Abstract
Sleep contributes importantly to energy homeostasis, and may impact hormones regulating appetite, such as leptin, an adipocyte derived hormone. There is increasing evidence that sleep duration, and reduced REM sleep, are linked to obesity. Leptin has central neural effects beyond modulation of appetite alone. As sleep is not a unifrom process, interactions between leptin and sleep stages including REM sleep may play a role in the relationship between sleep and obesity. This study examined the relationship between serum leptin and REM sleep in a sample of healthy adults.
Participants were 58 healthy adults who underwent polysomnography. Leptin was measured before and after sleep. We hypothesized that lower percentage of REM sleep would be related to lower leptin levels during sleep. The relationship between percentage of REM sleep and leptin was analyzed using hierarchical linear regression. Increased percentage of REM sleep was related to a greater reduction in leptin during sleep even when controlling for age, gender, percent body fat and total sleep time. A greater percentage of REM sleep was accompanied by more marked reductions in leptin. Studies examining the effects of selective REM sleep deprivation on leptin levels, and hence on energy homeostasis in humans, are needed.
Keywords: adipokines, obesity
Obesity is epidemic both in the developed world and in emerging countries (Balkau et al., 2007). Approximately two-thirds of adult Americans are overweight or obese, defined by a body mass index (BMI) of 30 or greater. Although obesity is primarily caused by the imbalance between energy intake and expenditure, an increasing body of literature suggests a connection between obesity and sleep. Epidemiologic studies document that shorter sleep times are correlated with a higher BMI (Rontoyanni et al., 2007, Taheri et al., 2004). Shorter sleep duration may also predict future weight gain (Chaput et al., 2008, Hasler et al., 2004). While epidemiologic and experimental evidence link sleep and obesity, sleep is not a uniform process. There is a need to better understand the role of NREM and REM sleep in hormonal pathways linking sleep and obesity. Thus, the present study examined the relationship between REM sleep and overnight change in leptin levels.
Sleep plays an important role in energy homeostasis. Hormones that regulate appetite include leptin, an adipocyte derived hormone that supresses appetite, and one potential mechanism linking sleep to weight gain. Leptin receptors are found in the central nervous system and peripheral tissue (Tartaglia, 1997). Circulating leptin levels reflect the amount of energy storage and are positively correlated with the percentage of body fat (Maffei et al., 1995). While leptin levels typically do not change following a normal meal (Korbonits et al., 1997), leptin levels may be impacted by the timing of meal patterns (Schoeller et al., 1997). Plasma leptin levels exhibit a diurnal pattern, with levels rising during the first part of the night and then declining during the latter part of the night (Licinio et al., 1997).
Past studies suggest a relationship between sleep duration and leptin. For example, Spiegel et al (2004) found decreased leptin levels and a decrease in the nocturnal applitude of leptin in eleven male subjects allowed a four hour sleep opportunity compared to when those subjects were well rested. However, later findings have been mixed with some studies reporting increased (van Leeuwen et al., 2010) or unchanged leptin levels (St-Onge et al., 2012) following sleep restriction. Sleep deptivation may more consistently impact the diurnal amplitude of leptin (Mullington et al., 2003, Spiegel et al., 2004, Guilleminault et al., 2003). In a study of healthy males, total sleep deprivation resulted in a decrease in the nocturnal amplitude of leptin, an effect that was normalized during recovery sleep. (Mullington et al., 2003). In animal studies, leptin deficiency leads to changes in sleep architecture, and impairment in the sleep wake cycle (Laposky et al., 2006). In a studies of sleep deprived rats, sleep deprivation resulted in increased energy expenditure and decreased leptin levels (Barf et al., 2012, Moraes et al., 2014).
While past studies suggest a relationship between sleep duration and leptin, sleep is not a uniform process. The relationship between sleep duration and weight gain may be a function of loss of REM sleep, rather than a result of less overall sleep. A large epidemiological study found reduced REM sleep was associated with central obesity in women (Theorell-Haglow et al., 2010). Children and teens who sleep less, particularly those who spend less time in REM sleep, are also more likely to be overweight (Liu et al., 2008). After adusting for other factors, Lui et al (2008) found that in children and teens, a one hour decrease in REM sleep was associated with a three fold increase in the odds of being overweight. Results of animal studies also suggest a relationship between REM sleep and leptin levels (Sinton et al., 1999). We therefore sought to examine the relationship between REM sleep and circulating levels of leptin in healthy adults, while controlling for the effect of total sleep duration. We hypothesized that lower percentage of REM sleep would be related to a greater decrease in leptin levels during sleep.
Methods
Participants
We studied 58 healthy volunteers (15 women, 43 men) recruited from the Rochester, MN community. Subjects were recruited over a five-year period for a larger study examining genetic and cardiovascular factors related to sleep. The larger study included individuals with sleep apnea. For this research question, individuals with sleep apnea were excluded. Blood samples from individuals who were healthy with no acute or chronic medical conditions and who were not taking any medications were selected and leptin was measured. Mean participant age was 35.6 years (SD = 11.4 years, range = 20 to 64 years). All participants were healthy with no acute or chronic medical conditions and were not taking any medications. All participants were nonsmokers. Shift workers were excluded from the study. Table 1 provides descriptive statistics.
Table 1.
Descriptive Statistics
| Demographic Variables | Mean (SD) |
|---|---|
| Age | 35.6 (11.3) |
| Gender (%Male) | 74% |
| Body mass index | 26.1(3.2) |
| % Body fat | 23.8(5.9) |
| Sleep Variables | |
| Total sleep time (min) | 324 (69.8) |
| Sleep Efficiency | 80.4(14.8) |
| %REM sleep | 17.0(6.5) |
| REM duration (min) | 56.9(27.2) |
| %SW sleep | 21.4(9.5) |
| SWS duration (min) | 67.0(30.8) |
| Leptin Variables | |
| Evening leptin (ng/mL) | 8.7(6.9) |
| Morning leptin (ng/mL) | 6.7(5.4) |
| Morning/evening ratio | .81(.26) |
Procedure
Sleep duration and percentage of REM sleep were obtained by overnight polysomnography. Total sleep time was defined as the total amount of sleep in minutes each participant obtained from sleep onset to awakening, excluding periods of wakefulness. Polysomnographic data were scored in 30 second epochs according to standardized criteria (Rechtschaffen and Kales, 1968). In addition, respiration, airflow, and oxygen saturation were measured to exclude occult sleep apnea. An apnea was defined as a complete cessation of airflow for ten seconds or more. A hypopnea was defined as a reduction in airflow for at least ten seconds, accompanied by an oxygen desaturation of four percent or more. An apnea-hypopnea index (AHI) was calculated as the average number of respiratory events per hour of sleep. Individuals with evidence of sleep apnea (defined by an AHI of one or more events/hour) were excluded from the study. Demographic information, body mass index (BMI) and body fat measurements were obtained from all participants. Participants reported medical conditions and health behaviors known to impact sleep such as alcohol, nicotine use, and current medications. Percent body fat was measured by bioelectric impedance analysis (BIA-101S system, RJL Systems, Mt. Clemens, MI). Participants were instructed to eat according to their usual eating habits between breakfast and dinner on the day of study visit, but caloric intake was otherwise not monitored. Participants went to sleep according to their usual sleep time. Blood samples were obtained in the evening before sleep (9 pm) and upon awakening in the morning following overnight fasting (6 am). Following the evening blood draw, participants watched TV, engaged in reading or did other quiet activities in their room until they were ready for bed. Participants were able to request lights off any time after 9pm, and were woken at 6am (see Figure 1 for study timeline). Blood samples were centrifuged, aliquotted, and stored until assayed in the Immunochemical Core Laboratory at the Mayo Clinic. Leptin levels were measured using radioimmunoassay kits (Linco Research Inc) with a sensitivity of .5 ng/ml and an intra-assay coefficient of 6.0%. The study protocol was approved by the Mayo Clinic Institutional Review Board and informed written consent was obtained from all subjects.
Figure 1.

Study timeline.
Statistical Analysis
Descriptive and regression analyses were conducted using SPSS 16.0 software (SPSS, Chicago, IL). Table 1 presents descriptive statistics. A zero-order correlation matrix (presented in Table 2) shows the relationship between leptin, demographic and sleep variables. Hierarchical linear regression was used to examine the relationship between percentage of REM sleep and leptin levels. Covariates were selected based on past research and theoretical considerations. Because percentage of REM sleep and total sleep time were moderately correlated, percentage of REM sleep was orthogonalized to total sleep time via residual centering. The orthogonal variable was used for all regression analyses involving both total sleep time and percentage of REM sleep as covariates. This method improves the interpretability and stability of the model estimates (Little et al., 2006). Table 3 summarizes the results of hierarchical linear regression analyses.
Table 2.
Zero-Order Correlation Matrix
| REM %TST |
SWS (min) |
SWS %TST |
TST | Leptin evening |
Leptin morning |
Leptin ratio |
Age | Body fat |
|
|---|---|---|---|---|---|---|---|---|---|
| REM (min) | .92** | .26 | −.12 | .69** | .04 | .01 | −.19 | .07 | .10 |
| REM %TST | --- | .14 | −.16 | .41** | .05 | −.03 | −.28* | .16 | .02 |
| SWS (min) | --- | .84** | .32* | −.08 | −.13 | −.12 | −.34* | −.15 | |
| SWS %TST | --- | −.18 | −.04 | −.04 | −.05 | −.18 | −.21 | ||
| TST | --- | −.06 | −.08 | −.02 | −.29* | .15 | |||
| Leptin evening | --- | .91** | −.22 | .22 | .65** | ||||
| Leptin morning | --- | .13 | .33* | .69** | |||||
| Leptin ratio | --- | .14 | .08 | ||||||
| Age | --- | .02 | |||||||
| Body fat | --- |
Note:
p < .01.
p < .05 level.
Note: REM=Rapid Eye Movement Sleep, SWS=Slow Wave Sleep, TST=Total Sleep Time
Table 3.
Hierarchical Linear Regression Analysis
| Variable | B (SE) | β | T |
|---|---|---|---|
| Model 1: Percentage of REM Sleep as a Predictor of Morning Leptin | |||
| Step 1 | |||
| Evening Leptin | .555 (.050) | .702 | 10.99** |
| Age | .117 (.025) | .245 | 4.61** |
| Gender | 2.75 (.847) | .223 | 3.244** |
| Body Fat | .063 (.068) | .068 | .929 |
| Total Sleep Duration | −.002 (.004) | −.025 | −.521 |
| Step 2 | |||
| %REM | −.124 (.045) | −.135 | −2.77** |
| Model 2: REM Duration (mins) as a Predictor of Morning Leptin | |||
| Step 1 | |||
| Evening Leptin | .554(.052) | .700 | 10.58** |
| Age | .114(.027) | .238 | 4.20** |
| Gender | 2.62(.88) | .212 | 2.97** |
| Body Fat | .077(.070) | .083 | 1.10 |
| Total NREM Sleep Duration | .001(.005) | .010 | .86 |
| Step 2 | |||
| REM Sleep Duration (min) | −.022(.011) | −.101 | −2.03* |
| Model 3: Sleep Stages (%) as Predictors of Morning Leptin | |||
| Step 1 | |||
| Evening Leptin | .551(.054) | .697 | 10.29** |
| Age | .093(.025) | .194 | 3.66** |
| Gender | 2.25(.88) | .183 | 2.56* |
| Body Fat | .100(.071) | .108 | 1.42 |
| Total Sleep Duration (min) | −.003(.004) | −.037 | −.732 |
| Step 2 † | |||
| %SWS | .029(.03) | .051 | .980 |
| %Stage II | −.028(.026) | .055 | −1.05 |
| %Stage I | −.05(.053) | −.060 | −.944 |
| %REM | −.124(.045) | −.135 | −2.77* |
| Model 4: Sleep Stages Duration (min) as Predictors of Morning Leptin | |||
| Step 1 | |||
| Evening Leptin | .535(.055) | .676 | 9.73** |
| Age | .117(.029) | .244 | 4.08** |
| Gender | 2.75(.903) | .224 | 3.05** |
| Body Fat | .092(.073) | .099 | 1.27 |
| Step 2 | |||
| Stage I (min) | −.015(.02) | −.041 | −.74 |
| Stage II (min) | .004(.006) | .041 | .67 |
| SWS (min) | .008(.011) | .044 | .702 |
| REM (min) | −.024(.011) | −.121 | −2.17* |
Note:
p < .01
p < .05
For Model 3, each step 2 variable was run in a separate model to avoid multicollinearity.
Results
Relationship between Leptin, Sleep and Demographic Variables
For descriptive purposes, results of zero-order correlations are presented in Table 2. Evening and morning serum leptin correlated significantly with body fat (p <.001). Morning leptin was significantly related to participant age (p=.012). Neither evening nor morning leptin significantly correlated with any sleep variable (all p’s >.3). However, leptin ratio, a measure of the change in leptin from evening to morning, was associated significantly with percentage of REM sleep (p=.031). In other words, individuals who spent a greater portion of sleep in REM also had a steeper decrease in leptin from night to morning.
REM Sleep and Overnight Change in Leptin
We sought to examine whether percentage of REM sleep was related to the overnight change in leptin during sleep. In order to do this, we analyzed the relationship between percent REM sleep and morning leptin while controlling for evening leptin, age, gender, body fat and total sleep time. Evening leptin, gender, age, body fat and total sleep were entered in the first step of the hierarchical equation as covariates, percentage of REM sleep was entered in step two.
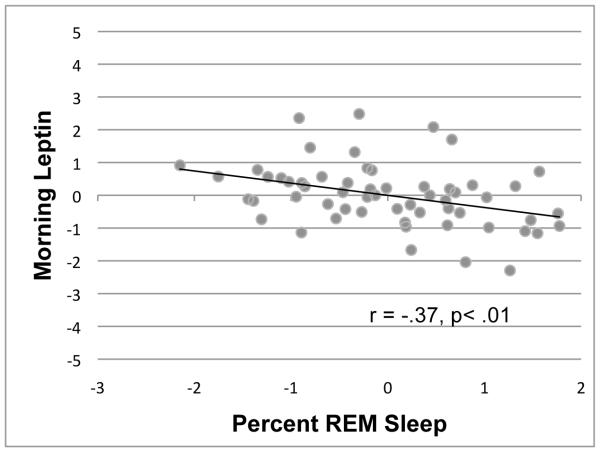
As shown in Table 3, morning leptin was correlated with older age, and gender (females had higher morning leptin than males), but not body fat or total sleep time, (R2 = .88; F(5, 57) = 77.88, p<.001). However, in and above these covariates, % REM predicted overnight change in leptin (R2 Δ =.02; F(6, 57) = 74.48, p<.001) . In other words, spending a greater proportion of the night in REM predicted a greater overnight change in leptin. A one percent increase in the percentage of REM sleep was associated with a .124 decrease in morning leptin, (p=.008). To determine whether the relationship between percent REM sleep and leptin differed between men and women, a gender by percent REM sleep interaction term was added to the model. The interaction term was not statistically significant (p=.14), and was therefore removed from the final model. Figure 2 shows the relationship between percentage of REM sleep and morning leptin levels, while controlling for evening leptin, age, body fat, and total sleep duration. As shown here, the individuals with the highest percentage of REM sleep had lower morning leptin levels. We conducted an additional subgroup analysis selecting only subjects who had an adequate sleep opportunity (≥ 7 hours) based on their usual sleep time. The relationship between overnight change in leptin and percentage of REM sleep remained significant (T6,20 = −2.64, p=.019), even when excluding subjects whose sleep may have been limited by the morning blood draw.
Figure 2.
Relationship between percentage of REM sleep and morning leptin, while controlling for the effects of evening leptin, age, gender, body fat, and total sleep time. A greater percentage of REM sleep was accompanied by more marked reductions in leptin.
We also sought to determine whether absolute duration of REM sleep was related to change in leptin during sleep. As shown in Table 3, Model 2, evening leptin, age, gender, total NREM sleep duration, and body fat were entered as covariates (step 1); next, duration of REM sleep was entered (step 2). As shown in Table 3, evening to morning change in leptin was correlated with older age, and gender, but not body fat or total NREM sleep duration, (R2 = .88; F(5, 57) = 76.98, p<.001). However, in and above these covariates, total REM sleep duration predicted overnight change in leptin (R2 Δ =.009; F(6, 57) = 68.69, p< .001). A one-minute increase in the total duration of REM sleep was associated with a .02 decrease in morning leptin, (p=.047). Again, we conducted an additional subgroup analysis selecting only subjects who had an adequate sleep opportunity (≥ 7 hours) based on their usual sleep time. The relationship between overnight change in leptin and REM sleep duration remained near significant (T6,20 = −2.14, p=.05), when excluding subjects whose sleep may have been limited by the morning blood draw.
NREM Sleep Stages and Change in Leptin
To further explore the data we sought to determine whether there was a relationship between other stages of sleep and overnight change in leptin. A separate model was run for percentage of stage I, II, and SWS. Consistent with earlier analyses, evening leptin, age, gender, percent body fat and total sleep time were entered as covariates, (R2 = .88; F(4, 57) = 98.07, p <.001). As shown in Table 3, no significant relationship was found between percentage of stage I, (p=.35), stage II, (p=.30) or SWS (p=.33) and morning leptin. We also examined absolute duration of stage I, stage II, and SWS as a predictor of morning leptin. Again, after controlling for the covariates (evening leptin, age, gender and body fat), stage I, (p=.46), stage II, (p= .51) and SWS (p=.49) were not significant predictors of overnight change in leptin. Thus, no significant relationship was observed between NREM sleep stages and overnight change in leptin levels.
Discussion
Our results suggest that a higher percentage of REM sleep is related to a greater reduction in leptin during sleep. This finding is consistent with animal studies, which have reported that higher percentage of REM sleep is related to lower leptin levels in rats (Sinton et al., 1999). Moreover, the relationship between REM sleep and change in leptin during sleep was not explained by total sleep duration. This finding is consistent with a study by Knutson et al (2011) that found no relationship between sleep duration, measured by wrist actigraphy, and leptin levels in a sample of eighty obese individuals. A large population based study found short sleep duration (measured by sleep diary) was related to lower morning leptin levels (Taheri et al., 2004). However, consistent with our study findings, in the same sample, no significant relationship was found between objective total sleep duration (measured by overnight polysomnography) and morning leptin levels (Taheri et al., 2004). Our study differs from Taheri et al. (2004) in that we excluded individuals with sleep apnea, and importantly leptin was measured both before and after sleep, allowing us to look at overnight changes in leptin, which may be more consistently impacted by changes in sleep.
Several studies have reported changes in leptin levels in individuals undergoing sleep restriction. For example, Spiegel et al. (2004) found decreased leptin levels in male subjects allowed a four hour sleep opportunity compared to subjects who were well rested. In a study by Mullington et al. (2003) eighty-eight hours of total sleep deprivation with scheduled meals resulted in a decrease in the diurnal amplitude of the leptin rhythm. Past research suggests that mean 24-hour leptin levels and amplitude of the 24-hour leptin profile are more consistently affected by sleep loss (Guilleminault et al., 2003, Mullington et al., 2003, Spiegel et al., 2004) than fasting morning leptin levels. Future studies examining diurnal changes in leptin in individuals undergoing selective REM sleep deprivation are needed. We did not find a significant relationship between the overnight change in leptin and any other sleep stage. Although no relationship was observed between overnight change in leptin and SWS, we did not examine SW activity, which may have a relationship to leptin levels.
The findings of this study are that REM sleep is associated with a decrease in leptin during sleep. Previous studies suggest that mean 24-hour leptin levels and the amplitude of the 24-hour leptin profile respond to change of energy expenditure and predict subsequent food intake, but that morning levels do not (van Aggel-Leijssen et al., 1999, Chin-Chance et al., 2000). Thus, the greater overnight reduction in leptin observed in this study may reflect greater amplitude of the 24-hour leptin profile when REM sleep is increased. This would be consistent with prior research suggesting that sleep loss is associated with a reduction in the amplitude of leptin (Mullington et al., 2003). Greater amplitude of diurnal leptin, facilitated by REM sleep, thus may contribute to suppression of appetite. However, future studies employing caloric monitoring in individuals undergoing REM sleep deprivation are needed.
Possible mechanisms linking REM sleep and change in leptin during sleep may include hormones involved in goal directed behavior such as orexin and melanin-concentrating hormone (MCH). Orexin neuropeptides are thought to play a role in both the regulation of food intake and sleep. Leptin may impact REM sleep through orexin-containing neurons. In an experimental study, ablation of orexin neurons in the rat resulted in increased bouts of REM sleep (Beuckmann et al., 2004). Orexin neurons activate cholinergic REM cells and may also impact regulation of REM sleep through serotonergic neurons in the dorsal raphe (Brown et al., 2001, Sinton and McCarley, 2004) Metabolic signals, including leptin, are thought to influence orexin neuronal activity to regulate energy homeostasis and arousal (Zhu et al., 2002).
Melanin-concentrating hormone (MCH) has also been recognized as an important regulator of energy balance, which may play a role in REM homeostasis. The distribution of MCH neurons overlaps with those of orexin. In animal models, MCH neurons have been found to be active during REM sleep (Arnulf et al., 2006). In addition, pharmacological administration of MCH in rats induces a dose dependent increase in REM sleep (Schuld et al., 2000). MCH levels rise dramatically in the absence of leptin suggesting these neurons may also be influenced by metabolic processes (Schuld et al., 2000). A study by Verret (2003) using an animal model found that administration of MCH stimulated leptin mRNA synthesis and secretion of leptin. Although research has largely been limited to animal models, results of these studies suggest that these hormones may play a role in sleep and metabolic homeostasis.
Our findings suggest that REM sleep is associated with change in leptin during sleep. This association was independent of body fat, age, gender, and total sleep time. This finding may be relevant to our understanding of the mechanisms underlying the relationship between sleep and obesity. Strengths of our study include the exclusion of individuals with chronic or acute medical conditions. Overnight polysomnography also allowed for an objective measure of sleep duration and the exclusion of individuals with occult sleep apnea. While some prior studies included individuals with mild sleep apnea (AHI < 10), we used a low threshold for exclusion of individuals with sleep apnea (AHI ≥ 1). Shift workers were also excluded. In addition to these strengths, this study also has some important limitations. Leptin displays a 24-hour profile that is dependent on circadian rythymicity. Although this study measured leptin at two time points, we were unable to examine comprehensively the diurnal changes in leptin. While shift workers were excluded from this study, we did not control for individual differences in circadian phase. Future studies should examine the relationship between leptin and REM sleep taking into account circadian phase, as circadian mechanisms may play a role in hunger and satiety. The study protocol used a standard wake and blood draw time. While this was necessary in order to allow us to compare leptin levels at a set clock time across all participants, we were not able to take into account individual differences in habitual wake time. However, when we selected a subset of participants who, based on their chosen sleep time, had an adequate sleep opportunity, the relationship between leptin and percent REM sleep remained significant. Other limitations include a modest sample size (n=58), and the fact that the caloric intake prior to the study was not standardized. Leptin levels reflect long-term changes in fuel status. Although leptin does not change acutely following a normal meal (Korbonits et al., 1997), the timing of meal patterns throughout the day may impact the diurnal rhythm of leptin (Schoeller et al., 1997). While prior to the study participants slept and ate according to their usual schedule, and shift workers were excluded from the study, we were not able to control for caloric timing or intake prior to the study. Thus, we cannot completely rule out an effect of prior caloric intake on the study findings. Future studies examining the relationship between leptin and REM should control for prior caloric intake. Another limitation of the study is that while individuals with apnea were excluded from the study sample, mean total sleep time (324 min) and sleep efficiency (80%) was somewhat low; this was likely due to a “first night effect.” Respiratory measurements may also have reduced sleep efficiency in the study sample. Thus, total sleep duration and architecture may be impacted by this well known “first night effect.” However, the relationship between REM sleep and overnight change in leptin remained significant even when accounting for total sleep time, suggesting that the observed relationship between leptin and REM was not accounted for by individual differences in total sleep duration. This study focused on the acute effects of REM sleep immediately preceding morning blood draw. These findings may not correspond to the chronic effects of sleep deprivation.
The findings of this study are that REM sleep is associated with a decrease in leptin during sleep. Studies examining the effects of selective REM sleep deprivation on leptin levels in humans are needed. Further research should examine the mechanisms by which sleep, and specific sleep stages, impact appetite and obesity, as increased understanding of these mechanisms may lead to more targeted therapeutic interventions.
Acknowledgments
Dr. Somers has received research support from Phillips Respicardia, Glaxo Smith Kline, U-Health, Rhonda Grey and Res Med, and is working with Mayo Health Solutions and their industry partners on intellectual property related to sleep, obesity, and cardiovascular disease. The other authors have no financial conflicts of interest to report.
Drs. Olson and Somers contributed significantly to the study conception and design. Dr. Olson analyzed the study data. Drs. Olson, Hamilton, and Somers contributed to the interpretation of the data. All authors contributed to the intellectual content and manuscript revisions.
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health (NIH) under Award Number R01HL065176, R01HL114024, and R01HL114676. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
This work was conducted at the Mayo Clinic, Rochester, MN.
References
- Arnulf I, Lin L, Zhang J, et al. CSF versus serum leptin in narcolepsy: is there an effect of hypocretin deficiency? Sleep. 2006;29:1017–24. doi: 10.1093/sleep/29.8.1017. [DOI] [PubMed] [Google Scholar]
- Balkau B, Deanfield JE, Despres JP, et al. International Day for the Evaluation of Abdominal Obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation. 2007;116:1942–51. doi: 10.1161/CIRCULATIONAHA.106.676379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barf RP, Van Dijk G, Scheurink AJ, et al. Metabolic consequences of chronic sleep restriction in rats: changes in body weight regulation and energy expenditure. Physiol Behav. 2012;107:322–8. doi: 10.1016/j.physbeh.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Beuckmann CT, Sinton CM, Williams SC, et al. Expression of a poly-glutamine-ataxin-3 transgene in orexin neurons induces narcolepsy-cataplexy in the rat. J Neurosci. 2004;24:4469–77. doi: 10.1523/JNEUROSCI.5560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology. 2001;40:457–9. doi: 10.1016/s0028-3908(00)00178-7. [DOI] [PubMed] [Google Scholar]
- Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 2008;31:517–23. doi: 10.1093/sleep/31.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin-Chance C, Polonsky KS, Schoeller DA. Twenty-four-hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake. J Clin Endocrinol Metab. 2000;85:2685–91. doi: 10.1210/jcem.85.8.6755. [DOI] [PubMed] [Google Scholar]
- Guilleminault C, Powell NB, Martinez S, et al. Preliminary observations on the effects of sleep time in a sleep restriction paradigm. Sleep Med. 2003;4:177–84. doi: 10.1016/s1389-9457(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–6. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Galli G, Zhao X, Mattingly M, Cizza G. No association between leptin levels and sleep duration or quality in obese adults. Obesity (Silver Spring) 2011;19:2433–5. doi: 10.1038/oby.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbonits M, Trainer PJ, Little JA, et al. Leptin levels do not change acutely with food administration in normal or obese subjects, but are negatively correlated with pituitary-adrenal activity. Clinical endocrinology. 1997;46:751–7. doi: 10.1046/j.1365-2265.1997.1820979.x. [DOI] [PubMed] [Google Scholar]
- Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW. Altered sleep regulation in leptin-deficient mice. American journal of physiology. Regulatory, integrative and comparative physiology. 2006;290:R894–903. doi: 10.1152/ajpregu.00304.2005. [DOI] [PubMed] [Google Scholar]
- Licinio J, Mantzoros C, Negrao AB, et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med. 1997;3:575–9. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- Little TD, Bovaird JA, Widaman KF. On the Merits of Orthogonalizing Power Terms: Implications for Modeling Interactions Among Latent Variables. Struct Equ Modeling. 2006;13:497–519. [Google Scholar]
- Liu X, Forbes EE, Ryan ND, Rofey D, Hannon TS, Dahl RE. Rapid eye movement sleep in relation to overweight in children and adolescents. Arch Gen Psychiatry. 2008;65:924–32. doi: 10.1001/archpsyc.65.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–61. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- Moraes DA, Venancio DP, Suchecki D. Sleep deprivation alters energy homeostasis through non-compensatory alterations in hypothalamic insulin receptors in Wistar rats. Hormones and behavior. 2014;66:705–12. doi: 10.1016/j.yhbeh.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Chan JL, Van Dongen HP, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15:851–4. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. US Department of Health, Bethesda, MD; 1968. [DOI] [PubMed] [Google Scholar]
- Rontoyanni VG, Baic S, Cooper AR. Association between nocturnal sleep duration, body fatness, and dietary intake in Greek women. Nutrition. 2007;23:773–7. doi: 10.1016/j.nut.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Schoeller DA, Cella LK, Sinha MK, Caro JF. Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest. 1997;100:1882–7. doi: 10.1172/JCI119717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuld A, Blum WF, Uhr M, et al. Reduced leptin levels in human narcolepsy. Neuroendocrinology. 2000;72:195–8. doi: 10.1159/000054587. [DOI] [PubMed] [Google Scholar]
- Sinton CM, Fitch TE, Gershenfeld HK. The effects of leptin on REM sleep and slow wave delta in rats are reversed by food deprivation. J Sleep Res. 1999;8:197–203. doi: 10.1046/j.1365-2869.1999.00158.x. [DOI] [PubMed] [Google Scholar]
- Sinton CM, Mccarley RW. Neurophysiological mechanisms of sleep and wakefulness: a question of balance. Semin Neurol. 2004;24:211–23. doi: 10.1055/s-2004-835067. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, L'hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- St-Onge MP, O'keeffe M, Roberts AL, Roychoudhury A, Laferrere B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012;35:1503–10. doi: 10.5665/sleep.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- Theorell-Haglow J, Berne C, Janson C, Sahlin C, Lindberg E. Associations between short sleep duration and central obesity in women. Sleep. 2010;33:593–8. [PMC free article] [PubMed] [Google Scholar]
- Van Aggel-Leijssen DP, Van Baak MA, Tenenbaum R, Campfield LA, Saris WH. Regulation of average 24h human plasma leptin level; the influence of exercise and physiological changes in energy balance. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1999;23:151–8. doi: 10.1038/sj.ijo.0800784. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen WM, Hublin C, Sallinen M, Harma M, Hirvonen A, Porkka-Heiskanen T. Prolonged sleep restriction affects glucose metabolism in healthy young men. International journal of endocrinology. 2010;2010:108641. doi: 10.1155/2010/108641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret L, Goutagny R, Fort P, et al. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Yamanaka A, Kunii K, Tsujino N, Goto K, Sakurai T. Orexin-mediated feeding behavior involves both leptin-sensitive and -insensitive pathways. Physiol Behav. 2002;77:251–7. doi: 10.1016/s0031-9384(02)00843-0. [DOI] [PubMed] [Google Scholar]