been possible. We thank Cathryn Garvey for her expert technical assistance. This work was supported by a Louisiana Board of Regents Research Competitiveness Award LEQSF-RD-A-13, by a Research Commercialization grant from the Louisiana Board of Regents through the Louisiana Vaccine Center, and by the National Institutes of Health from the National Center for Research Resources (P20RR021970) and the National Institute of General Medical Sciences (P20GM103501 & P30GM106392). In addition, this work was supported in part by R01AI112402 from the National Institute of Allergy and Infectious Diseases and U54GM104940 from the National Institute of General Medical Sciences through the Louisiana Clinical and Translational Science Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the National Institute of General Medical Sciences, the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archard LC, Williamson JD. The effect of arginine deprivation on the replication of vaccinia virus. The Journal of general virology. 1971;12:249–258. doi: 10.1099/0022-1317-12-3-249. [DOI] [PubMed] [Google Scholar]
- Armengou A, Hurtado O, Leira R, Obon M, Pascual C, Moro MA, Lizasoain I, Castillo J, Davalos A. L-arginine levels in blood as a marker of nitric oxide-mediated brain damage in acute stroke: a clinical and experimental study. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23:978–984. doi: 10.1097/01.WCB.0000080651.64357.C6. [DOI] [PubMed] [Google Scholar]
- Bansal V, Ochoa JB. Arginine availability, arginase, and the immune response. Current opinion in clinical nutrition and metabolic care. 2003;6:223–228. doi: 10.1097/00075197-200303000-00012. [DOI] [PubMed] [Google Scholar]
- Betz UA, Fischer R, Kleymann G, Hendrix M, Rubsamen-Waigmann H. Potent in vivo antiviral activity of the herpes simplex virus primase-helicase inhibitor BAY 57 1293. Antimicrob Agents Chemother. 2002;46:1766–1772. doi: 10.1128/AAC.46.6.1766-1772.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Jennens L, Field HJ. Single amino acid substitutions in the HSV-1 helicase protein that confer resistance to the helicase-primase inhibitor BAY 57-1293 are associated with increased or decreased virus growth characteristics in tissue culture. Archives of virology. 2007;152:1489–1500. doi: 10.1007/s00705-007-0964-7. [DOI] [PubMed] [Google Scholar]
- Cheng PN, Lam TL, Lam WM, Tsui SM, Cheng AW, Lo WH, Leung YC. Pegylated recombinant human arginase (rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res. 2007;67:309–317. doi: 10.1158/0008-5472.CAN-06-1945. [DOI] [PubMed] [Google Scholar]
- Christophers J, Clayton J, Craske J, Ward R, Collins P, Trowbridge M, Darby G. Survey of resistance of herpes simplex virus to acyclovir in northwest England. Antimicrob Agents Chemother. 1998;42:868–872. doi: 10.1128/aac.42.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen DM, Schaffer PA. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A, Griffiths P, Leone P, Mindel A, Patel R, Stanberry L, Whitley R. Current management and recommendations for access to antiviral therapy of herpes labialis. J Clin Virol. 2012;53:6–11. doi: 10.1016/j.jcv.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsky DI, Crumpacker CS. Drugs five years later: acyclovir. Annals of internal medicine. 1987;107:859–874. doi: 10.7326/0003-4819-107-6-859. [DOI] [PubMed] [Google Scholar]
- Duan R, de Vries RD, van Dun JM, van Loenen FB, Osterhaus AD, Remeijer L, Verjans GM. Acyclovir susceptibility and genetic characteristics of sequential herpes simplex virus type 1 corneal isolates from patients with recurrent herpetic keratitis. J Infect Dis. 2009;200:1402–1414. doi: 10.1086/606028. [DOI] [PubMed] [Google Scholar]
- Englund JA, Zimmerman ME, Swierkosz EM, Goodman JL, Scholl DR, Balfour HH., Jr Herpes simplex virus resistant to acyclovir. A study in a tertiary care center. Annals of internal medicine. 1990;112:416–422. doi: 10.7326/0003-4819-76-3-112-6-416. [DOI] [PubMed] [Google Scholar]
- Feun L, Savaraj N. Pegylated arginine deiminase: a novel anticancer enzyme agent. Expert Opin Investig Drugs. 2006;15:815–822. doi: 10.1517/13543784.15.7.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feun L, You M, Wu CJ, Kuo MT, Wangpaichitr M, Spector S, Savaraj N. Arginine deprivation as a targeted therapy for cancer. Current pharmaceutical design. 2008;14:1049–1057. doi: 10.2174/138161208784246199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field HJ. Persistent herpes simplex virus infection and mechanisms of virus drug resistance. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 1989;8:671–680. doi: 10.1007/BF01963751. [DOI] [PubMed] [Google Scholar]
- Field HJ, Coen DM. Pathogenicity of herpes simplex virus mutants containing drug resistance mutations in the viral DNA polymerase gene. J Virol. 1986;60:286–289. doi: 10.1128/jvi.60.1.286-289.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife KH, Crumpacker CS, Mertz GJ, Hill EL, Boone GS. Recurrence and resistance patterns of herpes simplex virus following cessation of >or = 6 years of chronic suppression with acyclovir. Acyclovir Study Group. J Infect Dis. 1994;169:1338–1341. doi: 10.1093/infdis/169.6.1338. [DOI] [PubMed] [Google Scholar]
- Fleming HE, Jr, Coen DM. Herpes simplex virus mutants resistant to arabinosyladenine in the presence of deoxycoformycin. Antimicrob Agents Chemother. 1984;26:382–387. doi: 10.1128/aac.26.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Khan A, Coe D, Zaher S, Chai JG, Kropf P, Muller I, Larkin DF, George AJ. Arginine depletion as a mechanism for the immune privilege of corneal allografts. European journal of immunology. 2011;41:2997–3005. doi: 10.1002/eji.201141683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer ES, Piccirillo M, Albino V, Di Giacomo R, Palaia R, Mastro AA, Beneduce G, Castello G, De Rosa V, Petrillo A, Ascierto PA, Curley SA, Izzo F. Phase II study of pegylated arginine deiminase for nonresectable and metastatic hepatocellular carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:2220–2226. doi: 10.1200/JCO.2009.26.7765. [DOI] [PubMed] [Google Scholar]
- Gnann JW, Jr, Whitley RJ. Clinical practice. Herpes zoster. The New England journal of medicine. 2002;347:340–346. doi: 10.1056/NEJMcp013211. [DOI] [PubMed] [Google Scholar]
- Goldblum N, Ravid Z, Becker Y. Effect of withdrawal of arginine and other amino acids on the synthesis of tumour and viral antigens of SV 40 virus. The Journal of general virology. 1968;3:143–146. doi: 10.1099/0022-1317-3-1-143. [DOI] [PubMed] [Google Scholar]
- Gonczol E, Boldogh I, Vaczi L. Effect of arginine deficiency on the reproduction of human cytomegalovirus. Acta microbiologica Academiae Scientiarum Hungaricae. 1975;22:263–270. [PubMed] [Google Scholar]
- Griffiths A, Coen DM. High-frequency phenotypic reversion and pathogenicity of an acyclovir-resistant herpes simplex virus mutant. J Virol. 2003;77:2282–2286. doi: 10.1128/JVI.77.3.2282-2286.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM, Ball MJ, Neumann DM, Azcuy AM, Bhattacharjee PS, Bouhanik S, Clement C, Lukiw WJ, Foster TP, Kumar M, Kaufman HE, Thompson HW. The high prevalence of herpes simplex virus type 1 DNA in human trigeminal ganglia is not a function of age or gender. J Virol. 2008;82:8230–8234. doi: 10.1128/JVI.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh BC, Chen SH, Hu A, Mulamba GB, Burns WH, Coen DM. Recurrent acyclovir-resistant herpes simplex in an immunocompromised patient: can strain differences compensate for loss of thymidine kinase in pathogenesis? J Infect Dis. 1998;178:618–625. doi: 10.1086/515375. [DOI] [PubMed] [Google Scholar]
- Kadeppagari RK, Sanchez RL, Foster TP. HSV-2 inhibits type-I interferon signaling via multiple complementary and compensatory STAT2-associated mechanisms. Virus research. 2012;167:273–284. doi: 10.1016/j.virusres.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan IL, Hajas K, Halasz A. The significance of the arginine and arginase of tears in experimentally-induced herpes simplex corneae. Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. Albrecht von Graefe's archive for clinical and experimental ophthalmology. 1979;209:219–224. doi: 10.1007/BF00414614. [DOI] [PubMed] [Google Scholar]
- Kimberlin DW. Herpes simplex virus infections in neonates and early childhood. Seminars in pediatric infectious diseases. 2005;16:271–281. doi: 10.1053/j.spid.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Kleymann G, Fischer R, Betz UA, Hendrix M, Bender W, Schneider U, Handke G, Eckenberg P, Hewlett G, Pevzner V, Baumeister J, Weber O, Henninger K, Keldenich J, Jensen A, Kolb J, Bach U, Popp A, Maben J, Frappa I, Haebich D, Lockhoff O, Rubsamen-Waigmann H. New helicase-primase inhibitors as drug candidates for the treatment of herpes simplex disease. Nature medicine. 2002;8:392–398. doi: 10.1038/nm0402-392. [DOI] [PubMed] [Google Scholar]
- Koutsky LA, Ashley RL, Holmes KK, Stevens CE, Critchlow CW, Kiviat N, Lipinski CM, Wolner-Hanssen P, Corey L. The frequency of unrecognized type 2 herpes simplex virus infection among women. Implications for the control of genital herpes. Sexually transmitted diseases. 1990;17:90–94. doi: 10.1097/00007435-199004000-00009. [DOI] [PubMed] [Google Scholar]
- Kriesel JD, Spruance SL, Prichard M, Parker JN, Kern ER. Recurrent antiviral-resistant genital herpes in an immunocompetent patient. J Infect Dis. 2005;192:156–161. doi: 10.1086/430612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston AA, Redei I, Caliendo AM, Somani J, Hutcherson D, Lonial S, Bucur S, Cherry J, Allen A, Waller EK. Development of drug-resistant herpes simplex virus infection after haploidentical hematopoietic progenitor cell transplantation. Blood. 2002;99:1085–1088. doi: 10.1182/blood.v99.3.1085. [DOI] [PubMed] [Google Scholar]
- Levin MJ, Bacon TH, Leary JJ. Resistance of herpes simplex virus infections to nucleoside analogues in HIV-infected patients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;39(Suppl 5):S248–S257. doi: 10.1086/422364. [DOI] [PubMed] [Google Scholar]
- Luneburg N, Xanthakis V, Schwedhelm E, Sullivan LM, Maas R, Anderssohn M, Riederer U, Glazer NL, Vasan RS, Boger RH. Reference intervals for plasma L-arginine and the L-arginine:asymmetric dimethylarginine ratio in the Framingham Offspring Cohort. J Nutr. 2011;141:2186–2190. doi: 10.3945/jn.111.148197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard ND, Gutschow MV, Birch EW, Covert MW. The virus as metabolic engineer. Biotechnology journal. 2010;5:686–694. doi: 10.1002/biot.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SM., Jr Arginine: beyond protein. The American journal of clinical nutrition. 2006;83:508S–512S. doi: 10.1093/ajcn/83.2.508S. [DOI] [PubMed] [Google Scholar]
- Morris SM., Jr Arginine metabolism: boundaries of our knowledge. J Nutr. 2007;137:1602S–1609S. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- Morris SM., Jr Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol. 2009;157:922–930. doi: 10.1111/j.1476-5381.2009.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussai F, Egan S, Higginbotham-Jones J, Perry T, Beggs A, Odintsova E, Loke J, Pratt G, U KP, Lo A, Ng M, Kearns P, Cheng P, De Santo C. Arginine dependence of acute myeloid leukemia blast proliferation: a novel therapeutic target. Blood. 2015;125:2386–2396. doi: 10.1182/blood-2014-09-600643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muylaert I, Zhao Z, Elias P. UL52 primase interactions in the herpes simplex virus 1 helicase-primase are affected by antiviral compounds and mutations causing drug resistance. The Journal of biological chemistry. 2014;289:32583–32592. doi: 10.1074/jbc.M114.609453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugier F, Colin JN, Aymard M, Langlois M. Occurrence and characterization of acyclovir-resistant herpes simplex virus isolates: report on a two-year sensitivity screening survey. J Med Virol. 1992;36:1–12. doi: 10.1002/jmv.1890360102. [DOI] [PubMed] [Google Scholar]
- Pelosi E, Mulamba GB, Coen DM. Penciclovir and pathogenesis phenotypes of drug-resistant Herpes simplex virus mutants. Antiviral Res. 1998;37:17–28. doi: 10.1016/s0166-3542(97)00054-5. [DOI] [PubMed] [Google Scholar]
- Reyes M, Shaik NS, Graber JM, Nisenbaum R, Wetherall NT, Fukuda K, Reeves WC. Acyclovir-resistant genital herpes among persons attending sexually transmitted disease and human immunodeficiency virus clinics. Archives of internal medicine. 2003;163:76–80. doi: 10.1001/archinte.163.1.76. [DOI] [PubMed] [Google Scholar]
- Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunological reviews. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, Antonia S, Ochoa JB, Ochoa AC. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- Rodriguez PC, Zea AH, Culotta KS, Zabaleta J, Ochoa JB, Ochoa AC. Regulation of T cell receptor CD3zeta chain expression by L-arginine. The Journal of biological chemistry. 2002;277:21123–21129. doi: 10.1074/jbc.M110675200. [DOI] [PubMed] [Google Scholar]
- Rodriguez PC, Zea AH, DeSalvo J, Culotta KS, Zabaleta J, Quiceno DG, Ochoa JB, Ochoa AC. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J Immunol. 2003;171:1232–1239. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- Rouse HC, Schlesinger RW. An arginine-dependent step in the maturation of type 2 adenovirus. Virology. 1967;33:513–522. doi: 10.1016/0042-6822(67)90128-6. [DOI] [PubMed] [Google Scholar]
- Sanchez RL, Ramsay AJ, Foster TP. Efficient generation and rapid isolation via stoplight recombination of Herpes simplex viruses expressing model antigenic and immunological epitopes. Journal of virological methods. 2012;179:116–126. doi: 10.1016/j.jviromet.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan JP, Hill MA, Davis MJ. Lymphatic vascular integrity is disrupted in type 2 diabetes due to impaired nitric oxide signalling. Cardiovascular research. 2015;107:89–97. doi: 10.1093/cvr/cvv117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002;186(Suppl 1):S3–S28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- Spring SB, Roizman B, Spear PG. Selective failure of protein synthesis in herpesvirus-infected cells deprived of arginine. Virology. 1969;38:710–712. doi: 10.1016/0042-6822(69)90193-7. [DOI] [PubMed] [Google Scholar]
- Sweirkosz EMHRL, Moore BM, Sacks S, Scholl DR, Wright DK. Antiviral Susceptibility Testing: Herpes Simplex Virus by Plaque Reduction Assay; Approved Standard, M33-A ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2004. CLSI. [Google Scholar]
- Tankersley RW., Jr Amino Acid Requirements of Herpes Simplex Virus in Human Cells. J Bacteriol. 1964;87:609–613. doi: 10.1128/jb.87.3.609-613.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson BK, Thomson JA, Bomalaski JS, Diaz M, Akande T, Mahaffey N, Li T, Dutia MP, Kelly K, Gong IY, Semrad T, Gandara DR, Pan CX, Lara PN., Jr Phase I Trial of Arginine Deprivation Therapy with ADI-PEG 20 Plus Docetaxel in Patients with Advanced Malignant Solid Tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:2480–2486. doi: 10.1158/1078-0432.CCR-14-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui SM, Lam WM, Lam TL, Chong HC, So PK, Kwok SY, Arnold S, Cheng PN, Wheatley DN, Lo WH, Leung YC. Pegylated derivatives of recombinant human arginase (rhArg1) for sustained in vivo activity in cancer therapy: preparation, characterization and analysis of their pharmacodynamics in vivo and in vitro and action upon hepatocellular carcinoma cell (HCC) Cancer Cell Int. 2009;9:9. doi: 10.1186/1475-2867-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang Q, Zhu Q, Zhou R, Liu J, Peng T. Identification and characterization of acyclovir-resistant clinical HSV-1 isolates from children. J Clin Virol. 2011;52:107–112. doi: 10.1016/j.jcv.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Weller SK, Kuchta RD. The DNA helicase-primase complex as a target for herpes viral infection. Expert opinion on therapeutic targets. 2013;17:1119–1132. doi: 10.1517/14728222.2013.827663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley RJ. Herpes simplex virus infection. Seminars in pediatric infectious diseases. 2002;13:6–11. doi: 10.1053/spid.2002.29752. [DOI] [PubMed] [Google Scholar]
- Whitley RJ. Herpes simplex encephalitis: adolescents and adults. Antiviral Res. 2006;71:141–148. doi: 10.1016/j.antiviral.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Whitley RJ, Gnann JW. Viral encephalitis: familiar infections and emerging pathogens. Lancet. 2002;359:507–513. doi: 10.1016/S0140-6736(02)07681-X. [DOI] [PubMed] [Google Scholar]
- Wigand R, Kumel G. Amino acid requirement of adenovirus multiplication. The Journal of general virology. 1978;39:281–292. doi: 10.1099/0022-1317-39-2-281. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc Rhoads J, Carey Satterfield M, Smith SB, Spencer TE, Yin Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–168. doi: 10.1007/s00726-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Meininger CJ. Arginine nutrition and cardiovascular function. J Nutr. 2000;130:2626–2629. doi: 10.1093/jn/130.11.2626. [DOI] [PubMed] [Google Scholar]
- Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. The Biochemical journal. 1998;336(Pt 1):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. Jama. 2006;296:964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- Yau T, Cheng PN, Chan P, Chan W, Chen L, Yuen J, Pang R, Fan ST, Poon RT. A phase 1 dose-escalating study of pegylated recombinant human arginase 1 (Peg-rhArg1) in patients with advanced hepatocellular carcinoma. Invest New Drugs. 2013;31:99–107. doi: 10.1007/s10637-012-9807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyaeyan M, Alborzi A, Japoni A, Kadivar M, Davarpanah MA, Pourabbas B, Abassian A. Frequency of acyclovir-resistant herpes simplex viruses isolated from the general immunocompetent population and patients with acquired immunodeficiency syndrome. International journal of dermatology. 2007;46:1263–1266. doi: 10.1111/j.1365-4632.2007.03449.x. [DOI] [PubMed] [Google Scholar]